Sir,
Humankind's exposure to chemicals and pharmaceuticals has undergone an unparalleled paradigm shift in recent decades.[1] Pharmacological products, including pharmaceuticals, nutraceuticals, food, beverages, and industrial and domestic goods, are being consumed at a significantly higher rate. When evaluating acute toxicity and as the first step in the process of screening pharmaceutical and pharmacological compounds for potential toxicity, the lethal dosage (LD50) evaluation is utilized as a key criterion.[2]
Methotrexate (MTX), a common chemotherapeutic agent utilized in clinical practice, may have diminished clinical efficacy due to its short half-life, drug-resistance patterns, and the extremely high dosage required for cancer cell suppression.[3] An MTX-layered double hydroxides (LDH) nanohybrid with an average particle size of about 130 nm was created by coprecipitating MTX with LDHs and an inorganic drug delivery vehicle, to overcome the limitations of MTX.[4] In recent years, computer models of nanoparticle-based medication delivery in the field of clinical pharmacology have also found utility for LDHs as delivery systems. By ion-exchange reactions with bodily fluid, the interpolated LDH molecules can be released in a controlled manner and may even be protective against unfavorable biological situations.[5]
Due to their low toxicity, high reserving capacity, and superior cellular absorption, the double-layered hydroxides are regarded as attractive inorganic matrices for the delivery of genes or drugs. It is typically the initial stage in assessing and evaluating a substance's hazardous properties. Thus, the goal of the current investigation was to determine the rat LD50 of LDH-MTX.
A modest pilot research was conducted on a group of Wistar rats before ascertaining the drugs LD50, largely to clarify the dose range to be chosen for a follow-up investigation. The “staircase method” or “up-and-down method” was applied. Two rats of each sex were chosen, given 135 mg/kg orally, and monitored for 24 h to check for any deaths. The second dose was given orally to two additional rats of either sex after the first dose, which had been well tolerated. The animals appeared to tolerate this dose as well; therefore, the following dose was increased by a factor of 1.5 and administered orally to one male and one female. Rats who received a dose of 1265.62 had symptoms such as depression, anorexia, piloerection, diarrhea, dirty anal region, and gasping-like signs before they died within 24 h. The highest nonfatal and lowest deadly doses were noted and utilized to calculate the LD50. For the oral LD50 investigation, 60 Wistar albino rats were split into six groups, each with 10 animals of either sex. Before administering the medication, each rat's body weight was recorded after a 12 h feeding period. With the aid of a gavage needle, LDH-MTX was dissolved in phosphate-buffered saline (PBS) pH 7.2–7.4 and sonicated to make a homogenous suspension. Animals in groups II, III, IV, V, and VI received 500, 750, 1000, 1250, and 1500 mg/kg of LDH-MTX, whereas the first group was kept as the control (group I) and received PBS only (0.5 ml, orally) and then cage side observation was made for 14 days. During the 14-day observation period, salient indicators and the quantity of animal death in separate groups were documented. Probit analysis was used to calculate the LD50 of LDH-MTX. 100% of the treated rats passed away within the observation period of 48 h, and the intensity peaked at 1250 mg/kg and 1500 mg/kg. With the increase in dose, the death rate also gradually rose. The symptoms suggested that LDH-MTX exposure in rats affected the automatic nervous system and central nervous system. After receiving one oral dosage of LDH-MTX at various escalating dose levels, the mortality rate of rats was observed. The probit analysis method, as depicted in Figure 1, was used to determine the oral LD50 value of LDH-MTX in rats. Following extrapolation, the determined LD50 value was found to be 1096.47 mg/kg body weight, which was classified as a somewhat hazardous chemical and is equivalent to 293.02 mg/kg MTX. However, the LD50 of MTX (the naked medication) is 135 mg/kg in rats, suggesting that LDH-MTX is less harmful than MTX alone. In Balb/C mice, polymer LDH-MTX has been found to have a greater LD50 than bare MTX, which has an LD50 of 60 mg/kg. Although bare MTX and LDH-MTX had similar mouse kinetics, LDH-MTX-inhibited tumor growth in mice models of human osteosarcoma when compared to bare MTX in the same dose. The enormous potential of LDH-MTX as an anticancer treatment is further demonstrated by the statistically considerable quantity of MTX transport from LDH-MTX into the targeted tumor cells as opposed to the lower drug levels detected in the normal tissues, along with a good side effect profile.
Figure 1.
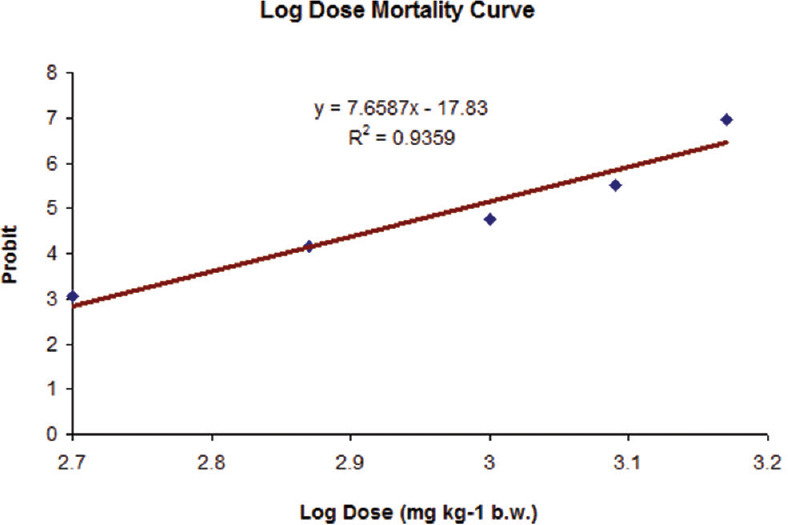
Graphical method of determination of LD50 of LDH-MTX formulation in rats. LDH-MTX = Layered double hydroxides-methotrexate
A stronger electron paramagnetic resonance effect, in which the nanocarriers become lodged in the solid tumor and persist with a higher concentration for a longer time period while bare MTX tends to diffuse back into circulation sooner, may be responsible for the increased delivery of LDH-MTX.
In addition, the tumor's acidic environment helps speed the delivery of MTX to the tumor and degrades the pH-sensitive ceramic matrix. This shows that LDH-MTX has a higher therapeutic efficacy in rats. Specific details about the LD50, the therapeutic index, and the general safety of pharmacological drugs are revealed by acute toxicity studies. Hence, a pharmacological compound must undergo a thorough toxicity assessment before being put through testing, receiving approval, and going on the market.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Sperling F. Introduction to toxicity evaluation session. Environ Health Perspect. 1979;32:259. doi: 10.1289/ehp.7932259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chinedu E, Arome D, Ameh FS. A new method for determining acute toxicity in animal models. Toxicol Int. 2013;20:224–6. doi: 10.4103/0971-6580.121674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gaies E, Jebabli N, Trabelsi S, Salouage I, Charfi R, Lakhal M, et al. Methotrexate side effects. J Drug Metab Toxicol. 2012;3:125. [Google Scholar]
- 4.Choi G, Kwon OJ, Oh Y, Yun CO, Choy JH. Inorganic nanovehicle targets tumor in an orthotopic breast cancer model. Sci Rep. 2014;4:4430. doi: 10.1038/srep04430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chakraborty M, Dasgupta S, Bose P, Mishra A, Mandal TK, Mitra M, et al. Layered double hydroxide: Inorganic organic conjugate nanocarrier for methotrexate. J Phys Chem Solids. 2011;72:779–83. [Google Scholar]


