Abstract
Ca2+/calmodulin-dependent protein kinase II α (CaMKIIα) signaling in the brain plays a critical role in regulating neuronal Ca2+ homeostasis. Its dysfunctional activity is associated with various neurological and neurodegenerative disorders, including Parkinson's disease (PD). Using computational modeling analysis, we predicted that, two essential cysteine residues contained in CaMKIIα, Cys30 and Cys289, may undergo redox modifications impacting the proper functioning of the CaMKIIα docking site for Ca2+/CaM, thus impeding the formation of the CaMKIIα:Ca2+/CaM complex, essential for a proper modulation of CaMKIIα kinase activity. Our subsequent in vitro investigations confirmed the computational predictions, specifically implicating Cys30 and Cys289 residues in impairing CaMKIIα:Ca2+/CaM interaction. We observed CaMKIIα:Ca2+/CaM complex disruption in dopamine (DA) nigrostriatal neurons of post-mortem Parkinson's disease (PD) patients' specimens, addressing the high relevance of this event in the disease. CaMKIIα:Ca2+/CaM complex disruption was also observed in both in vitro and in vivo rotenone models of PD, where this phenomenon was associated with CaMKIIα kinase hyperactivity. Moreover, we observed that, NADPH oxidase 2 (NOX2), a major enzymatic generator of superoxide anion (O2●-) and hydrogen peroxide (H2O2) in the brain with implications in PD pathogenesis, is responsible for CaMKIIα:Ca2+/CaM complex disruption associated to a stable Ca2+CAM-independent CaMKIIα kinase activity and intracellular Ca2+ accumulation. The present study highlights the importance of oxidative stress, in disturbing the delicate balance of CaMKIIα signaling in calcium dysregulation, offering novel insights into PD pathogenesis.
Graphical abstract
Visual Abstract. NOX2 activity plays a critical role in inducing redox modifications to Cys30 and Cys289, which in turn affect the functionality of the CaMKIIα docking site for Ca2+/CaM. This cascade ultimately shifts the equilibrium towards a Ca2+/CaM-independent CaMKIIα kinase activity, affecting Ca2+ homeostasis in DA neurons. This phenomenon is particularly relevant in Parkinson's disease and lends further support to the idea of inhibiting NOX2 as a potential therapeutic approach for PD.
Highlights
-
•
Oxidative stress and Impaired calcium signaling are mayor events in PD.
-
•
CaMKIIα/Ca2+/CaM complex sustains the proper cellular functions of calcium.
-
•
Cys30 and Cys289 residues in CaMKIIα are responsible for CaMKIIα/Ca2+/CaM interaction.
-
•
NADPH oxidase 2-mediated disruption of CaMKIIα/Ca2+/CaM complex is relevant in PD.
1. Introduction
The CaMKII protein kinase family are serine/threonine kinases regulated by the Ca2+/CaM complex involved in the coordination and execution of Ca2+ signaling in many biological processes [1], including modulation of neuronal excitability and synaptic plasticity [2,3], synthesis of catecholamines [4] and neuroimmune responses [5].
There are four CaMKII genes in humans, α, β, γ and δ, which have similar sequence identity and are expressed in at least 38 isoforms [6] through alternative splicing. CaMKIIα is highly expressed in the mammalian brain [7] and performs its function as a dodecameric protein homocomplex composed of two stacked rings, each formed by six subunits. These subunits contain a hub domain (C-terminal), a kinase domain (N-terminal), and a linker connecting the hub to the kinase domain (Fig. 1A). This specific conformation confers to CaMKIIα subunits the ability to co-exist in an “auto-inhibited” (closed) and an “active” (open) conformation depending on the state of the linker extension and the relative exposure of the docking site for the Ca2+/CaM complex.
Fig. 1.
Dodecameric model of CaMKIIα and dynamic molecular dynamics prediction of C30 and C289 distance.(A) Top and side views of the full dodecamer model of CaMKIIα. In this conformer there are four CaMKII subunits in the open conformation and the remaining eight in the closed conformation. (B) A detailed view of two adjacent CaMKII subunits. The C30 and C289 are represented by two larger yellow spheres. (C) Snapshots from one of the “all atoms” molecular dynamics simulations highlighting the open and closed conformations. The double yellow arrows indicate the distance between C30 and C289. The graph shows the time evolution of the distance between those two residues. Note that the minimum Cys-Cys distance reached during this simulation is less than 6 Å.
The binding of Ca2+/CaM initiates conformational changes that liberate the kinase domain from the regulatory domain, thereby initiating the activation of the enzyme [[8], [9], [10], [11]]. A later trans-phosphorylation of Thr286 (located within the autoinhibitory region of CaMKII) in a neighboring subunit leads to a Ca2+/CaM independent CaMKII activity by preventing the kinase domain from reassociating with the autoinhibitory region [[11], [12], [13], [14], [15]]. This bidirectional dynamic transition from Ca2+/CaM dependent to Ca2+/CaM independent is pivotal for CaMKIIα, enabling the conversion of a transient Ca2+ stimulus into sustained physiological or disease-related activity.
Dysregulation of Ca2+ homeostasis/signaling and redox imbalance have been suggested as key factors in various forms of neurodegeneration, including PD [[16], [17], [18], [19]]. DA nigrostriatal neurons are characterized by a unique physiology based on their “pace-making” activity relying on Ca2+. This requires precise regulation of certain systems, including CAMKIIα-Ca2+/CaM signaling and mitochondrial Ca2+ buffering to avoid elevated and harmful concentrations of cytosolic Ca2+. Ca2+ cycling and signaling is strictly associated with organelles generated O2●-,H2O2 [20]. These physiological features of nigrostriatal DA neurons correlate with increased basal levels of intracellular Ca2+ and oxidants production [19] and reduce their ability to tolerate further intracellular oxidative insults and storage of Ca2+.
Mitochondrial defects and oxidative stress are widely implicated in PD pathogenesis. While it's commonly believed that the detrimental redox changes observed in PD stem from mitochondria, emerging evidence suggests that oxygen-related oxidant species production is influenced by a functional interplay between mitochondria and NOX2 [21], which may be significant in neurodegenerative processes like PD [22]. Our prior research has highlighted the critical involvement of NOX2 in various aspects of PD pathogenesis [17]. In the current investigation, we aim to clarify the role of CaMKIIα as a potential link between neuronal oxidative injury and Ca2+ dysregulation.
The redox sensitivity of CaMKII kinase activity has already been described [23]. A more recent study demonstrated that oxidation of two methionine residues directly alters the autoinhibitory motif in CaMKII, resulting in increased autophosphorylation of Thr287 and relative Ca2+/CaM independent CaMKII activity [24]. These findings suggest an alternative modulatory mechanism for activating CaMKII, linking oxidative stress with Ca2+ signaling. However, while recent studies report redox sensitive properties of CaMKIIα in the heart and lung [24] and a large amount of information addresses the pathogenic role of dysfunctional CaMKIIα in neurodegeneration [25,26], there is still poor information on redox modulation of CaMKIIα activity in the brain.
Previous research described the pivotal role of CaMKII in modulating the voltage-gated CaV2.1 channels, primarily through its influence on the channels' C-terminal, which effectively slows down voltage-dependent inactivation [27]. Additional experimental evidence underscores CaMKII-mediated phosphorylation of specific isoforms of voltage gated Ca2+ channels (VGCCs) in neurons, by generally prolonging the opening of these channels, thereby promoting a sustained inward current for calcium [[28], [29], [30]]. All together, these findings support the notion that under certain pathological conditions, such as oxidative stress, sustained aberrant CaMKII kinase activity exacerbates the function of several VGCCs. This heightened activity culminates in a detrimental cascade, characterized by excessive Ca2+ influx with a consequent dysregulated Ca2+ signaling. Deeper insights into the axis: Oxidative stress, aberrant CaMKII kinase activity and Ca2+ dysregulation, may shed light on the mechanisms underlying CaMKII-mediated cellular dysfunction and offer the potential for the discover of therapeutic targets for mitigating pathological consequences in PD.
2. Methods
2.1. Reagents
To develop our study, we used the following antibodies.
| Host | Antibody | Catalog # | Vendor | Dilution | Use |
|---|---|---|---|---|---|
| Rabbit | β-actin | ab8227 | Abcam | 1:10,000 (WB) | Protein loading control |
| Sheep | Tyrosine Hydroxylase | AB1542 | Millipore | 1:2000 (IHC) | DA neurons marker |
| Mouse | CaMKIIα | ab2260 | Abcam | 1:500 (WB) 1:1000 (PLA) |
Redox WB PL CaMKIIα; CaM |
| Rabbit | Calmodulin | ab45689 | Abcam | 1:500 (WB) 1:1000 (PLA) |
WB PL CaMKIIα; CaM |
| Rabbit | CaMKIIα pThr286 | ab124880 | Abcam | 1:1000 (IHC) | Immunohistochemistry |
| Mouse | NOX2/gp91phox | ab80897 | Abcam | 1:1000 (ICC) | PLA NOX2 activity |
| Rabbit | NCF1[FPR13131–25] (p47phox) | ab181090 | Abcam | 1:1000 (ICC) | PLA NOX2 activity |
All reagents were purchased from Sigma-Aldrich, unless otherwise specified. The selective NOX2 inhibitors, Nox2ds-tat (for in vitro studies) and CPP11–H (for in vivo studies) were provided by Dr. Patrick J. Pagano, Dept of Pharmacology and Cellular Biology – University of Pittsburgh.
2.2. Molecular dynamics simulations
Computational simulations of CaMKIIα were performed using the AMBER15 software package, with all of them - except for the minimizations - using the GPU version of the PMEMD program. The crystal structure of CaMKIIα in a closed/auto-inhibited conformation (PDB structure 3SOA) was used as the starting structure [31] of human CaMKII in its α isoform, with a β7 linker (the shortest linker in all known splice variants). Some of the subunits in this dodecamer were converted computationally to open conformations based on the 2WEL PDB crystal structure of the CaMKIIδ isoform, which is in the open conformation. This was feasible since the kinase domains of both isoforms have a high sequence similarity of about 93 %. The final structure contained eight closed and four open sub-units of CaMKIIα in a dodecameric configuration (Fig. 1).
In order to bring the modeled molecule to a stable conformation, we ran several cycles of minimization and equilibration. We employed the Amber12 force field and the TIP3P water model. The systems were kept at a temperature of 298 K, using Langevin dynamics with a collision frequency of 2 ps−1, and part of the protocol used pressure control, via a weak-coupling Berendsen barostat, with a relaxation time of 2 ps. The SHAKE algorithm was adopted, allowing the use of a 2-fs time step. The protocol followed for minimization and equilibration was: 1) 100 cycles of minimization, using the XMIN method, followed by 5000 cycles using steepest descent and another 5000 steps using conjugate gradient; 2) 1 ns of heating to 298 K, followed by another ns at constant T and P (1 atm), and finally 20 ns with constant T. The simulation box consisted of one CaMKIIα dodecamer complex with eight subunits in the closed conformation and four subunits in the open conformation. All-atom simulations were performed on CaMKIIα in a box with explicit water plus ions to neutralize electrostatic charges. Systems were >1.3x106 atoms and all runs were >300ns. For all the runs we used Nvidia GeForce TITAN-X GPUs.
2.3. Ventral midbrain primary cultures
Neuronal cultures were prepared from Sprague-Dawley (Charles River, Wilmington, MA, USA) rats’ embryos at day 17 (E−17) obtained from two to three pregnant dams. Brains extracted from E−17 were immersed in cold MEM containing 2 % FBS, 2 % HS, 1 mM Sodium Pyruvate, 1 % NEAA, 1 g/l Glucose, 200 I·U./ml Penicillin and 200 l U./ml Streptomycin (Cellgro). Following anatomical landmarks, the SN, without the VTA, was dissected from a coronal midbrain slice. The cells obtained by enzymatic dissociation using trypsin followed by mechanical trituration, were plated at a density of 5X104 cells/cm2. The cultures were maintained at 37 OC in a humidified atmosphere of 5 % CO2 and 95 %. After 48 h, the medium was replaced with Neurobasal medium supplemented with B27 (1:50 from stock), Glutamax (1:100 from stock), Albumax (1:100 from stock), 200 I·U./ml Penicillin and 200 l U./ml Streptomycin (Cellgro). The medium was, subsequently replaced two times/week. Experiments were performed at day-in vitro 14 (DIV-14). As previously reported, primary ventral midbrain cultures, under the described culture conditions, contain about 2–3% glial cells, 5–10 % DA neurons and a majority of other neurons (MAP2-positive) [32].
All procedures were performed with the approval of the University of Pittsburgh Animal Care and Use Committee.
2.4. Mutagenesis experiments in Drosophila S2R + adherent cells
CaMKIIα and calmodulin expression in Drosophila S2R + cells was accomplished as follows: CaMKIIα and CaM coding sequences were copied from human cDNA clones CAMK2A transcript variant 2 (catalog SC109000, Origene, Rockville MD) and CALM1 Human calmodulin 1 transcript variant 1 (catalog SC115829, Origene, Rockville MD), using PCR primers to add flanking restriction sites, with the following primers: EcoRI-CAMK2A-FWD; GAGAGAATTCATGGCCACCATCACCTGCAC, CAMK2A-STP-SalI-REV; TCTCGT- CGACTTAGTGGGGCAGGACGGAGGGCG, MfeI-CALM1-FWD; GAGACAATTGATGGCT GATCAGCTGACCGA, CALM1-STP-SalI-REV; TCTCGTC GACTCATTTTGCAGTCATCAT- CT. Products were ligated into appropriately gapped vector pRmHA3 (Drosophila Genomics Research Center, Catalog 1145) to generate pRmHA3- CaMKII and pRmHA3-CALM1. Point mutation variants (pRmHA3-CaMKIIC30A, pRmHA3-CaMKIIC289A, and pRmHA3-CaMKIIC30A, C289A) were generated by site directed mutagenesis of pRmHA3-CaMKII using QuickChange Lighting Kit (Agilent, Catalog 210518.) The full coding sequence of all pRmHA3-CaMKII constructs was verified by Sanger sequencing. pRmHA3- CaMKII constructs were introduced to Drosophila S2R + cells (Drosophila Genomics Research Center, catalog 181) cultured under standard conditions in Schneider's media with 10 % FBS and Pen-Strep at densities between 0.5 and 2.0 x 106, by transient “Effectene” transfection (Qiagen 301425) according to manufacturer recommendations. Expression was induced with 500 μM CuSO4 at the time of transfection and transfected cells were incubated for 48 h under standard conditions. pRmHA3-mCherry was co-transfected with pRmHA3-CaMKII constructs to evaluate transfection efficiency and rule out morphological indications of toxicity in transfected cells. Western blot analysis was performed to assess the expression levels of human CaMKIIα and CaM in cells (Supplemental Fig. 1). Oxidative stress was induced by treating S2R+ cells with 5 nM H2O2 in normal culture media for 30 min before immunocytochemistry and lysis for immunoblot assays as described below.
2.5. Immunocytochemistry
At the endpoint, cells were fixed in 4 % paraformaldehyde (PFA), washed with phosphate buffered saline (PBS) to remove the excess of PFA. The cells were also permeabilized with 0.03 % Triton X-100 in 10 % NDS for 1 h and incubated overnight with primary antibodies, including tyrosine hydroxylase (TH), as marker of dopaminergic neurons (refer to Table 1). Fluorescently labeled secondary antibodies were then added for a 1-h incubation at room temperature. After washing with PBS to remove secondary antibodies, the coverslips were mounted on microscopy glass slides using Gelvatol. Proximity ligation assay (PLA; see in the methods section) was also performed in cell cultures to assess levels of CaMKIIα:CaM interaction.
Table 1.
List of the validated antibodies used in this study
| Host | Antibody | Catalog # | Vendor | Dilution | Use |
|---|---|---|---|---|---|
| Rabbit | β-actin | ab8227 | Abcam | 1:10,000 (WB) | Protein loading control |
| Sheep | Tyrosine Hydroxylase | AB1542 | Millipore | 1:2000 (IHC) | DA neurons marker |
| Mouse | CaMKIIα | ab2260 | Abcam | 1:500 (WB) 1:1000 (PLA) |
Redox WB PL CaMKIIα; CaM |
| Rabbit | Calmodulin | ab45689 | Abcam | 1:500 (WB) 1:1000 (PLA) |
WB PL CaMKIIα; CaM |
| Rabbit | CaMKIIα pThr286 | ab124880 | Abcam | 1:1000 (IHC) | Immunohistochemistry |
| Mouse | NOX2/gp91phox | ab80897 | Abcam | 1:1000 (ICC) | PLA NOX2 activity |
| Rabbit | NCF1[FPR13131–25] (p47phox) | ab181090 | Abcam | 1:1000 (ICC) | PLA NOX2 activity |
2.6. Calcium live imaging
Primary ventral midbrain cultures were grown in 35 mm petri dishes with a glass coverslip inserted at the bottom (Thermo Fisher). At DIV 15, the medium was replaced with HEPES-buffered salt solution (standard HBSS) of the following composition (in mM): NaCl 137, KCl 5, NaHCO3 10, KH2PO4 0.6, Na2HPO4 0.6, MgSO4 0.9, CaCl2 1.4, HEPES 20, and glucose 5.5 (pH adjusted to 7.4 with NaOH). The cultures were then exposed to (i) rotenone (50 nM), (ii) rotenone + Autocamtide 2 related inhibitor peptide myristoylated (AIP; 50 nM), or (iii) rotenone + Nox2ds-tat (10 μM) for 1, 2, or 4 h.
Thirty minutes before the end points, Fluo 8-AM (Abcam) was added at a concentration of 5 μM and incubated at 37 °C with the cells. Subsequently, cells were washed with standard HBSS. Fluo-8-AM related fluorescence was detected at excitation/emission wavelengths of 490/525 nm using a Nikon Eclipse Ti2 inverted confocal microscope. Fluorescence intensity was measured using Nikon software in 15–25 individual neurons for each microscopic field at 60X magnification (4 fields per coverslip). Background fluorescence, determined from three or four cell-free regions of the coverslips, was subtracted from all signals.
2.7. Animal studies
All experiments utilizing animals were approved by the Institutional Animal Care and Use Committee of the University of Pittsburgh in accordance with published National Institutes of Health guidelines. For the in vivo experiments, we used retired male breeder Lewis rats (7–9 months old - Envigo). The animals were maintained under standard conditions of 12 h light/dark cycle in a 22 ± 1 °C temperature-controlled room with 50%–70 % humidity. Water and food were provided ad libitum. Animals were adapted for one week before the experiments, and randomly divided into the following groups of treatments: (i) Vehicle, (ii) Rotenone and (iii) Rotenone + CPP11H.
Before each daily treatment, body weights were recorded for each animal. Rotenone was administered intraperitoneally once a day at a dose of 2.8 mg/kg until the end of the treatment (5 days). The solution was prepared as a 50X stock dissolved in pure dimethyl sulfoxide at final concentration of 2 %, then diluted in Miglyol 812 N (Sasol North America, Inc, Houston, TX; distributed by Warner Graham, Baltimore, MD, USA) at final concentration of 98 %, and administered at 1 ml/kg. This five-day rotenone regimen does not produce nigrostriatal lesions, but significantly enhances several key PD-related pathogenic events [[33], [34], [35]]. Control animals received an equivalent amount of vehicle (2 % dimethyl sulfoxide 98 % Miglyol; i.p.). For CPP11H treatment, rats were dosed with 15 mg/kg by oral gavage concomitantly with rotenone. Control animals received an equal amount of vehicle (PEG 400; gavage). At the end point (5 days) animals were euthanized and Brains were collected from each animal.
2.8. Histology and immunohistochemistry
Animals were euthanized by CO2 inhalation followed by decapitation. The brains were removed following an intracardial perfusion with saline solution (NaCl 0.9 %) and a fixing perfusion with 4 % PFA. After 48 h, the brains were placed in 30 % sucrose in PBS for cryoprotection until infiltration was complete (at least 3 days).
For immunohistochemistry, brain slices (35 μm) containing Substantia nigra pars compacta were washed in PBS, treated with a blocking/permeabilizing PBS solution containing 10 % FBS and 3 % Triton X-100 for 1 h. The tissue was then exposed to a primary antibody PSS/FBS solution (for antibody dilutions, refer to Table 1). Finally, the slices were exposed to secondary antibodies for canonical immunohistochemistry or placed on glass microscopy slides to perform PLA [36].
2.9. Human tissue
Paraffin-embedded midbrain sections were obtained from the University of Pittsburgh Brain Bank. All banked specimens underwent standardized premortem neurological and postmortem neuropathological assessment. Diagnoses were confirmed and staging performed by the study neuropathologist (JKK) by examination of H&E, alpha-synuclein, tau, silver and ubiquitin stains of key sections needed for Braak staging (37). The study design was reviewed and approved by the University of Pittsburgh Committee for Oversight of Research Involving the Dead. Midbrain sections from 6 PD patients and 7 control subjects, matched for age and postmortem intervals, were used for the analysis; 10–15 cells were imaged per brain section, 3 sections for each patient.
| Sample | Male/Female | Age | Brain Weight | Postmortem Interval |
|---|---|---|---|---|
| Control | 4/3 | 67 ± 5 | 1248 ± 33 | 7.0 ± 0.8 |
| PD | 5/1 | 73 ± 4 | 1297 ± 29 | 9.8 ± 1.9 |
To eliminate endogenous fluorescence, human tissue was pre-treated with Sudan Black (Chemicon, Temecula, CA), an autofluorescence eliminating reagent according to the manufacturer's instructions.
2.10. Fluorescence measurements
Quantitative fluorescence measurements were conducted using a Nikon Eclipse Ti2 inverted confocal microscope, ensuring that images did not contain saturated pixels. To enable accurate comparisons, all imaging parameters (such as laser power, exposure, and pinhole) were kept constant across specimens. Fluorescence intensity was measured in predefined regions of interest drawn around the single cells (i.e. tyrosine hydroxylase (TH)-positive dopaminergic neurons).
In ventral midbrain (VMB) cultures or S2R + cell cultures, analyses were performed in three independent experiments. In VMB cultures, fluorescence signals were measured in 100–150 TH positive neurons per group of treatment per each independent experiment, while in S2R + cell cultures, 200–250 cells were analyzed per group of treatment per each independent experiment. The final analysis represents the normalized intensity average (assuming Vehicle group as 100 %) for each group of treatment in a single independent experiment.
For analyses conducted in rat substantia nigra pars compacta (SNpc), fluorescence in 60–100 TH-positive nigrostriatal neurons were assessed in a slice (with four slices per animal), totaling six animals per treatment group.
2.11. Proximity Ligation Assay
Proximity Ligation Assay (PLA) (Duolink; Sigma Aldrich) was performed as previously described [36] in 4 % PFA-fixed brain tissue (rat or human) or cell cultures (Drosophila S2R + or rat ventral midbrain cell cultures) to assess the level of interaction CaMKIIα:CaM under our experimental conditions. Samples were incubated with specific primary antibodies (refer to Table 1). PLA probes consisting of secondary antibodies (anti-Rabbit and anti-Mouse) conjugated with complementary oligos (plus and minus respectively) were added to the reaction and incubated. Ligation solution, consisting of two oligos and ligase, was added. In this assay, the oligos hybridize to the two PLA probes and join to a closed loop if they are in close proximity. An amplification solution, consisting of nucleotides and fluorescently labeled oligos, was added together with polymerase. The oligonucleotide arm of one of the PLA probes acts as a primer for “rolling-circle amplification” (RCA) using the ligated circle as a template, and this generates a concatemeric product.
Fluorescently labeled oligonucleotides hybridize to the RCA product. The PLA signal is visible as distinct fluorescent spots. Fluorescent images were acquired with a Nikon Eclipse Ti2 inverted confocal microscope and quantification was carried out at 60 -100X magnification. PLA validation was performed by primary antibody deletion (Suppl. Fig. 2).
2.12. Redox western blot
Levels of oxidized thiols (S–S) in CaMKII were measured with a redox Western blot technique. VMB primary cultures or Drosophila S2R + cells were lysed in 50 mM Tris-HCl pH 7.0, 2 % sodium dodecyl sulfate (SDS), 1 mM EDTA, protease inhibitor cocktail and 100 μM N-ethyl- maleimide (NEM), to stably alkylate free thiol residues (SH). The lysate was heated at 65 °C for 5 min to denature proteins and incubated for 20 min at room temperature. Proteins were precipitated in ice-cold acetone to remove un-reacted reagents, resuspended in 50 mM Tris-HCl pH 7, 2 % SDS, and 10 mM TCEP and heated at 65 °C for 5 min and incubated 20 min at room temperature to reduce oxidized thiol residues (e.g. S–S bridges) to free SH groups. Proteins were precipitated again, and the pellet was re-suspended in 50mMTris-HCl pH 7, 2 % SDS, and 100 μM polyethylene glycol-maleimide (PEG-NEM; 10 KDa) to label the formerly oxidized thiols. After 20 min of incubation, the proteins were precipitated and a Western blot for CaMKIIα was performed. PEG-NEM labeling of formerly oxidized thiols increases the molecular weight of the protein by 10 KDa for each labeled thiol. Blotted membranes were imaged with an Odyssey infrared scanner (LiCor), and the signal was quantified with the scanner's software. S-Nitrosation was performed replacing TCEP with the selective S–NO reductant solution, ascorbate 1 mM, and CuCl2 1 μM [37].
The “total protein” S–NO assay shown in Supplementary Fig. 3 was performed on SHSY-5Y cell lysates using the same procedure previously described, with the exception of the NEM-mediated alkylating reactions. Briefly, the lysates were first exposed to Alexa Fluor 680-NEM to label the pool of reduced thiols. After a reduction step with 1 mM ascorbate and 1 μM CuCl2, former S–NO residues were labeled with Alexa Fluor 800-NEM. The samples were then run on 4–12 % polyacrylamide gels, transferred to a PVDF membrane, and imaged with an Odyssey scanner. The fluorescent signals at 680 nm and 800 nm were quantified and analyzed as the ratio of S–NO to SH.
2.13. Dihydroethidium (DHE) staining
A 3 mM stock solution of DHE (D11347, Thermo Fisher) was prepared in DMSO and stored at −20 °C. Before use, the 3 mM DHE solution was thawed and kept protected from light due to DHE's light sensitivity. The 3 mM solution was then diluted with cell culture media to a final concentration of 3 μM DHE. The old media was aspirated from each well and replaced with fresh media containing 3 μM DHE. Cells were incubated for 20 min at 37 °C, protected from light. Subsequently, the cells were gently washed with 1 × PBS at room temperature for 5 min and then fixed with 4 % PFA for 20 min. After fixation, the cells were washed three times for 10 min each with 1 × PBS. Single coverslips were then extracted from the wells and mounted on glass slides for confocal microscopy imaging. Imaging parameters were set to avoid signal saturation under oxidative stress conditions and were maintained consistently across all experimental conditions. The final analysis represents the normalized intensity average, with the Vehicle group set as 100 %, for each treatment group in a single independent experiment and repeated for 3 independent experiments.
2.14. Statistical analyses
Each result presented here was derived from three (in vitro) to six independent experiments (in vivo). For simple comparisons of two experimental conditions, two-tailed, unpaired t-tests were used. Where variances were not equal, Welch's correction was used. For comparisons of multiple experimental conditions, one-way ANOVA was used, and if significant overall, post hoc corrections (Bonferroni) for multiple pairwise comparisons were made. P-values less than 0.05 were considered significant.
3. Results
Computational analysis of dodecameric CaMKIIα reveals Cys30/Cys289-mediated conformational changes in the docking site for Ca2+/CaM binding.
Examining the structural dynamics of CaMKIIα required a structure with protein subunits in the open/active conformation within the dodecameric complex. In the absence of such a structure, we used existing crystal structures of CaMKIIα in the closed conformation and CaMKIIα in an open configuration to create a model of a CaMKIIα dodecamer with four open subunits and eight closed subunits (see Methods). We ran 8 independent all-atoms Molecular Dynamics simulations with explicit water (see Methods) of the modeled dodecamer, for a total simulation time of around 2.6 μs. We evaluated the distance between the α-carbons of all the cysteines and identified two of them, Cys30 and Cys289, belonging to adjacent subunits that get very close to each other due to the overall motions of the protein complex (Fig. 1 B–C).
The analysis of the distances between α-carbons (CA) of Cys30 and Cys289 in independent simulations shows different outcomes emphasizing the stochasticity of the phenomenon. We found that in 4 cases the proximity between CAs (<10 Å) is transient and it survives for just a few nanoseconds. In two of the simulations we ran, the Cys30-Cys289 interaction is vanishingly rare or does not occur. Finally, in the remaining two cases, the distance between those two cysteines stabilizes around 10-15 Å for a significant period (Fig. 1 B–C).
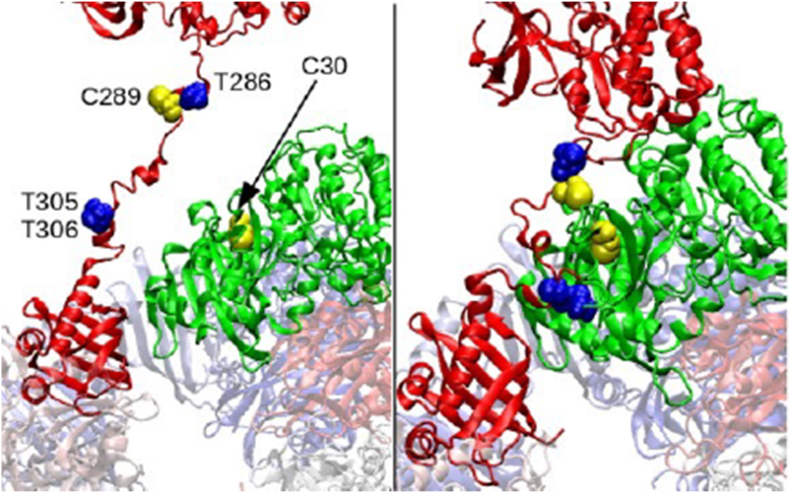
In most of the simulations, after ∼100ns, the CA-CA distance drops to less than 20 Å with a minimum separation below 6 Å. Our modeling revealed that Cys 30 and Cys 289 residues of two adjacent CaMKIIα subunits remain generally in proximity. The evidence provides prediction for a possible disulfide bridge (S–S bridge) formation between the thiol residues of Cys 30 and Cys 289 under oxidative stress conditions. Moreover, Cys30-Cys289 proximity and their relative potential in forming a S–S bridge may lead to a switch mechanism, where the presence of the S–S bridge locks the docking site for the Ca2+/CaM complex (Threonine residues 305 and 306) [38,39], forbidding its binding, while the absence of the S–S bridge allows it (Fig. 2).
Fig. 2.
Close-up view of two subunits of CaMKIIα. The computational analysis predicts a localization of Cys30 and Cys289 compatible with the formation of a disulfide bridge (S–S). As showed, T305 and T306 (binding region of CaM), are exposed when the C30–C289 bridge is not formed (left panel), allowing CaMKII-CaM interaction. When the bridge is formed (right panel), the accessible area to the binding site becomes greatly reduced, possibly preventing CaM binding.
3.1. Redox changes of Cys30 and Cys289 are critical in the formation of CaMKIIα-Ca2+/CaM complex
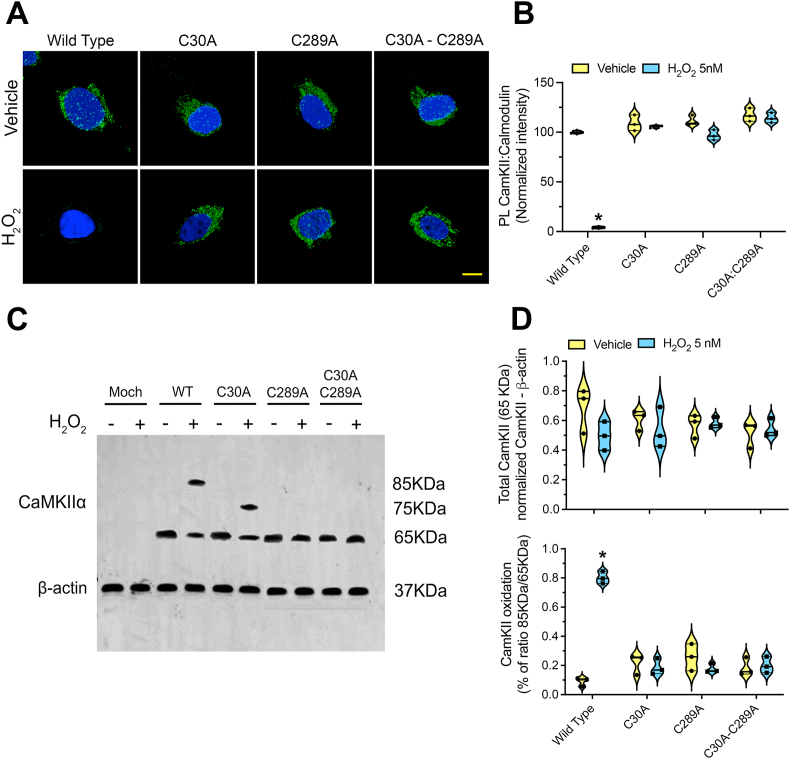
Our computational investigations have revealed a transient proximity between Cys30 and Cys298 residues of CaMKIIα, suggesting the potential formation of a S–S bridge that could impact the formation of CaMKIIα:Ca2+/CaM complex. To assess the significance of these cysteine residues in the CaMKIIα:Ca2+/CaM interaction and to circumvent potential cross-immunoreactivity issues with native proteins in mammalian cells, we expressed various mutant forms of human CaM and CaMKIIα in Drosophila S2R + adherent cells (Validation: Suppl. Fig. 1). Utilizing PLA, we examined the CaMKIIα:CaM interaction (Validation: Suppl. Fig. 2) in response to 5 nM H2O2 treatment in the mutant cell lines. Notably, wild-type CaM/CaMKIIα cells exhibited disrupted CaMKIIα:CaM interaction upon H2O2 exposure, whereas this effect was prevented in CaMKIIαC30A, CaMKIIαC289A, and CaMKIIαC30A−C289A mutant cell lines (Fig. 3 A-B).
Fig. 3.
Redox changes of Cys30 and Cys289 affect CaMKIIα:CaM interaction.(A) Representative image of PLA for CaMKIIα:CaM interaction (green) in Drosophila S2R + cells expressing human CaMWT associated with the expression of CaMKIIαWT, CaMKIIαC30A, CaMKIIαC289A or CaMKIIαC30A−C289A (scale bar: 15μm).(B) Quantification of the CaMKIIα:CaM PLA-related fluorescence intensity in Drosophila S2R + cell lines. Symbols represent the normalized means of the intensities (with vehicle treatment being set at 100 %) analyzed for each independent experiment (200–250 cells/treatment group per experiment). Statistical analysis was performed as one-way ANOVA with Bonferroni's correction (n = 3 independent experiments). (C) Redox WB of lysates from genetically modified Drosophila S2R + cells. (D) Quantification of the bands intensity reported as normalized ratio CaMKIIα/β-actin (upper graph) or as normalized CaMKIIα85KDa/CaMKIIα65KDa (lower graph). Symbols represent the normalized bands intensities ratios (with vehicle treatment being set at 1) analyzed for each independent experiment Statistical analysis was performed as one-way ANOVA with Bonferroni's correction (n = 3 independent experiments). In plots B and D, *denotes p < 0.0001 significance compared vehicle.
Furthermore, employing redox Western blot assays, which rely on the alkylation of thiol residues with a 10 kDa PEG-NEM tag to detect oxidized thiols, we observed distinct molecular weight shifts indicative of thiol oxidation. Specifically, exposure to 5 nM H2O2 induced a 20 kDa molecular weight shift in wild-type CaMKIIα, while in C30A mutants, a 10 kDa shift was observed. However, no such shift was detected in C289A mutant lines, suggesting that Cys289 may undergo alternative forms of thiol oxidation rather than participating in S–S bridge formation (Fig. 3 B–C). Remarkably, despite observing redox changes in Cys289 in the absence of Cys30, these alterations were not sufficient to disrupt the CaMKIIα:CaM interaction, as evidenced in Fig. 3 A-B. Moreover, the double mutant C30A/C289A prevented molecular weight shifts in CaMKIIα, indicating that both Cys30 and Cys289 are actively involved in the redox perturbations leading to impaired CaMKIIα:CaM interaction.
Rotenone-induced oxidative stress affects CaMKIIα:CaM complex integrity in Ventral Midbrain DA neurons.
To investigate the relevance of this phenomenon in PD, we designed experiments applied to the rotenone model of PD in rat primary VMB dopamine (DA) neuronal cultures. Proximity ligation assay was used to assess in situ interaction between CaMKIIα and CaM in DA (TH-positive) neurons. Under basal conditions, a robust fluorescent signal indicative of CaMKIIα:CaM interaction was observed. However, upon 24 h of exposure to rotenone (50 nM), a significant reduction in this signal was noted. Notably, co-administration of the ROS scavenger, N-acetylcysteine (NAC; 250 μM) prevented rotenone-induced CaMKIIα:CaM complex disruption, suggesting the involvement of redox-sensitive mechanisms in CaMKIIα:Ca2+/CaM complex formation (Fig. 4 A-B).
Fig. 4.
Oxidative stress-related CaMKIIα:CaM interaction.(A) Image of PLA for CaMKIIα:CaM interaction (green) in DA neurons (Red: TH) in primary VMB cultures. (Scale bar: 35 μm). (B) Quantification of the CaMKIIα:CaM PLA-related fluorescence intensity in primary VMB cultures. Symbols represent the normalized means of the intensities (with vehicle treatment being set at 100 %) analyzed for each independent experiment (100–150 neurons/treatment group per experiment). Statistical analysis was performed as one-way ANOVA with Bonferroni's correction (n = 3 independent experiments). (C) CaMKIIα Redox WB assay performed with a non-selective reduction of thiol residues (TCEP) or with a S–NO selective reduction step performed with ascorbic acid. (D) Quantification of the bands intensity reported as normalized ratio CaMKIIα/β-actin (upper graph) or as normalized CaMKIIα85KDa/CaMKIIα65KDa (lower graph). Symbols represent the normalized bands' intensities ratios (with vehicle treatment being set at 1) analyzed for each independent experiment. Statistical analysis was performed as one-way ANOVA with Bonferroni's correction (n = 3 independent experiments) In plots B and D, *denotes p < 0.0001 significance compared to vehicle.
To further explore the redox state of CaMKIIα cysteine residues in response to oxidative conditions, we conducted redox Western blot assays in the same experimental model. Using two different thiol reduction steps - TCEP for non-specific thiol reduction and ascorbic acid for selective reduction of S–NO residues (Validated: Suppl. Fig. 3) - we observed a 20 kDa shift in the CaMKIIα band in TCEP-treated lysates from cells exposed to rotenone. However, this shift was not observed in lysates treated with ascorbic acid, indicating the presence of two oxidized cysteine residues in CaMKIIα under oxidative stress conditions and excluding the formation of S–NO residues (Fig. 4 C-D).
These findings underscore the involvement of redox-sensitive mechanisms in the regulation of CaMKIIα:Ca2+/CaM interaction and highlight the potential relevance of such mechanisms in the pathogenesis of Parkinson's disease.
NOX2 modulates CaM-independent CaMKIIa kinase activity and causes Ca2+ dysregulation in rotenone treated ventral midbrain neurons.
We investigated the role of NOX2 in disrupting the CaMKIIα:CaM complex and triggering hyperactivation of CaM-independent CaMKIIα kinase due to rotenone-induced mitochondrial dysfunction in VMB DA neurons.
Our findings, illustrated in Fig. 5 A-B, reveal a significant reduction in the CaMKIIα:CaM complex in DA (TH positive) neurons following 4 h of rotenone exposure. Notably, co-treatment with the cell-permeable selective NOX2 inhibitor peptide, Nox2ds-tat (10 μM) prevented the rotenone induced disruption of the CaMKIIα:CaM complex. Furthermore, experiments conducted in CRISPR/Cas9 gene-edited NOX2 knockout (NOX2−/−) HEK-293 cells exposed to rotenone, revealed no NOX2 activity [17] and a significant prevention of rotenone-induced disruption of the CaMKIIα:CaM complex compared to wild type cells (Suppl. Fig. 4). Overall, the data suggest a critical role for NOX2 activity in promoting the formation of an oxidized post-translational modification (PTM) form of CaMKIIα, rendering it unresponsive to Ca2+/CaM modulation. This is supported by evidence from redox Western blot analyses conducted in Drosophila S2R + implicating Cys30 and Cys289 residues in the binding capacity of CaMKIIα with CaM under oxidative stress conditions. Moreover, DHE staining performed under the same experimental conditions, showed a significant increase of cellular oxidant species production in neurons after 4 h of rotenone exposure, that was prevented by the co-administration of Nox2ds-tat (Suppl. Fig. 5). Consistently with previous study [17], this evidence supports the role of NOX2 activity as major early oxidative event in PD.
Fig. 5.
NOX2 activity affects CaMKIIα:CaM interaction and modulates CaM-independent CaMKIIa kinase activity in rotenone-treated ventral midbrain neurons.(A) Representative image of PLA for CaMKIIα:CaM interaction (green) in DA neurons (Red: TH) in primary VMB cultures. (Scale bar: 35 μm). (B) Image representing levels of pThr286CaMKIIα (blue) in DA neurons (Red: TH) in primary VMB cultures (Scale bar: 35 μm). (C) Quantification of the CaMKIIα:CaM PLA-related fluorescence intensity in primary VMB cultures (light blue violins) associated with quantification of fluorescence relative to phosphor Thr286 (yellow violins). Symbols represent the normalized means of the intensities (with vehicle treatment being set at 100 %) analyzed for each independent experiment (100–150 neurons/treatment group per experiment). Statistical analysis was performed as one-way ANOVA with Bonferroni's correction (n = 3 independent experiments). C, * denotes p < 0.0001 significance of PLA compared vehicle. # denotes p < 0.0001 significance of normalized intensity for pThr286CaMKIIα compared to vehicle.
Additionally, the observed increased levels in CaMKIIα(Thr286) phosphorylation in rotenone exposed VMB cultures, indicative of prolonged CaM-independent CaMKIIα kinase activity, were consistently prevented by Nox2ds-tat co-treatment, suggesting a role of NOX2 activity in sustaining elevated CaM-independent CaMKIIα kinase activity in the context of cellular oxidative stress.
Interestingly, the selective cell-permeable CaMKIIα kinase inhibitor AIP; (50 nM) did not prevent rotenone-induced disruption of the CaMKIIα:CaM complex but did prevent the elevated levels of CaMKIIα(Thr286) phosphorylation induced by rotenone exposure. This suggests a direct inhibitory action of AIP on CaMKIIα in a redox-independent manner.
Furthermore, we investigated the downstream effects of CaMKIIα hyperactivity on Ca2+ homeostasis. Using the green, fluorescent calcium binding dye Fluo-8-AM (5 μM; Abcam) and live imaging confocal microscopy, we observed a time-dependent increase in intracellular calcium levels in VMB neurons upon rotenone treatment. Importantly, this abnormal calcium accumulation was prevented by both Nox2ds-tat and AIP co-treatment (Fig. 6).
Fig. 6.
NOX2 activity-induced CaM-independent CaMKIIα kinase activity affects Ca2+homeostasis in rotenone-treated ventral midbrain neurons.(A) Representative images acquired by live imaging assay of Fluo-8 AM related fluorescent signal (green) in primary VMB cultures. (Scale bar: 50 μm). (B) Quantification of the time-dependent accumulation of intracellular Ca2+ in response to rotenone measured as fluorescence intensity relative to Fluo-8 AM in primary VMB cultures. Symbols represent the normalized means of the intensities (with vehicle treatment being set at 100 %) analyzed in 3 independent experiments (100–150 neurons/treatment group per independent experiment). Statistical analysis was performed as one-way ANOVA with Bonferroni's correction (n = 3 independent experiments). In plot C, * denotes p < 0.0001 significance compared to vehicle.
Together, this experimental evidence highlights the involvement of CaM-independent CaMKII kinase activity in calcium dysregulation in a PD-relevant model, shedding light on potential novel mechanisms underlying PD pathogenesis.
CaMKIIα-Ca2+/CaM complex is disrupted in DA nigrostriatal neurons in the rotenone model of PD in rat.
The mitochondrial complex I inhibitor rotenone recapitulates many features of PD-related pathogenic events including mitochondrial dysfunction, oxidative stress, altered Ca2+ homeostasis [40], and accumulation of toxic forms of α-synuclein [19,41]. We further assayed the CaMKIIα:CaM interaction in this experimental paradigm to examine if the disruption of binding is manifested in the rotenone model of PD in rat. A robust CaMKIIα:CaM interaction was observed in DA nigrostriatal neurons under basal conditions, whereas rotenone treated animals showed a significant reduction of PLA signal for CaMKIIα:CaM complex (by over 70 %) in DA nigrostriatal neurons of substantia nigra pars compacta (SNpc) (Fig. 7 A-B). Interestingly, the integrity of the CaMKIIα:CaM complex was preserved in rotenone-exposed animals co-treated with the highly selective NOX2 inhibitor, CPP11–H, suggesting a critical role of NOX2 – as major
Fig. 7.
NOX2-mediates CaMKIIα/CaM disruption the rotenone model of PD in rat.
(A) Representative image of PLA for CaMKIIα:CaM interaction (red) in rat nigrostriatal DA neurons (Blue: TH) (Scale bar: 50 μm). (B) Quantification of the CaMKIIα:CaM PLA-related fluorescence intensity in rat SNpc. Symbols represent the normalized means of the intensities (with vehicle treatment being set at 100 %) analyzed for each independent experiment (35–50 nigrostriatal neurons per hemisphere). Statistical analysis was performed as one-way ANOVA with Bonferroni's correction (n = 6 animals/group). (C) Image representing levels of phospho-Thr286 (red) in nigrostriatal DA neurons in rats (blue: TH). (Scale bar: 50 μm) (D) Quantification of CaMKIIα(pThr286) in rat SNpc. Symbols represent the normalized means of the intensities (with vehicle treatment being set at 100 %) analyzed for each independent experiment (35–50 nigrostriatal neurons per hemisphere). Statistical analysis was performed as one-way ANOVA with Bonferroni's correction (n = 6 animals/group). In plots B and D, *denotes p < 0.0001 significance compared to vehicle.
source of ROS in DA neurons - in inducing redox changes of CaMKIIα. Previous evidence in PD models report CaMKIIα hyperactivity related to high levels of phosphorylation at the Thr286 residue [21,22], an event linked to an uncontrolled Calmodulin-independent CaMKIIα activity. Similarly, as shown in Fig. 7 C-D, we detected a significant increase of CaMKIIα(Thr286) phosphorylation in rotenone treated animals. This event suggests that, in absence of Ca2+/CaM modulation, CaMKIIα undergoes uncontrolled kinase activation. Consistently with the PLA-based results, levels of CaMKIIα(Thr286) phosphorylation were reduced to control in rotenone/CPP11–H co-treated rats.
3.2. Altered CaMKIIα-Ca2+/CaM interaction is associated with idiopathic PD
To determine whether this process is relevant to idiopathic human PD, we performed PLA for CaMKIIα:CaM interaction in blinded postmortem substantia nigra sections from PD patients (N = 5) and from controls (N = 4). Compared to controls, nigrostriatal dopamine neurons from all PD cases showed very low PLA signal, indicating loss of interaction between CaMKIIα and CaM (Fig. 8). This evidence strongly suggests that CaM-mediated modulation of CaMKIIα is impaired in the human disease and may be a relevant factor in PD pathophysiology.
Fig. 8.
Relevance of CaMKIIα/CaM interaction in idiopathic PD.(A) PLA assay for CaMKIIα and CaM in human brain. (Scale bar: 30 μm). (B) Quantification of the CaMKIIα:CaM PLA-related fluorescence intensity in Human SNpc. Symbols represent the normalized means of the intensities (with controls being set at 100 %) analyzed for each independent experiment (10–20 nigrostriatal neurons/sample). Statistical testing by 2-tailed unpaired t-test with Welch's correction. *Denotes p < 0.0001 significance compared controls.
4. Discussion
This study presents a comprehensive investigation into the redox regulation of CaMKIIα and its implications in PD pathogenesis. Combining computational modeling with experimental validation, we reveal a novel mechanism by which oxidative stress disrupts the interaction between CaMKIIα and its modulator, the Ca2+/CaM complex, resulting in aberrant kinase activity and calcium dysregulation in PD-relevant models.
Previous studies have highlighted the redox sensitivity of CaMKIIα activity, noting that oxidative events can lead to Ca2+/CaM-independent CaMKIIα activation by implicating methionine 281/282 residues within the same subunit [24]. The present study advances the understanding of CaMKIIα regulation by uncovering a cysteine-mediated mechanism governing oxidative stress-related post-translational modifications. These modifications cause CaMKIIα to lose its responsiveness to the modulatory effects of the Ca2+/CaM complex, resulting in aberrant kinase activity.
Our novel computational modeling approach explored the dynamics and regulation of a CaMKIIα dodecameric complex in its active conformation. The computational analysis performed in this more complex model allowed the prediction that Cys289, located in the linker region, and Cys30, located in the adjacent subunit hub, are in close proximity. This suggests a high probability of disulfide bridge formation between these cysteine residues. This computational approach, thus, provides critical insights into more complex mechanisms at play within the fully active conformation of CaMKIIα.
Subsequent in vitro and in vivo studies confirmed this computational prediction and supported the hypothesis that this post-translational modification may interfere with the docking site for the Ca2+/CaM complex, thereby hindering its modulatory function on CaMKIIα.
Moreover, this study enhances our understanding of the specific mechanisms driving oxidative stress-mediated aberrant CaMKIIα activity and its contribution to Parkinson's disease-related neurodegeneration.
PD pathogenesis is characterized by a complexity of convergent pathways with significant contributions of oxidative stress impacting several PD-related neurodegenerative events, including mitochondrial dysfunction [42], α-synuclein aggregation [43], LRRK2 kinase activation [44] and neuroinflammation [45]. Importantly, several experimental evidence support a critical role of calcium dysregulation and altered signaling in PD pathogenesis [18].
The mammalian brain's high oxidative metabolic rate is especially pronounced in nigrostriatal DA neurons. Their reliance on VGCC-dependent Ca2+ currents for “pace-making” activity necessitates continuous Ca2+ buffering by the endoplasmic reticulum and mitochondria. This process is tightly linked to mitochondrial ROS generation, increasing the vulnerability of these neurons to oxidative stress [19,46,47]. In this context, administration of the complex I inhibitor rotenone, which induces ROS production through mitochondrial dysfunction, selectively damages the nigrostriatal DA system, mimicking key aspects of PD pathogenesis [41,48].
NADPH oxidases, particularly the isoform 2 (NOX2) - the most abundant isoform in the brain - are increasingly implicated in neurodegenerative diseases, including PD [49]. Genetic manipulation or pharmacological inhibition of NOX2 has shown neuroprotective effects and reduced pathology in experimental models [17,50], highlighting the therapeutic potential of NOX2 inhibition.
Our previous study demonstrated NOX2 activation in SNpc nigrostriatal DA neurons in the rotenone model of PD in rat. We found that neuronal NOX2 activation - in a bidirectional interplay with mitochondria previously described in endothelial cells [21] - amplifies O2●- and H2O2 production and initiates an intracellular oxidative signaling able to enhance several PD-related pathogenic events [17].
In this study we assessed the impact of this PD-relevant phenomenon on the redox modulation of CaMKIIα kinase activity and its implications Ca2+ dysregulation. CaMKIIα is implicated in a positive modulation of VGCC, enhancing prolonged Ca2+ currents [29,30]. It is, thus, conceivable that aberrant CaM-independent CaMKII activity may contribute to nigrostriatal degeneration by the induction of a dysregulated Ca2+ homeostasis and signaling.
Our findings obtained in this multidisciplinary study contribute to the understanding of PD pathogenesis by elucidating a novel mechanism linking oxidative stress, CaMKIIα dysfunction, and calcium dysregulation in a “vicious” feed forward cycle. The identification of specific cysteine residues involved in redox-mediated modulation of CaMKIIα activity provides insights into potential targets for therapeutic intervention in PD. Future studies should aim to further dissect the molecular mechanisms underlying CaMKIIα redox regulation and its contribution to PD pathology, ultimately paving the way for the development of novel therapeutic strategies targeting this pathway.
CRediT authorship contribution statement
Filippo Pullara: Writing – review & editing, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Madison C. Forsmann: Writing – review & editing, Methodology. Ignacio J. General: Writing – review & editing, Methodology, Data curation, Conceptualization. Joseph C. Ayoob: Methodology, Investigation. Emily Furbee: Investigation. Sandra L. Castro: Methodology. Xiaoping Hu: Methodology. J. Timothy Greenamyre: Writing – review & editing, Conceptualization. Roberto Di Maio: Writing – review & editing, Writing – original draft, Validation, Supervision, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization.
Declaration of competing interest
The Authors declare no conflicts of interest.
Acknowledgements
We thank Dr. Pagano for providing Nox2ds-tat and CPP11–H. The graphical abstract was realized with Bio-Render (License # TT26KH1190). This work was financially supported by NIH grants R21-NS112787 (R.D.M.), R01-NS126203-01A1 (R.D.M.).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.redox.2024.103254.
Contributor Information
Filippo Pullara, Email: filippo@predxbio.com.
Roberto Di Maio, Email: rod16@pitt.edu.
Appendix A. Supplementary data
The following is the Supplementary data to this article.
Data availability
Data will be made available on request.
References
- 1.Coultrap S.J., Bayer K.U. CaMKII regulation in information processing and storage. Trends Neurosci. 2012;35(10):607–618. doi: 10.1016/j.tins.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rotenberg A., et al. Mice expressing activated CaMKII lack low frequency LTP and do not form stable place cells in the CA1 region of the hippocampus. Cell. 1996;87(7):1351–1361. doi: 10.1016/s0092-8674(00)81829-2. [DOI] [PubMed] [Google Scholar]
- 3.Baudat F., et al. [Prdm9, a key control of mammalian recombination hotspots] Med. Sci. 2010;26(5):468–470. doi: 10.1051/medsci/2010265468. [DOI] [PubMed] [Google Scholar]
- 4.Itagaki C., et al. Stimulus-coupled interaction of tyrosine hydroxylase with 14-3-3 proteins. Biochemistry. 1999;38(47):15673–15680. doi: 10.1021/bi9914255. [DOI] [PubMed] [Google Scholar]
- 5.O'Day D.H. Calmodulin binding domains in critical Risk proteins involved in neurodegeneration. Curr. Issues Mol. Biol. 2022;44(11):5802–5814. doi: 10.3390/cimb44110394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tombes R.M., Faison M.O., Turbeville J.M. Organization and evolution of multifunctional Ca(2+)/CaM-dependent protein kinase genes. Gene. 2003;322:17–31. doi: 10.1016/j.gene.2003.08.023. [DOI] [PubMed] [Google Scholar]
- 7.Cook S.G., et al. Analysis of the CaMKIIalpha and beta splice-variant distribution among brain regions reveals isoform-specific differences in holoenzyme formation. Sci. Rep. 2018;8(1):5448. doi: 10.1038/s41598-018-23779-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hudmon A., Schulman H. Structure-function of the multifunctional Ca2+/calmodulin-dependent protein kinase II. Biochem. J. 2002;364(Pt 3):593–611. doi: 10.1042/BJ20020228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rosenberg O.S., et al. Structure of the autoinhibited kinase domain of CaMKII and SAXS analysis of the holoenzyme. Cell. 2005;123(5):849–860. doi: 10.1016/j.cell.2005.10.029. [DOI] [PubMed] [Google Scholar]
- 10.Pullara F., Asciutto E.K., General I.J. Mechanisms of activation and subunit release in Ca(2+)/calmodulin-dependent protein kinase II. J. Phys. Chem. B. 2017;121(45):10344–10352. doi: 10.1021/acs.jpcb.7b09214. [DOI] [PubMed] [Google Scholar]
- 11.Asciutto E.K., Pantano S., General I.J. Physical interactions driving the activation/inhibition of calcium/calmodulin dependent protein kinase II. J. Mol. Graph. Model. 2021;105 doi: 10.1016/j.jmgm.2021.107875. [DOI] [PubMed] [Google Scholar]
- 12.Miller S.G., Patton B.L., Kennedy M.B. Sequences of autophosphorylation sites in neuronal type II CaM kinase that control Ca2(+)-independent activity. Neuron. 1988;1(7):593–604. doi: 10.1016/0896-6273(88)90109-2. [DOI] [PubMed] [Google Scholar]
- 13.Schworer C.M., et al. Ca2+/calmodulin-dependent protein kinase II. Identification of a regulatory autophosphorylation site adjacent to the inhibitory and calmodulin-binding domains. J. Biol. Chem. 1988;263(27):13486–13489. [PubMed] [Google Scholar]
- 14.Lou L.L., Schulman H. Distinct autophosphorylation sites sequentially produce autonomy and inhibition of the multifunctional Ca2+/calmodulin-dependent protein kinase. J. Neurosci. 1989;9(6):2020–2032. doi: 10.1523/JNEUROSCI.09-06-02020.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Myers J.B., et al. The CaMKII holoenzyme structure in activation-competent conformations. Nat. Commun. 2017;8 doi: 10.1038/ncomms15742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shioda N., Fukunaga K. Physiological and pathological roles of CaMKII-PP1 signaling in the brain. Int. J. Mol. Sci. 2017;19(1) doi: 10.3390/ijms19010020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Keeney M.T., et al. NADPH oxidase 2 activity in Parkinson's disease. Neurobiol. Dis. 2022;170 doi: 10.1016/j.nbd.2022.105754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schapira A.H. Calcium dysregulation in Parkinson's disease. Brain. 2013;136(Pt 7):2015–2016. doi: 10.1093/brain/awt180. [DOI] [PubMed] [Google Scholar]
- 19.Horowitz M.P., et al. Single-cell redox imaging demonstrates a distinctive response of dopaminergic neurons to oxidative insults. Antioxid Redox Signal. 2011;15(4):855–871. doi: 10.1089/ars.2010.3629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gleichmann M., Mattson M.P. Neuronal calcium homeostasis and dysregulation. Antioxid Redox Signal. 2011;14(7):1261–1273. doi: 10.1089/ars.2010.3386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dikalov S.I., et al. Nox2-induced production of mitochondrial superoxide in angiotensin II-mediated endothelial oxidative stress and hypertension. Antioxid Redox Signal. 2014;20(2):281–294. doi: 10.1089/ars.2012.4918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hernandes M.S., Cafe-Mendes C.C., Britto L.R. NADPH oxidase and the degeneration of dopaminergic neurons in parkinsonian mice. Oxid. Med. Cell. Longev. 2013;2013 doi: 10.1155/2013/157857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Howe C.J., et al. Redox regulation of the calcium/calmodulin-dependent protein kinases. J. Biol. Chem. 2004;279(43):44573–44581. doi: 10.1074/jbc.M404175200. [DOI] [PubMed] [Google Scholar]
- 24.Erickson J.R., et al. A dynamic pathway for calcium-independent activation of CaMKII by methionine oxidation. Cell. 2008;133(3):462–474. doi: 10.1016/j.cell.2008.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Picconi B., et al. Abnormal Ca2+-calmodulin-dependent protein kinase II function mediates synaptic and motor deficits in experimental parkinsonism. J. Neurosci. 2004;24(23):5283–5291. doi: 10.1523/JNEUROSCI.1224-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang Y.J., et al. The expression of calcium/calmodulin-dependent protein kinase II-alpha in the hippocampus of patients with Alzheimer's disease and its links with AD-related pathology. Brain Res. 2005;1031(1):101–108. doi: 10.1016/j.brainres.2004.10.061. [DOI] [PubMed] [Google Scholar]
- 27.Jiang X., et al. Modulation of CaV2.1 channels by Ca2+/calmodulin-dependent protein kinase II bound to the C-terminal domain. Proc. Natl. Acad. Sci. U. S. A. 2008;105(1):341–346. doi: 10.1073/pnas.0710213105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hell J.W., et al. Differential phosphorylation of two size forms of the neuronal class C L-type calcium channel alpha 1 subunit. J. Biol. Chem. 1993;268(26):19451–19457. [PubMed] [Google Scholar]
- 29.Blaich A., et al. Facilitation of murine cardiac L-type Ca(v)1.2 channel is modulated by calmodulin kinase II-dependent phosphorylation of S1512 and S1570. Proc. Natl. Acad. Sci. U. S. A. 2010;107(22):10285–10289. doi: 10.1073/pnas.0914287107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dzhura I., et al. Calmodulin kinase determines calcium-dependent facilitation of L-type calcium channels. Nat. Cell Biol. 2000;2(3):173–177. doi: 10.1038/35004052. [DOI] [PubMed] [Google Scholar]
- 31.Chao L.H., et al. A mechanism for tunable autoinhibition in the structure of a human Ca2+/calmodulin- dependent kinase II holoenzyme. Cell. 2011;146(5):732–745. doi: 10.1016/j.cell.2011.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Valian N., et al. Comparison of rat primary midbrain neurons cultured in DMEM/F12 and neurobasal mediums. Basic Clin. Neurosci. 2021;12(2):205–212. doi: 10.32598/bcn.12.2.1568.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rocha E.M., et al. LRRK2 inhibition prevents endolysosomal deficits seen in human Parkinson's disease. Neurobiol. Dis. 2020;134 doi: 10.1016/j.nbd.2019.104626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Di Maio R., et al. alpha-Synuclein binds to TOM20 and inhibits mitochondrial protein import in Parkinson's disease. Sci. Transl. Med. 2016;8(342):342ra78. doi: 10.1126/scitranslmed.aaf3634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Di Maio R., et al. LRRK2 activation in idiopathic Parkinson's disease. Sci. Transl. Med. 2018;10(451) doi: 10.1126/scitranslmed.aar5429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Keeney M.T., et al. Measurement of LRRK2 kinase activity by proximity ligation assay. Bio Protoc. 2021;11(17):e4140. doi: 10.21769/BioProtoc.4140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Milanese C., et al. Mitochondrial complex I reversible S-nitrosation improves bioenergetics and is protective in Parkinson's disease. Antioxid Redox Signal. 2018;28(1):44–61. doi: 10.1089/ars.2017.6992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hanson P.I., Schulman H. Inhibitory autophosphorylation of multifunctional Ca2+/calmodulin-dependent protein kinase analyzed by site-directed mutagenesis. J. Biol. Chem. 1992;267(24):17216–17224. [PubMed] [Google Scholar]
- 39.Colbran R.J. Inactivation of Ca2+/calmodulin-dependent protein kinase II by basal autophosphorylation. J. Biol. Chem. 1993;268(10):7163–7170. [PubMed] [Google Scholar]
- 40.Freestone P.S., et al. Acute action of rotenone on nigral dopaminergic neurons--involvement of reactive oxygen species and disruption of Ca2+ homeostasis. Eur. J. Neurosci. 2009;30(10):1849–1859. doi: 10.1111/j.1460-9568.2009.06990.x. [DOI] [PubMed] [Google Scholar]
- 41.Sanders L.H., Timothy Greenamyre J. Oxidative damage to macromolecules in human Parkinson disease and the rotenone model. Free Radic. Biol. Med. 2013;62:111–120. doi: 10.1016/j.freeradbiomed.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen C., et al. Parkinson's disease neurons exhibit alterations in mitochondrial quality control proteins. NPJ Parkinsons Dis. 2023;9(1):120. doi: 10.1038/s41531-023-00564-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Virdi G.S., et al. Protein aggregation and calcium dysregulation are hallmarks of familial Parkinson's disease in midbrain dopaminergic neurons. NPJ Parkinsons Dis. 2022;8(1):162. doi: 10.1038/s41531-022-00423-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rosenbusch K.E., Kortholt A. Vol. 2016. Parkinsons Dis; 2016. (Activation Mechanism of LRRK2 and its Cellular Functions in Parkinson's Disease). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zundorf G., Reiser G. Calcium dysregulation and homeostasis of neural calcium in the molecular mechanisms of neurodegenerative diseases provide multiple targets for neuroprotection. Antioxid Redox Signal. 2011;14(7):1275–1288. doi: 10.1089/ars.2010.3359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Blesa J., et al. Oxidative stress and Parkinson's disease. Front. Neuroanat. 2015;9:91. doi: 10.3389/fnana.2015.00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cannon J.R., et al. A highly reproducible rotenone model of Parkinson's disease. Neurobiol. Dis. 2009;34(2):279–290. doi: 10.1016/j.nbd.2009.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Drolet R.E., et al. Chronic rotenone exposure reproduces Parkinson's disease gastrointestinal neuropathology. Neurobiol. Dis. 2009;36(1):96–102. doi: 10.1016/j.nbd.2009.06.017. [DOI] [PubMed] [Google Scholar]
- 49.Fiadeiro M.B., et al. NADPH oxidases in neurodegenerative disorders: mechanisms and therapeutic opportunities. Antioxid. Redox Signal. 2024;1 doi: 10.1089/ars.2023.0002. 2023.0002-54. [DOI] [PubMed] [Google Scholar]
- 50.Hernandes M.S., et al. Microglial cells are involved in the susceptibility of NADPH oxidase knockout mice to 6-hydroxy-dopamine-induced neurodegeneration. PLoS One. 2013;8(9) doi: 10.1371/journal.pone.0075532. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.