Abstract
Background & Aims
Pancreatitis is characterized by acinar cell death and persistent inflammation. Ferroptosis is a type of lipid peroxidation-dependent necrosis, which is negatively regulated by glutathione peroxidase 4. We studied how trypsin, a serine protease secreted by pancreatic acinar cells, affects the contribution of ferroptosis to triggering pancreatitis.
Methods
In vitro, the mouse pancreatic acinar cell line 266-6 and mouse primary pancreatic acinar cells were used to investigate the effect of exogenous trypsin on ferroptosis sensitivity. Short hairpin RNAs were designed to silence gene expression, whereas a library of 1080 approved drugs was used to identify new ferroptosis inhibitors in 266-6 cells. In vivo, a Cre/LoxP system was used to generate mice with a pancreas-specific knockout of Gpx4 (Pdx1-Cre;Gpx4flox/flox mice). Acute or chronic pancreatitis was induced in these mice (Gpx4flox/flox mice served as controls) by cerulein injections or a Lieber–DeCarli alcoholic liquid diet. Pancreatic tissues, acinar cells, and serum were collected and analyzed by histology, immunoblot, quantitative polymerase chain reaction, enzyme-linked immunosorbent assay, or immunohistochemical analyses.
Results
Supraphysiological doses of trypsin (500 or 1000 ng/mL) alone did not trigger significant cell death in 266-6 cells and mouse primary pancreatic acinar cells, but did increase the sensitivity of these cells to ferroptosis upon treatment with cerulein, L-arginine, alcohol, erastin, or RSL3. Proteasome 26S subunit, non–adenosine triphosphatase 4–dependent lipid peroxidation caused ferroptosis in pancreatic acinar cells by promoting the proteasomal degradation of glutathione peroxidase 4. The drug screening campaign identified the antipsychotic drug olanzapine as an antioxidant inhibiting ferroptosis in pancreatic acinar cells. Mice lacking pancreatic Gpx4 developed more severe pancreatitis after cerulein infection or ethanol feeding than control mice. Conversely, olanzapine administration protected against pancreatic ferroptotic damage and experimental pancreatitis in Gpx4-deficient mice.
Conclusions
Trypsin-mediated sensitization to ferroptotic damage increases the severity of pancreatitis in mice, and this process can be reversed by olanzapine.
Keywords: Acinar Cells, Animal Model, Cell Death, Digestive Enzyme, Sterile Inflammation
Abbreviations used in this paper: ACSL4, acyl-CoA synthetase long chain family member 4; ARNTL, aryl hydrocarbon receptor nuclear translocator-like; cDNA, complementary DNA; CoQ10, coenzyme Q10; DPPH, 2,2-diphenyl-1-picrylhydrazyl; GPX4, glutathione peroxidase 4; GSH, glutathione; HMGB1, high mobility group box 1; IL, interleukin; IL1B, interleukin 1β; KO, knockout; LDH, lactate dehydrogenase; MDA, malondialdehyde; mPAC, mouse primary pancreatic acinar cell; MPO, myeloperoxidase; mRNA, messenger RNA; p-MLKL, phosphorylated mixed-lineage kinase domain-like pseudokinase; PSMD4, proteasome 26S subunit, non–adenosine triphosphatase 4; PTGS2, prostaglandin-endoperoxide synthase 2; qPCR, quantitative polymerase chain reaction; shRNA, short hairpin RNA; SLC40A1, solute carrier family 40 member 1; STI, soybean trypsin inhibitor; TBST, Tris-buffered saline with 0.1% Tween 20 detergent; WT, wild-type
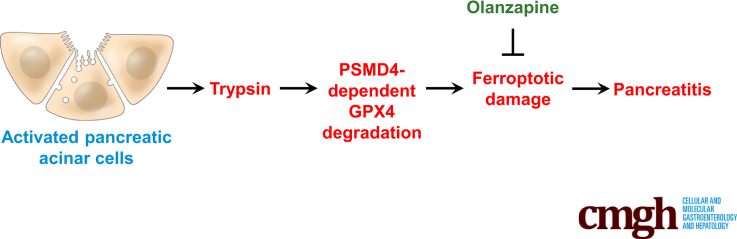
Graphical abstract
Summary.
Trypsin-mediated sensitization of pancreatic acinar cells to ferroptosis may be targeted for the prevention and treatment of pancreatitis in mice. An unbiased drug screening campaign identified olanzapine as a novel ferroptosis inhibitor that prevents pancreatitis in mice.
Pancreatitis is a progressive inflammatory disease caused by premature activation of digestive enzymes, such as trypsin.1, 2, 3 Trypsin is normally produced in pancreatic acinar cells in a latent, inactive form (also known as trypsinogen), and is activated only in the lumen of the small intestine to digest proteins. The premature activation and release of trypsin may contribute to the pathogenesis of pancreatitis, as indicated by several lines of evidence. First, the levels of trypsin in serum or urine correlate with the severity of human pancreatitis.4,5 Second, mutations affecting genes in the trypsin activation (eg, PRSS1 and SPINK1) are implicated in human pancreatitis.6, 7, 8, 9, 10, 11, 12 Third, transgenic expression of human PRSS1 mutations in mice results in a phenotype that is highly susceptible to experimental pancreatitis.13, 14, 15 Fourth, the activation of trypsinogen resulting in excessive trypsin production can cause pancreatic acinar cell damage and/or activate immune pathways (such as nuclear factor-κB) to trigger pancreatitis in mice.16, 17, 18, 19, 20 However, despite the introduction of this trypsin paradigm for almost 40 years, the mechanism of action of trypsin in pancreatitis still is elusive.21
The pathogenesis of pancreatitis involves the excessive induction of multiple distinct cell death pathways.22,23 The severity of pancreatitis is tightly related to necrosis.24,25 Ferroptosis is a type of regulated necrosis driven by iron-dependent lipid peroxidation and subsequent plasma membrane rupture.26,27 Excessive ferroptotic cell death causes sterile inflammation through the release of endogenous damage-associated molecular patterns.28 Although it is well established that iron metabolism is altered in pancreatitis,29 the contribution of iron-dependent ferroptotic damage to pancreatitis still is poorly understood.30, 31, 32 Here, we investigated the previously elusive relationship between trypsin and ferroptosis during the progression of experimental pancreatitis. Using a combination of drug screening experiments and conditional knockout mouse models, we found that trypsin can enhance ferroptotic death in acinar cells, thus exacerbating pancreatitis. Our results suggest that trypsin-mediated ferroptotic death might constitute a therapeutic target for mitigating pancreatitis.
Results
Trypsin Increases the Sensitivity of Pancreatic Acinar Cells to Ferroptosis
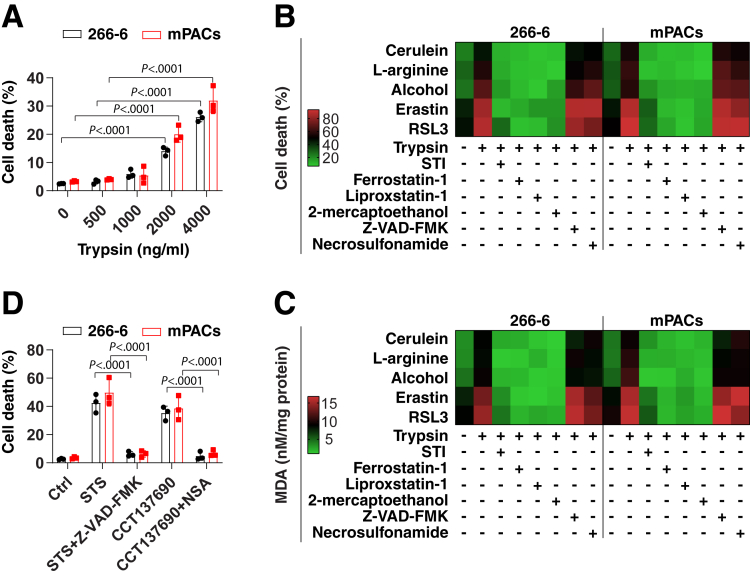
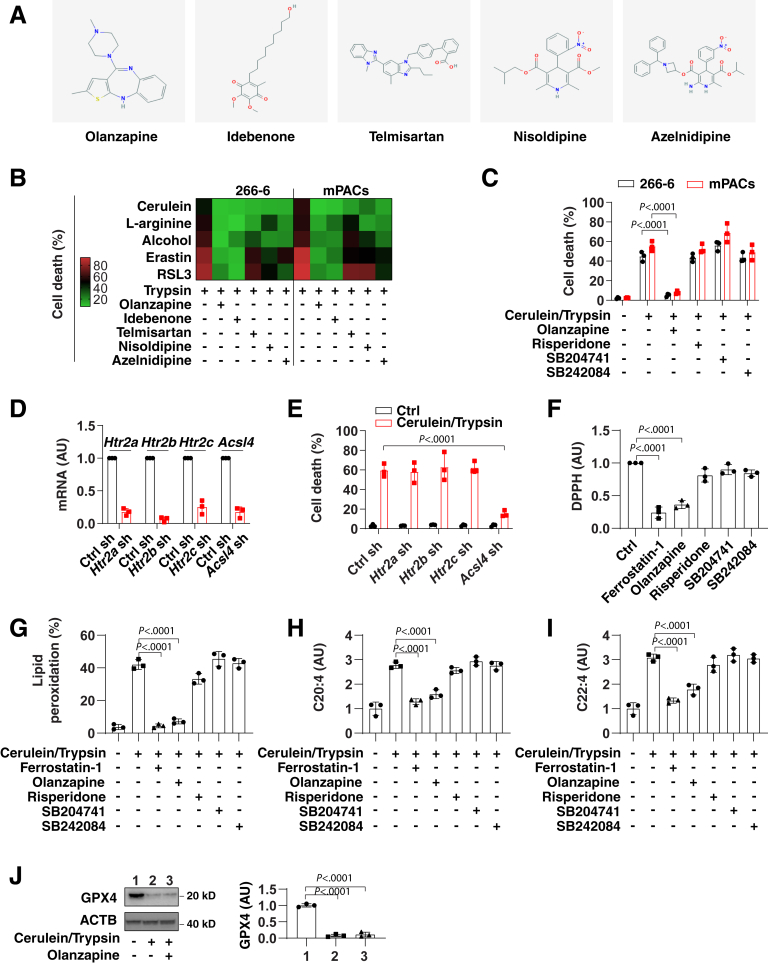
In patients with acute pancreatitis or in the acute phase of chronic pancreatitis, serum trypsin levels reportedly increase to 500–1000 ng/mL.33,34 To determine the effects of trypsin on cell death, we treated the mouse pancreatic acinar cell line 266-6 and mouse primary pancreatic acinar cells (mPACs) with cell culture–grade trypsin, which was devoid of endotoxin or other biological contaminants. Trypsin used in the reported pathogenic range (500 or 1000 ng/mL) did not trigger significant cell death in 266-6 cells and mPACs, but higher doses (>1000 ng/mL) resulted in cell killing (Figure 1A). Next, we exposed 266-6 cells and mPACs to pancreatitis-inducing toxins, including cerulein, L-arginine, or alcohol, in the absence or presence of trypsin (500 ng/mL). Of note, trypsin increased the sensitivity of acinar cells to cell death induction by these pancreatitis-related stimuli, while a widely used trypsin inhibitor, namely soybean trypsin inhibitor (STI),35 reduced cell killing by cerulein, L-arginine, or alcohol (Figure 1B). These findings indicate that trypsin sensitizes pancreatic acinar cells to cell death.
Figure 1.
Trypsin increases the sensitivity of pancreatic acinar cells to ferroptosis. (A) Analysis of cell death in indicated acinar cells after treatment with trypsin (500–4000 ng/mL) for 24 hours. Data are presented as means ± SD; n = 3 biologically independent samples; 2-way analysis of variance with the Tukey multiple comparisons test on all pairwise combinations. (B and C) Analysis of cell death and MDA in indicated acinar cells after treatment with cerulein (100 nmol/L), L-arginine (5 mg/mL), alcohol (50 mmol/L), erastin (2 μmol/L), and RSL3 (500 nmol/L) in the absence or presence of trypsin (500 ng/mL), STI (500 ng/mL), ferrostatin-1 (1 μmol/L), liproxstatin-1 (500 nmol/L), 2-mercaptoethanol (20 μmol/L), Z-VAD-FMK (10 μmol/L), or necrosulfonamide (1 μmol/L) for 24 hours. Data are shown in a heat map as the mean of 3 biologically independent samples. (D) Analysis of cell death in indicated acinar cells after treatment with staurosporine (STS, 500 nmol/L) or CCT137690 (1 μmol/L) in the absence or presence of Z-VAD-FMK (10 μmol/L) or necrosulfonamide (NSA, 1 μmol/L) for 24 hours. Data are presented as means ± SD; n = 3 biologically independent samples; 2-way analysis of variance with the Tukey multiple comparisons test on all pairwise combinations. Ctrl, control.
To elucidate the mechanism of trypsin-enhanced acinar cell death, we monitored the levels of oxidative stress by means of the quantitation of malondialdehyde (MDA), a product of lipid peroxidation.36 Cerulein-, L-arginine-, or alcohol-induced MDA production was increased by addition of trypsin to the cell culture (Figure 1C). This increased MDA production was blocked by addition of the trypsin inhibitor STI (Figure 1C). Of note, trypsin-mediated cell death sensitivity and MDA production were completely reverted by ferroptosis inhibitors (eg, ferrostatin-1, liproxstatin-1, or 2-mercaptoethanol),37 but not by inhibitors of apoptosis (eg, Z-VAD-FMK) or necroptosis (eg, necrosulfonamide) (Figure 1B and C). As a positive control, Z-VAD-FMK or necrosulfonamide inhibited cell death initiated by staurosporine (an apoptosis inducer38) or CCT137690 (a necroptosis inducer39) in 266-6 cells and mPACs, respectively (Figure 1D). Trypsin enhanced the cell death and MDA production triggered by classic ferroptosis inducers (eg, erastin or RSL3)37 in 266-6 cells or mPACs, and these processes were blocked by ferrostatin-1, liproxstatin-1, 2-mercaptoethanol, or STI (Figure 1B and C). Altogether, these results indicate that the presence of trypsin sensitizes pancreatic acinar cells to the induction of ferroptosis.
Proteasome 26S Subunit, Non–Adenosine Triphosphatase 4–Dependent Lipid Peroxidation Promotes Ferroptosis in Acinar Cells
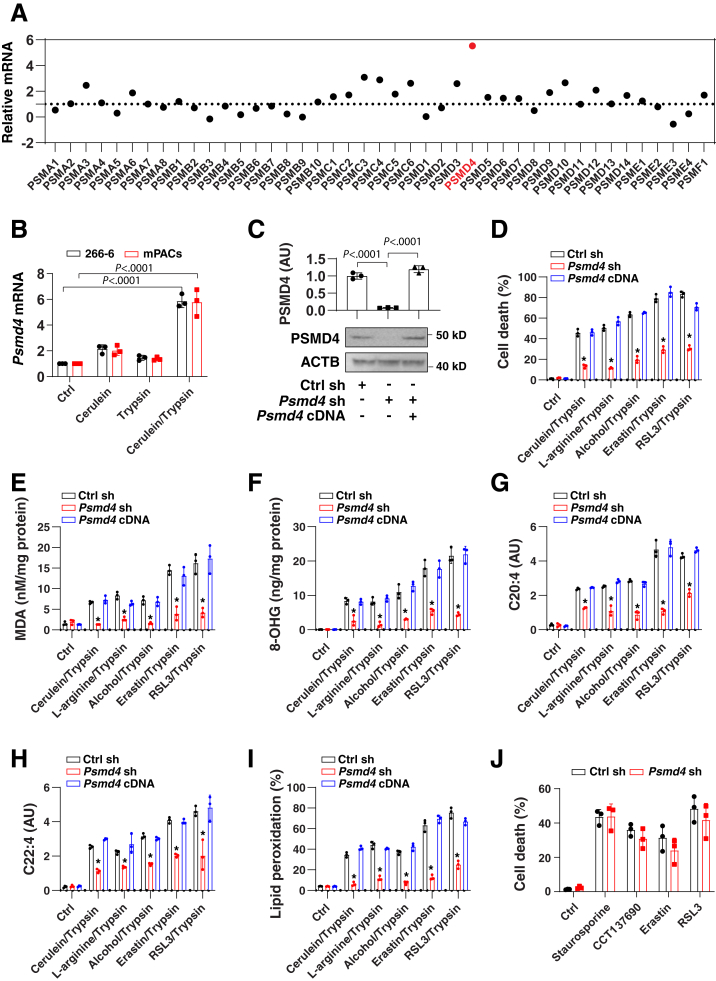
The proteasome is a large protein complex composed of 43 different subunits, which are jointly responsible for the selective degradation of proteins by proteolysis. The proteasome plays a dual (inhibitory or facilitatory) role in ferroptosis, depending on the specific substrate protein involved in the process.40, 41, 42, 43 To define the core components of the proteasome responsible for ferroptosis modulation in acinar cells, we used quantitative polymerase chain reaction (qPCR) to measure the levels of messenger RNAs (mRNAs) coding for the 43 proteasome subunits in 266-6 cells treated with cerulein alone or together with trypsin. The proteasome 26S subunit, non–adenosine triphosphatase 4 (Psmd4) ranked as the most up-regulated gene in 266-6 cells after treatment with cerulein plus trypsin (Figure 2A). In contrast, cerulein or trypsin alone did not cause significant up-regulation of Psmd4 mRNA in 266-6 cells and mPACs (Figure 2B).
Figure 2.
PSMD4-dependent lipid peroxidation promotes ferroptosis in acinar cells. (A) Analysis of mRNA expression of 43 proteasome subunits in 266-6 cells after treatment with cerulein (100 nmol/L) in the presence of trypsin (500 ng/mL) for 24 hours. Data are shown in a scatter map as the mean of 3 biologically independent samples. Psmd4 ranked as the most up-regulated gene as shown in red. (B) Analysis of mRNA expression of Psmd4 in 266-6 cells and mPACs after treatment with cerulein (100 nmol/L) in the absence or presence of trypsin (500 ng/mL) for 24 hours. Data are presented as means ± SD; n = 3 biologically independent samples; 2-way analysis of variance (ANOVA) with the Tukey multiple comparisons test on all pairwise combinations. (C) Analysis of protein expression of PSMD4 in indicated 266-6 cells. Semiquantitative data are presented as means ± SD; n = 3 biologically independent samples; 1-way ANOVA test on all pairwise combinations. (D–I) Analysis of (D) cell death, (E) MDA, (F) 8-hydroxy-2-deoxyguanosine (8-OHG), (G) C20:4, (H) C22:4, or (I) lipid peroxidation in indicated 266-6 cells after treatment with cerulein (100 nmol/L), L-arginine (5 mg/mL), alcohol (50 mmol/L), erastin (2 μmol/L), and RSL3 (500 nmol/L) in the presence of trypsin (500 ng/mL) for 24 hours. Data are presented as mean ± SD; n = 3 biologically independent samples; 2-way ANOVA test on all pairwise combinations (∗P < .0001 vs control shRNA group or Psmd4 cDNA group). (J) Analysis of cell death in indicated 266-6 cells after treatment with staurosporine (500 nmol/L), CCT137690 (1 μmol/L), erastin (2 μmol/L), or RSL3 (500 nmol/L) in the absence of trypsin for 24 hours. Data are presented as means ± SD; n = 3 biologically independent samples; 2-way ANOVA with the Tukey multiple comparisons test on all pairwise combinations. Ctrl, control.
To determine whether the proteasome 26S subunit, non–adenosine triphosphatase 4 (PSMD4) is involved in ferroptosis, we suppressed Psmd4 expression by specific short hairpin RNAs (shRNAs) (Figure 2C). In the presence of trypsin, the knockdown of Psmd4 limited the death of 266-6 cells caused by cerulein, L-arginine, alcohol, erastin, or RSL3 (Figure 2D). In contrast, transfection-enforced overexpression of Psmd4 (Figure 2C) restored the sensitivity of Psmd4-depleted 266-6 cells to these ferroptosis inducers (Figure 2D), confirming that PSMD4 is a positive regulator of ferroptosis in acinar cells.
Lipid peroxidation plays a major role in promoting ferroptosis owing to its implication in membrane damage as well as in DNA damage.44 Oxidized phosphatidylethanolamine-polyunsaturated fatty acids, such as arachidonic acid (C20:4) and adrenic acid (C22:4), play a direct role in promoting ferroptosis.45 We examined the effects of PSMD4 on the production of lipid peroxidation products, including MDA (Figure 2E), 8-hydroxy-2-deoxyguanosine (a product of oxidative DNA damage) (Figure 2F), C20:4 (Figure 2G), and C22:4 (Figure 2H). Of note, Psmd4 depletion blocked the generation of these lipid peroxidation products in 266-6 cells after treatment with cerulein, L-arginine, alcohol, erastin, or RSL3 in the presence of trypsin (Figure 2E–H). The measurement of lipid peroxidation products by means of the boron dipyrromethene difluoride (BODIPY) 581/591 C11 assay confirmed the decrease in oxidative reactions in Psmd4-knockdown 266-6 cells (Figure 2I). In contrast, Psmd4 knockdown failed to affect staurosporine- or CCT137690-induced cell death (Figure 2J). Moreover, cell death induced by erastin or RSL3 alone was not influenced by Psmd4 knockdown in 266-6 cells (Figure 2J). Altogether, these findings support the interpretation that PSMD4 selectively promotes trypsin-related ferroptosis by inducing lipid peroxidation.
PSMD4-Dependent Glutathione Peroxidase 4 Degradation Promotes Ferroptosis in Acinar Cells
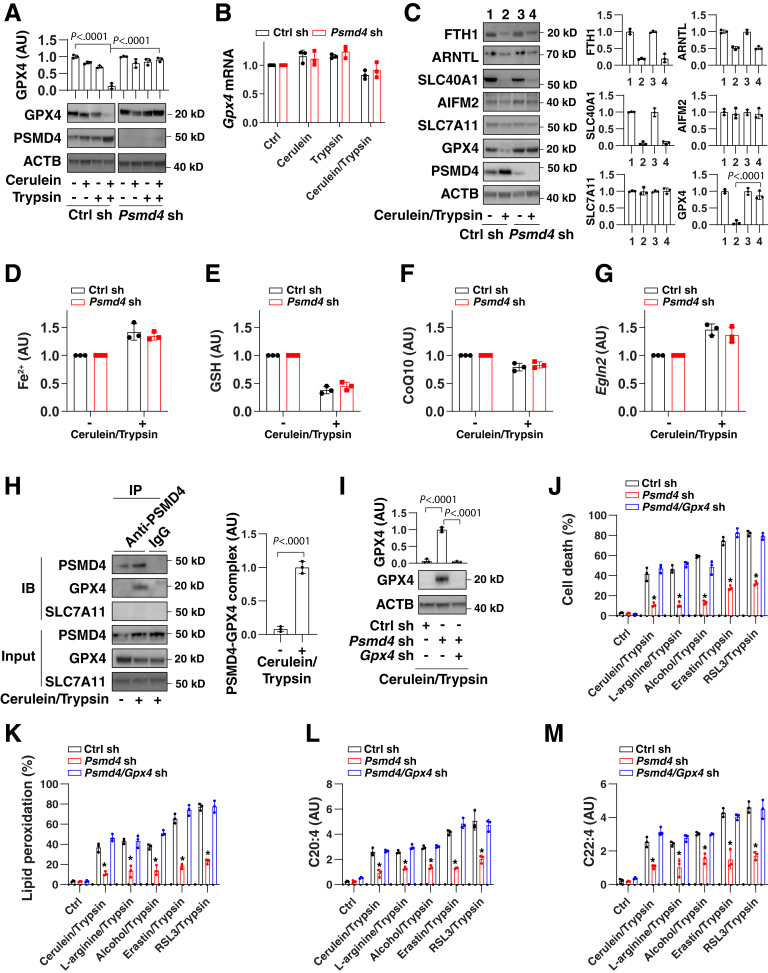
Glutathione peroxidase 4 (GPX4) is a key antioxidant enzyme that detoxifies lipid peroxides during ferroptosis.46,47 Accordingly, GPX4 degradation by proteasomes or autophagy favors the induction of ferroptosis.42,48 Driven by these considerations, we determined whether PSMD4 might affect GPX4 degradation in acinar cells. Western blot and qPCR analysis showed that, after treatment with cerulein in the presence of trypsin, the protein levels of GPX4 (but not the mRNA levels of Gpx4) were reduced in control cells, but not in Psmd4-depleted 266-6 cells (Figure 3A and B). These results indicate that, in acinar cells, PSMD4 inhibits cerulein-/trypsin-induced down-regulation of GPX4 protein, but not Gpx4 mRNA.
Figure 3.
PSMD4-dependent GPX4 degradation promotes ferroptosis in acinar cells. (A and B) Analysis of (A) protein or (B) mRNA expression of GPX4 in control or Psmd4-knockdown 266-6 cells after treatment with cerulein (100 nmol/L) in the absence or presence of trypsin (500 ng/mL) for 24 hours. Data for mRNA are presented as means ± SD; n = 3 biologically independent samples. Two-way analysis of variance (ANOVA) test on all pairwise combinations. (C–G) Analysis of (C) protein expression, (D) intracellular Fe2+, (E) intracellular GSH, (F) intracellular CoQ10, and (G) Egln2 mRNA in control or Psmd4-knockdown 266-6 cells after treatment with cerulein (100 nmol/L) in the presence of trypsin (500 ng/mL) for 24 hours. Data are presented as means ± SD; n = 3 biologically independent samples. One-way ANOVA test on all pairwise combinations. (H) Immunoprecipitation (IP) analysis of the interaction between PSMD4 and GPX4 in 266-6 cells after treatment with cerulein (100 nmol/L) in the presence of trypsin (500 ng/mL) for 6 hours. Semiquantitative data are presented as means ± SD; n = 3 biologically independent samples; t test. (I) Analysis of protein expression in indicated 266-6 cells after treatment with cerulein (100 nmol/L) in the presence of trypsin (500 ng/mL) for 24 hours. Semiquantitative data are presented as means ± SD; n = 3 biologically independent samples; 1-way ANOVA test on all pairwise combinations. (J–M) Analysis of (J) cell death, (K) lipid peroxidation, (L) C20:4, or (M) C22:4 in indicated 266-6 cells after treatment with cerulein (100 nmol/L), L-arginine (5 mg/mL), alcohol (50 mmol/L), erastin (2 μmol/L), and RSL3 (500 nmol/L) in the presence of trypsin (500 ng/mL) for 24 hours. Data are presented as means ± SD; n = 3 biologically independent samples; 2-way ANOVA test on all pairwise combinations. ∗P < .0001 vs control sh or Psmd4/Gpx4 sh group. ACTB, actin beta; Ctrl, control; IB, immunoblotting; sh, shRNA.
The selective autophagic degradation of the iron storage protein ferritin,49 the circadian clock regulator aryl hydrocarbon receptor nuclear translocator-like (ARNTL, best known as brain and mMuscle ARNT-like 1 [BMAL1]),50 and the iron export protein solute carrier family 40 member 1 (SLC40A1, also known as ferroportin-1)51 all promote ferroptosis. However, the knockdown of Psmd4 failed to affect the protein expression of ferritin heavy chain 1, ARNTL, and SLC40A1 during cerulein-/trypsin-induced ferroptosis (Figure 3C), indicating that PSMD4 is not required for autophagy-dependent ferroptosis.51,52 Other important regulators of ferroptosis, such as apoptosis inducing factor mitochondria-associated 2 (an oxidoreductase involved in producing reduced coenzyme Q10 [CoQ10])53, 54, 55 and SLC7A11 (a subunit of the cystine/glutamate antiporter system xc– that promotes cystine uptake and glutathione [GSH] biosynthesis),37 also remained unaffected by PSMD4 (Figure 3C). Accordingly, the knockdown of Psmd4 failed to affect the intracellular levels of iron, GSH, and CoQ10, as well as the mRNA expression of Egln2 (an ARNTL-targeted gene involved in ferroptosis50) in cerulein-/trypsin-treated 266-6 cells (Figure 3D–G).
In the early stages of the induction of ferroptosis by cerulein/trypsin, a protein complex containing PSMD4 and GPX4 was observed in 266-6 cells (Figure 3H). Functionally, the knockdown of Gpx4 by shRNAs (Figure 3I) restored the sensitivity of Psmd4-depleted 266-6 cells to ferroptosis (Figure 3J), which was associated with increased lipid peroxidation (Figure 3K), C20:4 (Figure 3L), or C22:4 (Figure 3M). Altogether, these findings indicate that PSMD4-mediated GPX4 degradation promotes lipid peroxidation-mediated ferroptosis in pancreatic acinar cells.
Olanzapine Is a New Ferroptosis Inhibitor in Acinar Cells
Most ferroptosis inhibitors are experimental drugs (eg, ferrostatin-1 or liproxstatin-1), but have not been characterized yet in clinical trials.56 Driven by this consideration, we screened a library of 1080 Food and Drug Administration–approved drugs for their capacity to inhibit ferroptosis caused by cerulein/trypsin in 266-6 cells. The top 5 compounds that blocked cerulein-/trypsin-induced cell death included olanzapine (an antipsychotic drug), idebenone (a synthetic analogue of CoQ10), telmisartan (an angiotensin II receptor blocker), nisoldipine (a calcium channel blocker), and azelnidipine (a dihydropyridine calcium channel blocker) (Figure 4A). These top 5 drugs were evaluated further in 266-6 cells or mPACs challenged with trypsin combined with cerulein, L-arginine, alcohol, erastin, or RSL. These results confirmed that olanzapine and idebenone can suppress ferroptosis in various conditions (Figure 4B ). Given that CoQ10 already has been characterized as a ferroptosis inhibitor,53,54 we focused on olanzapine in subsequent studies.
Figure 4.
Olanzapine is a new ferroptosis inhibitor in acinar cells. (A) Chemical structures of 5 potential ferroptosis inhibitors. (B) Analysis of cell death in 266-6 cells after treatment with cerulein (100 nmol/L), L-arginine (5 mg/mL), alcohol (50 mmol/L), erastin (2 μmol/L), and RSL3 (500 nmol/L) in the absence or presence of trypsin (500 ng/mL), olanzapine (10 μmol/L), idebenone (10 μmol/L), telmisartan (10 μmol/L), nisoldipine (10 μmol/L), and azelnidipine (10 μmol/L) for 24 hours. Data are shown in a heat map as the mean of 3 biologically independent samples. (C) Analysis of cell death in 266-6 cells after treatment with cerulein (100 nmol/L)/trypsin (500 ng/mL) in the absence or presence of olanzapine (10 μmol/L), risperidone (10 μmol/L), SB204741 (10 μmol/L), and SB242084 (10 μmol/L) for 24 hours. Data are presented as means ± SD; n = 3 biologically independent samples; 2-way analysis of variance (ANOVA) with the Tukey multiple comparisons test on all pairwise combinations. (D) Analysis of mRNA expression in indicated gene knockdown 266-6 cells. Data are presented as means ± SD; n = 3 biologically independent samples. (E) Analysis of cell death in indicated gene knockdown 266-6 cells after treatment with cerulein (100 nmol/L)/trypsin (500 ng/mL) for 24 hours. Data are presented as means ± SD; n = 3 biologically independent samples; 2-way ANOVA with the Tukey multiple comparisons test on all pairwise combinations. (F) Scavenging DPPH free radical activity of indicated drugs. Data are presented as means ± SD; n = 3 biologically independent samples; 1-way ANOVA test on all pairwise combinations. (G–I) Analysis of (G) lipid peroxidation, (H) C20:4, and (I) C22:4 in 266-6 cells after treatment with cerulein (100 nmol/L)/trypsin (500 ng/mL) in the absence or presence of ferrostatin-1 (1 μmol/L), olanzapine (10 μmol/L), risperidone (10 μmol/L), SB204741 (10 μmol/L), and SB242084 (10 μmol/L) for 24 hours. Data are presented as means ± SD; n = 3 biologically independent samples; 1-way ANOVA test on all pairwise combinations. (J) Analysis of GPX4 protein expression in 266-6 cells after treatment with cerulein (100 nmol/L)/trypsin (500 ng/mL) in the absence or presence of olanzapine (10 μmol/L) for 24 hours. Semiquantitative data are presented as means ± SD; n = 3 biologically independent samples; 1-way ANOVA test on all pairwise combinations. ACTB, actin beta; Ctrl, control; sh, shRNA.
Although the mechanism of action of olanzapine is unknown, it has been proposed that its antipsychotic action involves the inhibition of serotonin type 2 (5-hydroxytryptamine 2) receptors, including HTR2A/5-HT2A, HTR2B/5-HT2B, and HTR2C/5-HT2C. Unlike olanzapine, selective antagonists of HTR2A (risperidone), HTR2B (SB204741), or HTR2C (SB242084) failed to inhibit cerulein-/trypsin-induced ferroptotic death in 266-6 cells or mPACs (Figure 4C), suggesting that olanzapine acts through off-target effects to block ferroptosis. Indeed, the knockdown of Htr2a, Htr2b, and Htr2c by shRNAs also failed to affect cerulein-/trypsin-induced cell death in 266-6 cells (Figure 4D and E). As a positive control, the knockdown of ferroptotic promoter Acsl445,57,58 inhibited cerulein-/trypsin-induced cell death in 266-6 cells (Figure 4D and E).
We used the 2,2-diphenyl-1-picrylhydrazyl (DPPH) free radical scavenging assay in a cell-free system to determine whether the off-target activity of olanzapine involves antioxidant effects. Similar to ferrostatin-1 (positive control), the olanzapine-mediated suppression of ferroptosis correlated with its antioxidant activity (Figure 4F). In contrast, the 5-hydroxytryptamine 2 antagonists (risperidone, SB204741, and SB242084) failed to show such antioxidant effects (Figure 4F). Moreover, olanzapine inhibited cerulein-/trypsin-induced lipid peroxidation (Figure 4G), C20:4 (Figure 4H), and C22:4 (Figure 4I). In contrast, olanzapine had no significant impact on cerulein-/trypsin-induced GPX4 degradation in 266-6 cells (Figure 4J). Collectively, these data suggest that olanzapine protects against ferroptotic cell death through antioxidant effects.
Ferroptotic Damage Increases the Severity of Acute Pancreatitis in Mice
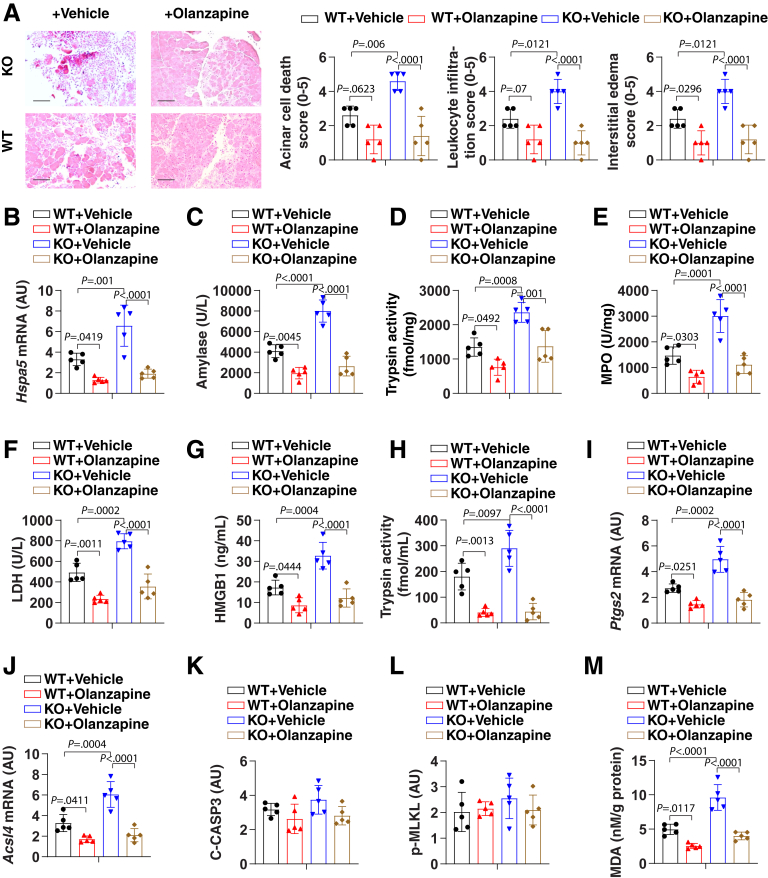
We recently showed that the conditional knockout (KO) of Gpx4 within the pancreas of mice (termed KO mice) accelerated cerulein-induced acute pancreatitis by ferroptotic damage.32 We used this model to evaluate whether olanzapine protects against ferroptosis-induced acute pancreatitis. Histologic assessment of pancreatic damage showed exaggerated acinar cell death, leukocyte infiltration, and interstitial edema in the KO mice compared with control mice (termed wild-type [WT] mice) (Figure 5A). These histologic changes were accompanied by significantly increased levels of pancreatitis parameters, including the activation of the endoplasmic reticulum stress signaling pathway (as measured by mRNA expression of pancreatic heat shock protein family A member 5 [Hspa5, also known as Bip or Grp78]) (Figure 5B), serum amylase (Figure 5C), pancreatic trypsin activity (Figure 5D), pancreatic neutrophil recruitment (as measured by pancreatic myeloperoxidase [MPO] activity) (Figure 5E), pancreatic necrosis (as reflected by serum lactate dehydrogenase [LDH] and high mobility group box 1 [HMGB1]) (Figure 5F and G, respectively), and serum trypsin activity (Figure 5H). The pancreatic expression of biomarkers of ferroptosis (eg, Ptgs2 and Acsl4) (Figure 5I and J, respectively), but not biomarkers of apoptosis (eg, cleaved caspase-3) (Figure 5K) and necroptosis (eg, phosphorylated mixed-lineage kinase domain-like pseudokinase [p-MLKL]) (Figure 5L), were increased in cerulein-treated KO mice. As expected, the level of pancreatic MDA was increased in cerulein-treated KO mice (Figure 5M). The administration of olanzapine protected against all features of cerulein-induced acute pancreatitis in mice (especially KO mice), meaning that it prevented pancreatic dysfunction, tissue injury, inflammation, ferroptosis, and MDA production (Figure 5).
Figure 5.
Ferroptotic damage increases the severity of acute pancreatitis in mice. (A) Representative images of pancreatic histology in cerulein-induced pancreatitis in Gpx4 WT and KO mice with or without olanzapine (5 mg/kg) treatment. Histologic scores. for acinar cell death, leukocyte infiltration, and edema at 12 hours after the last cerulein treatment were evaluated. Data are presented as means ± SD; n = 5 mice/group; 1-way analysis of variance (ANOVA) test on all pairwise combinations. Bar=100 µm (B–L) In parallel, (B) pancreatic Hspa5 mRNA, (C) serum amylase, (D) pancreatic trypsin activity, (E) pancreatic MPO activity, (F) serum LDH, (G) serum HMGB1, (H) serum trypsin activity, (I) pancreatic Ptgs2 mRNA, (J) pancreatic Acsl4 mRNA, (K) pancreatic cleaved caspase-3 (C-CASP3), (L) pancreatic p-MLKL, and (M) pancreatic MDA at 12 hours after the last cerulein treatment were assayed. Data are presented as means ± SD; n = 5 mice/group; 1-way ANOVA test on all pairwise combinations.
Ferroptotic Damage Accelerates the Development of Chronic Pancreatitis in Mice
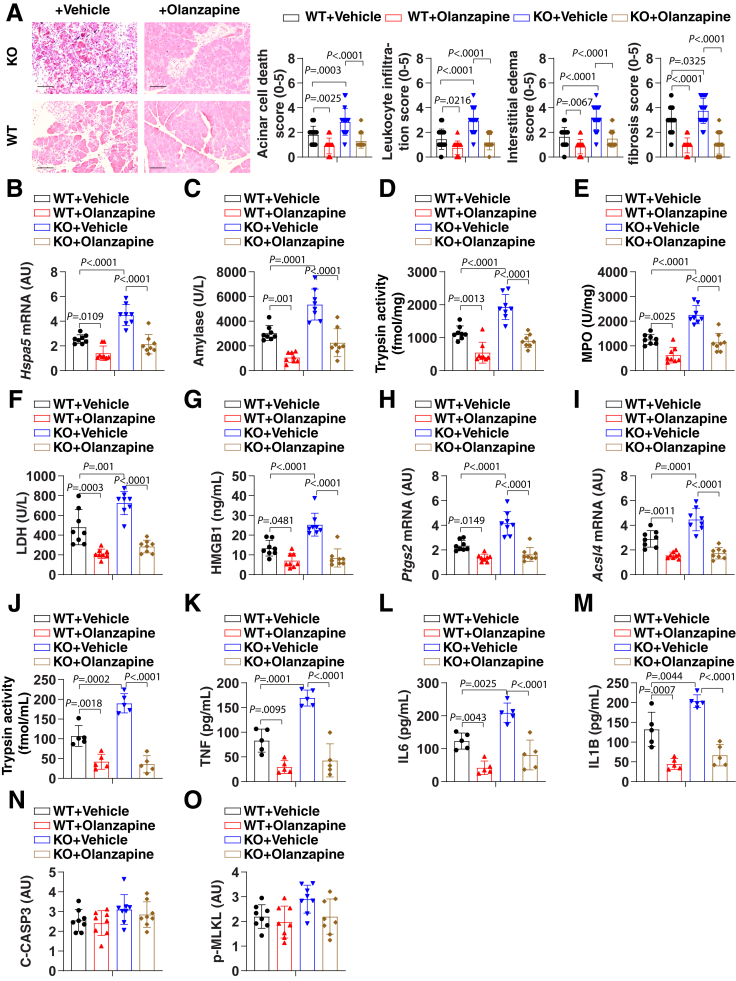
Epidemiologic studies have shown that high alcohol consumption is associated with an increased risk of pancreatitis.59 To further determine the interaction between ferroptosis and clinically relevant environmental insults, we first assessed the effects of pancreatic Gpx4 deletion on chronic pancreatitis initiated by a Lieber–DeCarli alcoholic liquid diet. Ethanol feeding caused histologic changes in the pancreas in 15% (3 of 20) of WT mice (Figure 6A). In contrast, ethanol feeding led to the development of chronic inflammatory changes and pancreatic tissue injury in 85% (17 of 20) of KO mice (Figure 6A). Pancreatic fibrosis, a feature of chronic pancreatitis, also was increased in KO mice (Figure 6A). Accordingly, serum amylase, LDH, HMGB1, pancreatic MPO and trypsin activity, serum trypsin activity, pancreatic Ptgs2 and Acsl4 mRNA expression, as well as serum cytokines (tumor necrosis factor, interleukin 6 [IL6], and IL1β [IL1B]) were increased in KO mice compared with WT mice (Figure 6B–M). This Gpx4 depletion-mediated chronic pancreatitis response was blocked by olanzapine, further supporting the hypothesis that ferroptotic damage promotes the development of chronic pancreatitis. As a control, Gpx4 depletion and olanzapine had no effects on biomarkers of apoptosis (cleaved caspase-3) or necroptosis (p-MLKL) (Figure 6N and O).
Figure 6.
Ferroptotic damage increases the severity of chronic pancreatitis in mice. (A) Representative images of pancreatic histology in Lieber–DeCarli alcoholic liquid diet–induced pancreatitis in Gpx4 WT and KO mice with or without olanzapine (5 mg/kg) treatment. Histologic scores for acinar cell death, leukocyte infiltration, edema, and fibrosis at 4 weeks after Lieber–DeCarli alcoholic liquid diet were evaluated. Data are presented as means ± SD; n = 20 mice/group; 1-way ANOVA test on all pairwise combinations. Bar=100 µm (B–O) In parallel, (B) pancreatic Hspa5 mRNA, (C) serum amylase, (D) pancreatic trypsin activity, (E) pancreatic MPO activity, (F) serum LDH, (G) serum HMGB1, (H) pancreatic Ptgs2 mRNA, (I) pancreatic Acsl4 mRNA, (J) serum trypsin activity, (K) serum tumor necrosis factor (TNF), (L) serum IL6, (M) serum IL1B, (N) pancreatic cleaved caspase-3 (C-CASP3), and (O) pancreatic p-MLKL at 4 weeks after Lieber–DeCarli alcoholic liquid diet were assayed. Data are presented as means ± SD; n = 5–8 mice/group; 1-way ANOVA test on all pairwise combinations.
Discussion
Understanding the mechanisms and functions of pancreatic necrosis is essential for the development of pancreatitis treatment strategies.22 In this study, we reported a role for extracellular trypsin in the sensitization of acinar cells to ferroptotic death by inducing PSMD4-dependent GPX4 degradation. We further showed that, in experimental models of acute or chronic pancreatitis, olanzapine inhibits ferroptosis, improves pancreatic function, blunts inflammation, and limits tissue damage in pancreatic Gpx4 KO mice. These findings enhance our understanding of the pathologic link between abnormal digestive enzyme changes and tissue damage in pancreatitis.
Although many endogenous factors or exogenous factors affect the function of the pancreas, the activation and secretion of digestive enzymes is considered to be one of the important initial signals of pancreatitis.60 For example, the activation of trypsin occurs early in the disease process, parallel to organ damage and inflammation.4,5 Mechanistically, the proteolytic activation of trypsin from trypsinogen (the precursor of trypsin) requires cathepsin B,61 and pharmacologic or genetic blockade of cathepsin B prevents pancreatitis in mice.61,62 Cathepsin B is also a mediator of ferroptosis.63,64 These findings suggest an important role for lysosomal hydrolases in both trypsin activation and ferroptosis.
Although early studies using genetic mouse models that express R122H or p.D23A trypsinogen suggested that trypsin activation alone may directly cause pancreatitis,65,66 recent studies using genetic mouse models that express human wild-type PRSS1 (which encodes cationic trypsinogen) or its mutant form (R122H or K23R) have shown that trypsin activation is not sufficient to induce spontaneous pancreatitis.13, 14, 15,67 In contrast, serine protease 1 mice are more sensitive to experimental pancreatitis induced by cerulein, ethanol, or a high-fat diet,13, 14, 15,67 indicating that trypsin needs to be combined with other risk factors to induce pancreatitis. Our current study provides a theoretical model to suggest that active trypsin increases the risk of pancreatitis caused by ferroptotic damage.
Trypsin has enzyme digestion–dependent and –independent functions, relying on its concentration. In addition to extracellular trypsin being able to activate the nuclear factor-κB inflammation pathway,20 our current data suggest that excessive levels of extracellular trypsin enhance the sensitivity of acinar cells to ferroptosis, but not apoptosis and necroptosis. As an oxidative stress–dependent cell death,68 ferroptosis is characterized by necrotic changes, such as plasma rupture and the release of damage-associated molecular patterns (eg, HMGB1).69,70 Different from intracellular HMGB1, which scavenges cellular damage, extracellular HMGB1 is a mediator of sterile inflammation in mouse models of pancreatitis.71 Unlike apoptosis and necrosis, ferroptosis can be initiated without the contribution of caspases and MLKL.26,27 Unrestricted lipid peroxidation and the abnormal lysosomal degradation pathways that are observed commonly in pancreatitis all are implicated in inducing ferroptosis in vitro and in vivo.69 Here, we documented that the presence of excessive trypsin dramatically increases the susceptibility of acinar cells to various ferroptosis activators.
Our study highlights a new mechanism by which PSMD4-dependent proteasomal degradation of GPX4 promotes ferroptosis in acinar cells. The lipid hydroperoxidase GPX4 has the unique ability to detoxify lipid hydroperoxide into harmless alcohol and water, thereby preventing ferroptotic damage.72, 73, 74 The degradation of GPX4 appears to be a common response to ferroptosis activators.42,48,75 In contrast to selective autophagy, which mediates GPX4 degradation in ferroptotic cancer cells,48 our current study suggests that the activation of the ubiquitin-proteasome system is responsible for GPX4 degradation in ferroptotic acinar cells. Although the core E3 ubiquitin ligase responsible for GPX4 degradation remains elusive, PSMD4 (a proteasome ubiquitin receptor) plays a significant role in mediating GPX4 degradation to induce ferroptosis. Consequently, blocking PSMD4-dependent GPX4 degradation inhibits trypsin-enhanced ferroptosis in acinar cells. In fact, administration of the proteasome inhibitor MG132 prevents the onset of acute pancreatitis in mice,76,77 supporting the notion that the proteasomal degradation pathway is one of the therapeutic targets of pancreatitis.
The depletion of pancreatic Gpx4 does not affect the normal development and physiological function of the pancreas, but exacerbates acute or chronic pancreatitis caused by pathologic (eg, cerulein) or clinically relevant stressors (eg, alcohol).32 We showed that pancreatic Gpx4 depletion triggers a feed-forward loop between lipid peroxidation and trypsin activation during experimental pancreatitis. In contrast to the pancreas, the depletion of Gpx4 in other, nonpancreatic tissues or cells, such as the kidney or T cells, may cause spontaneous tissue damage in a ferroptosis-dependent manner.72,73 In addition, the loss of Gpx4 in myeloid cells increased susceptibility to bacterial infections through lipid peroxidation–dependent pyroptosis, rather than ferroptosis.78 Therefore, GPX4 has physiological or pathologic functions that differ according to tissue type or stimulation.28,78, 79, 80 It is worth noting that a recent study showed that genetic ablation of the antioxidant enzyme peroxiredoxin 1 in the pancreas prevents cerulein-induced pancreatitis through a redox-independent mechanism.81 Further understanding of the overlap and different functions of antioxidant enzymes in pancreatitis may establish new strategies to limit sterile inflammation.
In summary, we provide evidence that trypsin-mediated sensitization of pancreatic acinar cells to ferroptosis may be targeted for the prevention and treatment of pancreatitis in mice. An unbiased drug screening campaign identified olanzapine (a drug used to treat certain psychiatric conditions, such as schizophrenia, bipolar disorder, and depression82) as a novel ferroptosis inhibitor that prevents pancreatitis in mice. These findings may accelerate translational research on the suppression of ferroptotic damage in human pancreatitis.
Materials and Methods
Reagents
Erastin (S7242), RSL3 (S8155), ferrostatin-1 (S7243), liproxstatin-1 (S7699), Z-VAD-FMK (S7023), necrosulfonamide (S8251), staurosporine (S1421), CCT137690 (S2744), olanzapine (S2493), idebenone (S2605), telmisartan (S1738), nisoldipine (S1748), azelnidipine (S3053), and risperidone (S1615) were obtained from Selleck Chemicals (Houston, TX). Cerulein (C9026), L-arginine (A5006), alcohol (PHR1373), 2-mercaptoethanol (M6250), trypsin (T1426), soybean trypsin inhibitor (650357), SB204741 (S0693), and SB242084 (5.06417) were obtained from Sigma-Aldrich (Burlington, MA). Dimethyl sulfoxide (472301; Sigma-Aldrich) was used to prepare the stock solution of most drugs. The final concentration of dimethyl sulfoxide in the drug working solution in the cells was less than 0.01%. In addition, 0.01% dimethyl sulfoxide was used as a vehicle control in the corresponding cell culture assays.
The antibodies to PSMD4 (sc-398033) and actin beta (ACTB, sc-8432) were obtained from Santa Cruz Biotechnology (Dallas, TX). The antibodies to GPX4 (ab125066) and SLC7A11 (ab175186) were obtained from Abcam (Cambridge, MA). The antibodies to ferritin heavy chain 1 (3998) and ARNTL (14020) were obtained from Abcam. The antibodies to SLC40A1 (NBP1-21502) and apoptosis inducing factor mitochondria-associated 2 (H00084883-D01P) were obtained from NOVUS (Saint Charles, MO).
Pancreatitis Models
The protocol for animal use was reviewed and approved by our institutional animal care and use committees. Pancreatic-specific Gpx4 knockout mice were produced and identified in our laboratory by crossing floxed Gpx4 (a gift from Dr Qitao Ran, University of Texas Health, San Antonio, TX) and Pdx1-Cre (014647; The Jackson Laboratory, Bar Harbor, ME) transgenic mice (C57BL/6J background) as previously described.32 Mouse were kept under standard pathogen-free conditions with an artificial 12-hour light/dark cycle (lights on: 8:00 AM) and constant 50%–60% humidity. Mice were allowed access to tap water and free (ad libitum) access to standard laboratory chow during the experimental period.
For cerulein-induced acute pancreatitis, male mice (age, 8–10 wk) received 7 hourly intraperitoneal injections of 50 μg/kg cerulein in sterile saline.83 Olanzapine was repeatedly administered orally by gavage at a dose of 5 mg/kg to mice at 3 and 12 hours after the first cerulein injection, while controls were treated by oral administration with vehicle (smooth peanut butter).84 The parameters of acute pancreatitis were assessed 12 hours after the last cerulein treatment. For the induction of chronic pancreatitis, male mice (age, 8–10 wk) were fed a Lieber–DeCarli ethanol (5% vol/vol) liquid diet for 4 weeks (F1258; Bio-Serv, Flemington, NJ).85 In parallel, olanzapine was administered orally by gavage at a dose of 5 mg/kg to mice (3 times per week, over 4 weeks), while controls were treated by oral administration with vehicle. The parameters of chronic pancreatitis were assessed in mice 4 weeks after feeding them the Lieber–DeCarli ethanol liquid diet.
Animals were killed at the indicated time by CO2 asphyxia, and a blood sample and tissue were collected. Serum was collected further immediately after centrifugation at 10,000 × g for 5 minutes at 4°C. Tissue samples were collected, snap-frozen in liquid nitrogen, and stored at -80°C. Formalin-fixed pancreas samples were processed, and 5-μm–thick paraffin sections were stained with H&E for histologic analysis. Pancreatitis was scored according to a revised scoring standard,86 with an independent pathologist evaluating histologic scores for acinar cell death, leukocyte infiltration, or edema in a blind manner. Histologic images were acquired using an EVOS FL Auto Cell Imaging System (Thermo Fisher Scientific, Pittsburgh, PA).
Cell Culture
The 266-6 (CRL-2151) cell line was obtained from the American Type Culture Collection and cultured in Dulbecco's modified Eagle medium (11995073; Thermo Fisher Scientific) supplemented with 10% heat-inactivated fetal bovine serum (A3840001; Thermo Fisher Scientific) and 1% penicillin and streptomycin (15070-063; Thermo Fisher Scientific) at 37°C, 95% humidity, and 5% CO2. Primary mouse acinar cells were cultured as described below. Briefly, the pancreas from male C57BL/6J mice (age, 8–10 wk) was removed and minced for 5 minutes in Hank's balanced salt solution (H8264; Sigma-Aldrich) plus 0.1% bovine serum albumin (05470; Sigma-Aldrich) and 10 mmol/L HEPES (PHG0001; Sigma-Aldrich). After washing, the pancreatic segments were incubated in 10 mL collagenase IA solution (Hank's balanced salt solution 1× containing 10 mmol/L HEPES, 200 U/mL of collagenase IA [C9891; Sigma-Aldrich], and 0.25 mg/mL of trypsin inhibitor) for 20–30 minutes at 37°C. The solution containing collagenase then was removed and replaced with Dulbecco's modified Eagle medium supplemented with 1% penicillin and streptomycin, 10% fetal bovine serum, 0.25 mg/mL trypsin inhibitor, and 25 ng/mL recombinant mouse epidermal growth factor (E5160; Sigma-Aldrich) at 37°C, 95% humidity, and 5% CO2. Cell line identity was validated by short tandem repeat profiling, and routine mycoplasma testing was negative for contamination.
Western Blot Analysis
Cells were lysed 3 times with 1× cell lysis buffer (9803; Cell Signaling Technology, Danvers, MA) containing protease inhibitor cocktail (P8340; Sigma-Aldrich) on ice for 10 minutes. Protein was quantified using the bicinchoninic acid assay (23225; Thermo Fisher Scientific) and 20–30 μg of each sample was resolved on 4%–12% Criterion XT Bis-Tris gels (3450124; Bio-Rad) in XT MES running buffer (1610789; Bio-Rad) and transferred to polyvinylidene difluoride membranes (1620233; Bio-Rad) using the Trans-Blot Turbo Transfer Pack and System (1704150; Bio-Rad). Membranes were blocked with Tris-buffered saline with 0.1% Tween 20 detergent (TBST) containing 5% nonfat milk (9999; Cell Signaling Technology) for 1 hour and incubated overnight at 4°C with various primary antibodies (1:500–1:1000). After 3 washes in TBST, membranes were incubated with goat anti-rabbit or anti-mouse IgG horseradish peroxidase–conjugated secondary antibody (1:1000, 7074 or 7076; Cell Signaling Technology) at room temperature for 1 hour. After being washed with TBST, the signals were visualized using enhanced chemiluminescence (32106; Thermo Fisher Scientific) and then visualized and analyzed with a ChemiDoc Touch Imaging System (Bio-Rad).
Immunoprecipitation Analysis
Cells were lysed at 4°C in pre-cold radioimmunoprecipitation assay lysis buffer (9806; Cell Signaling Technology) and cell lysates were cleared by brief centrifugation (12,000 × g for 10 minutes).87 Concentrations of proteins in the supernatant were determined by bicinchoninic acid assay (23225; Thermo Fisher Scientific). Before immunoprecipitation, samples containing equal amounts of proteins were precleared with protein A agarose beads (9863; Cell Signaling Technology) at 4°C for 3 hours and subsequently incubated with irrelevant IgG or specific antibodies (5 μg/mL) in the presence of protein A agarose beads overnight at 4°C with gentle shaking. After incubation, protein A agarose beads were washed extensively with phosphate-buffered saline and proteins were eluted by boiling in 2 × Laemmli sample buffer (161-0737; Bio-Rad) before sodium dodecyl sulfate–polyacrylamide gel electrophoresis.
Cytotoxicity Assays
A Countess II FL Automated Cell Counter (Thermo Fisher Scientific) was used to assay the percentage of dead cells after cell staining with 0.4% trypan blue solution (T10282; Thermo Fisher Scientific). Cells were suspended as single cells in buffered saline. If cell clumps (especially primary acinar cells) were seen in the sample tube, these samples were filtered with nylon mesh to remove aggregates. Samples then were prepared by adding 10 μL of cell suspension to 10 μL of 0.4% trypan blue stain.
Food and Drug Administration–Approved Drug Screening
The Food and Drug Administration–approved drug library was obtained from Selleck Chemicals (L1300). The 266-6 cells were seeded into 96-well plates and then treated with cerulein (100 nmol/L)/trypsin (500 ng/mL) in the absence or presence of a drug (10 μmol/L) for 24 hours. Subsequently, 100 μL fresh medium was added to cells containing 10 μL Cell Counting Kit-8 (CCK-8) solutions (CK04; Dojindo Laboratories, Rockville, MD) and incubated for 2 hours (37°C, 5% CO2). Cell viability was measured at 450-nm absorbance using a microplate reader (Cytation 5 Cell Imaging Multi-Mode Reader, Winooski, VA). The effects of top drug candidates on cerulein-/trypsin-induced cell death in 266-6 cells were assayed further using a Countess II FL Automated Cell Counter.
Quantitative Real-Time Polymerase Chain Reaction Assay
Total RNA was extracted and purified from cultured cells using the RNeasy Plus Mini Kit (74136; QIAGEN, Germantown, MD). First-strand complementary DNA (cDNA) was synthesized from 1 μg RNA using the iScript cDNA Synthesis Kit (1708890; Bio-Rad). Briefly, 20-μL reactions were prepared by combining 4 μL iScript Select reaction mix, 2 μL gene-specific enhancer solution, 1 μL reverse transcriptase, 1 μL gene-specific assay pool (20×, 2 μmol/L), and 12 μL RNA diluted in RNase-free water. Then cDNA from various cell samples was amplified by real-time qPCR with specific primers using a CFX96 Touch Real-Time PCR Detection System (Bio-Rad) with CFX Manager software 2.0 (Bio-Rad). The data were normalized to Rna18S and the fold change was calculated via the 2-ΔΔCt method. The relative concentrations of mRNA were expressed in arbitrary units based on the untreated group, which was assigned a value of 1. The predesigned primers were obtained from OriGene Technologies (Rockville, Maryland, USA).
RNAi and Gene Transfection
Psmd4 shRNA (GTACCGGGAAACTAGCTAAACGCCTTAACTCGAGTTAAGGCGTTTAGCTAGTTTCTTTTTTG), Gpx4 shRNA (CCGGCCGGCTACAACGTCAAGTTTGCTCGAGCAAACTTGACGTTGTAGCCGGTTTTTG), Htr2a shRNA (CCGGCCCACTGGTATATACGTTGTTCTCGAGAACAACGTATATACCAGTGGGTTTTT), Htr2b shRNA (CCGGGCGGTGATAATACCCACCATTCTCGAGAATGGTGGGTATTATCACCGCTTTTT), Htr2c shRNA (CCGGGCTTCCAAAGTCCTTGGCATTCTCGAGAATGCCAAGGACTTTGGAAGCTTTTT), and Acsl4 shRNA (CCGGGCAGAAGATTATTGTGTTGATCTCGAGATCAACACAATAATCTTCTGCTTTTT) were obtained from Sigma-Aldrich. Psmd4 cDNA (MC200621) was obtained from OriGene Technologies. The shRNA or indicated cDNA were transfected into cells using Lipofectamine 3000 (L3000-015; Invitrogen, Pittsburgh, PA) or Lipofectamine RNAiMAX (13778030; Invitrogen) when cells were at approximately 60%–70% confluence. Puromycin (ant-pr-1; InvivoGen, San Diego, CA) was used to generate stable knockdown cell lines. The efficiency of RNAi and gene transfection was verified by reverse-transcription PCR or Western blot.
Enzyme-Linked Immunosorbent Assay Analysis
The concentrations or activity of amylase (ab102523; Abcam), MPO (EMMPO; Thermo Fisher Scientific), trypsin (ab102531; Abcam), 8-hydroxy-2-deoxyguanosine (K4160; BioVision, Milpitas, CA), HMGB1 (326054329; Sino-Test Corporation, Tokyo, Japan), LDH (ab102526; Abcam), cleaved-caspase3 (DYC835-2; R&D Systems, Minneapolis, MN), p-MLKL (PEL-MLKL-S345-1; RayBiotech Life, Norcross, GA), MDA (ab118970; Abcam), iron (MAK025; Sigma-Aldrich), GSH (CS0260; Sigma-Aldrich), CoQ10 (MBS7233016; MyBioSource, San Diego, CA), tumor necrosis factor (BMS607-3; Thermo Fisher Scientific), IL6 (BMS603-2; Thermo Fisher Scientific), and IL1B (BMS6002; Thermo Fisher Scientific) in the indicated samples were measured using enzyme-linked immunosorbent assay kits according to the manufacturer's guidelines. Data were normalized to protein or DNA concentration.
Lipid Peroxidation Assay
BODIPY 581/591 C11 probe (D3861; Thermo Fisher Scientific) was used to detect lipid peroxidation according to the manufacturer’s instructions. Briefly, cells were incubated with BODIPY 581/591 C11 at a final concentration of 5 μmol/L for 30 minutes at 37°C and washed 3 times with phosphate-buffered saline. Oxidation of the polyunsaturated butadienyl portion of the dye resulted in a shift of the fluorescence emission peak from approximately 590 nm to approximately 510 nm, which was measured in a Bio-Tek fluorescence microplate reader (Winooski, VA).
DPPH Radical Scavenging Assay
The free radical scavenging ability of the indicated drugs was tested by a DPPH radical scavenging assay. DPPH produces purple/violet in methanol solution and fades to yellow in the presence of antioxidants. Briefly, a reaction mixture containing 2.4 mL of 0.1 mmol/L DPPH in methanol and 1.6 mL of tested drugs (1 μmol/L ferrostatin-1, 10 μmol/L olanzapine, 10 μmol/L risperidone, 10 μmol/L SB204741, and 10 μmol/L SB242084) was incubated in a dark environment at 25°C for 30 minutes. The optical absorbance at 517 nm of the mixture was measured in triplicate after centrifugation. The DPPH radical scavenging activity was expressed in arbitrary units based on the untreated group, which was assigned a value of 1. Ferrostatin-1 was used as a positive control.
Liquid Chromatography with Tandem Mass Spectrometry Method for Lipid Analysis
Reverse-phase chromatography was selected for LC separation using the CSH C18 column (1.7 μm, 2.1 mm × 100 mm; Waters Corp, Milford, MD). The lipid extracts were re-dissolved in 200 μL 90% isopropanol/acetonitrile, centrifuged at 14,000 × g for 15 minutes, and finally 3 μL of sample was injected. Solvent A was acetonitrile–water (6:4, vol/vol) with 0.1% formic acid and 0.1 mmol/L ammonium formate and solvent B was acetonitrile–isopropanol (1:9, vol/vol) with 0.1% formic acid and 0.1 mmol/L ammonium formate. The initial mobile phase was 30% solvent B at a flow rate of 300 μL/min. It was held for 2 minutes, and then increased linearly to 100% solvent B in 23 minutes, followed by equilibrating at 5% solvent B for 10 minutes.
Mass spectra were acquired with Q-Exactive Plus (Pittsburgh, PA) in positive and negative mode, respectively. Electrospray ionization (ESI) parameters were optimized and preset for all measurements as follows: source temperature, 300°C; capillary temperature, 350°C; ion spray voltage, 3000 V; S-lens RF level, 50%; and the scan range of the instruments, 200–1800 m/z. LipidSearch Software 5.0 (Thermo Fisher Scientific) was used to identify lipid species based on MS/MS data. The control group was assigned a value of 1, and the treatment group then was calculated relative to the control group.
Statistical Analysis
Data are presented as means ± SD except where otherwise indicated. GraphPad Prism 8.4.3 (San Diego, CA) was used to collect and analyze data. A t test was used to compare the means of 2 groups. A 1-way or 2-way analysis of variance with the Tukey multiple comparisons test was used for comparison among the different groups. A P value less than .05 was considered statistically significant. We did not exclude samples or animals. For every figure in this article, statistical tests are justified when appropriate. All data meet the assumptions of the tests (eg, normal distribution).
Acknowledgments
The authors thank Dave Primm (Department of Surgery, University of Texas Southwestern Medical Center) for critical reading of the manuscript.
CRediT Authorship Contributions
Ke Liu (Data curation: Lead)
Jiao Liu (Data curation: Lead)
Borong Zou (Data curation: Equal)
Changfeng Li (Data curation: Equal)
Herbert Zeh (Writing – review & editing: Equal)
Rui Kang (Data curation: Equal; Methodology: Equal)
Guido Kroemer (Writing – review & editing: Lead)
Jun Huang (Conceptualization: Lead; Formal analysis: Lead; Writing – original draft: Equal)
Daolin Tang, MD, PhD (Conceptualization: Lead; Data curation: Lead; Formal analysis: Lead; Investigation: Lead; Methodology: Lead; Project administration: Lead; Supervision: Lead; Writing – original draft: Lead; Writing – review & editing: Lead)
Footnotes
Conflicts of interest The authors disclose no conflicts.
Funding This work was supported by the American Cancer Society grant RSG-16-014-01 (D.T.), and by the National Natural Science Foundation of China grants 81872323 and 82073299 (C.L.).
Contributor Information
Jun Huang, Email: 505447@csu.edu.cn.
Daolin Tang, Email: daolin.tang@utsouthwestern.edu.
References
- 1.Beck I.T., Kahn D.S., Solymar J., McKenna R.D., Zylberszac B. The role of pancreatic enzymes in the pathogenesis of acute pancreatitis. III. Comparison of the pathologic and biochemical changes in the canine pancreas to intraductal injection with bile and with trypsin. Gastroenterology. 1964;46:531–542. [PubMed] [Google Scholar]
- 2.Hegyi E., Sahin-Toth M. Genetic risk in chronic pancreatitis: the trypsin-dependent pathway. Dig Dis Sci. 2017;62:1692–1701. doi: 10.1007/s10620-017-4601-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ji B., Logsdon C.D. Digesting new information about the role of trypsin in pancreatitis. Gastroenterology. 2011;141:1972–1975. doi: 10.1053/j.gastro.2011.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Neoptolemos J.P., Kemppainen E.A., Mayer J.M., Fitzpatrick J.M., Raraty M.G., Slavin J., Beger H.G., Hietaranta A.J., Puolakkainen P.A. Early prediction of severity in acute pancreatitis by urinary trypsinogen activation peptide: a multicentre study. Lancet. 2000;355:1955–1960. doi: 10.1016/s0140-6736(00)02327-8. [DOI] [PubMed] [Google Scholar]
- 5.Gudgeon A.M., Heath D.I., Hurley P., Jehanli A., Patel G., Wilson C., Shenkin A., Austen B.M., Imrie C.W., Hermon-Taylor J. Trypsinogen activation peptides assay in the early prediction of severity of acute pancreatitis. Lancet. 1990;335:4–8. doi: 10.1016/0140-6736(90)90135-r. [DOI] [PubMed] [Google Scholar]
- 6.Whitcomb D.C., Gorry M.C., Preston R.A., Furey W., Sossenheimer M.J., Ulrich C.D., Martin S.P., Gates L.K., Jr., Amann S.T., Toskes P.P., Liddle R., McGrath K., Uomo G., Post J.C., Ehrlich G.D. Hereditary pancreatitis is caused by a mutation in the cationic trypsinogen gene. Nat Genet. 1996;14:141–145. doi: 10.1038/ng1096-141. [DOI] [PubMed] [Google Scholar]
- 7.Teich N., Rosendahl J., Toth M., Mossner J., Sahin-Toth M. Mutations of human cationic trypsinogen (PRSS1) and chronic pancreatitis. Hum Mutat. 2006;27:721–730. doi: 10.1002/humu.20343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nemeth B.C., Sahin-Toth M. Human cationic trypsinogen (PRSS1) variants and chronic pancreatitis. Am J Physiol Gastrointest Liver Physiol. 2014;306:G466–G473. doi: 10.1152/ajpgi.00419.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Witt H., Luck W., Hennies H.C., Classen M., Kage A., Lass U., Landt O., Becker M. Mutations in the gene encoding the serine protease inhibitor, Kazal type 1 are associated with chronic pancreatitis. Nat Genet. 2000;25:213–216. doi: 10.1038/76088. [DOI] [PubMed] [Google Scholar]
- 10.Pfutzer R.H., Barmada M.M., Brunskill A.P., Finch R., Hart P.S., Neoptolemos J., Furey W.F., Whitcomb D.C. SPINK1/PSTI polymorphisms act as disease modifiers in familial and idiopathic chronic pancreatitis. Gastroenterology. 2000;119:615–623. doi: 10.1053/gast.2000.18017. [DOI] [PubMed] [Google Scholar]
- 11.Muller N., Sarantitis I., Rouanet M., de Mestier L., Halloran C., Greenhalf W., Ferec C., Masson E., Ruszniewski P., Levy P., Neoptolemos J., Buscail L., Rebours V. Natural history of SPINK1 germline mutation related-pancreatitis. EBioMedicine. 2019;48:581–591. doi: 10.1016/j.ebiom.2019.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chandak G.R., Idris M.M., Reddy D.N., Mani K.R., Bhaskar S., Rao G.V., Singh L. Absence of PRSS1 mutations and association of SPINK1 trypsin inhibitor mutations in hereditary and non-hereditary chronic pancreatitis. Gut. 2004;53:723–728. doi: 10.1136/gut.2003.026526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang H., Swidnicka-Siergiejko A.K., Daniluk J., Gaiser S., Yao Y., Peng L., Zhang Y., Liu Y., Dong M., Zhan X., Wang H., Bi Y., Li Z., Ji B., Logsdon C.D. Transgenic expression of PRSS1(R122H) sensitizes mice to pancreatitis. Gastroenterology. 2020;158:1072–1082 e7. doi: 10.1053/j.gastro.2019.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gui F., Zhang Y., Wan J., Zhan X., Yao Y., Li Y., Haddock A.N., Shi J., Guo J., Chen J., Zhu X., Edenfield B.H., Zhuang L., Hu C., Wang Y., Mukhopadhyay D., Radisky E.S., Zhang L., Lugea A., Pandol S.J., Bi Y., Ji B. Trypsin activity governs increased susceptibility to pancreatitis in mice expressing human PRSS1R122H. J Clin Invest. 2020;130:189–202. doi: 10.1172/JCI130172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jancso Z., Sahin-Toth M. Mutation that promotes activation of trypsinogen increases severity of secretagogue-induced pancreatitis in mice. Gastroenterology. 2020;158:1083–1094. doi: 10.1053/j.gastro.2019.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rakonczay Z., Jr., Hegyi P., Takacs T., McCarroll J., Saluja A.K. The role of NF-kappaB activation in the pathogenesis of acute pancreatitis. Gut. 2008;57:259–267. doi: 10.1136/gut.2007.124115. [DOI] [PubMed] [Google Scholar]
- 17.Huang H., Liu Y., Daniluk J., Gaiser S., Chu J., Wang H., Li Z.S., Logsdon C.D., Ji B. Activation of nuclear factor-kappaB in acinar cells increases the severity of pancreatitis in mice. Gastroenterology. 2013;144:202–210. doi: 10.1053/j.gastro.2012.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhan X., Wan J., Zhang G., Song L., Gui F., Zhang Y., Li Y., Guo J., Dawra R.K., Saluja A.K., Haddock A.N., Zhang L., Bi Y., Ji B. Elevated intracellular trypsin exacerbates acute pancreatitis and chronic pancreatitis in mice. Am J Physiol Gastrointest Liver Physiol. 2019;316:G816–G825. doi: 10.1152/ajpgi.00004.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gaiser S., Daniluk J., Liu Y., Tsou L., Chu J., Lee W., Longnecker D.S., Logsdon C.D., Ji B. Intracellular activation of trypsinogen in transgenic mice induces acute but not chronic pancreatitis. Gut. 2011;60:1379–1388. doi: 10.1136/gut.2010.226175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ji B., Gaiser S., Chen X., Ernst S.A., Logsdon C.D. Intracellular trypsin induces pancreatic acinar cell death but not NF-kappaB activation. J Biol Chem. 2009;284:17488–17498. doi: 10.1074/jbc.M109.005520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sendler M., Lerch M.M. The complex role of trypsin in pancreatitis. Gastroenterology. 2020;158:822–826. doi: 10.1053/j.gastro.2019.12.025. [DOI] [PubMed] [Google Scholar]
- 22.Kang R., Lotze M.T., Zeh H.J., Billiar T.R., Tang D. Cell death and DAMPs in acute pancreatitis. Mol Med. 2014;20:466–477. doi: 10.2119/molmed.2014.00117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Criddle D.N., Gerasimenko J.V., Baumgartner H.K., Jaffar M., Voronina S., Sutton R., Petersen O.H., Gerasimenko O.V. Calcium signalling and pancreatic cell death: apoptosis or necrosis? Cell Death Differ. 2007;14:1285–1294. doi: 10.1038/sj.cdd.4402150. [DOI] [PubMed] [Google Scholar]
- 24.Petrov M.S., Shanbhag S., Chakraborty M., Phillips A.R., Windsor J.A. Organ failure and infection of pancreatic necrosis as determinants of mortality in patients with acute pancreatitis. Gastroenterology. 2010;139:813–820. doi: 10.1053/j.gastro.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 25.Bakker O.J., van Santvoort H.C., Besselink M.G., van der Harst E., Hofker H.S., Gooszen H.G. Dutch Pancreatitis Study Group. Prevention, detection, and management of infected necrosis in severe acute pancreatitis. Curr Gastroenterol Rep. 2009;11:104–110. doi: 10.1007/s11894-009-0017-3. [DOI] [PubMed] [Google Scholar]
- 26.Chen X., Li J., Kang R., Klionsky D.J., Tang D. Ferroptosis: machinery and regulation. Autophagy. 2021;17:2054–2081. doi: 10.1080/15548627.2020.1810918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tang D., Chen X., Kang R., Kroemer G. Ferroptosis: molecular mechanisms and health implications. Cell Res. 2021;31:107–125. doi: 10.1038/s41422-020-00441-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen X., Kang R., Kroemer G., Tang D. Ferroptosis in infection, inflammation, and immunity. J Exp Med. 2021;218 doi: 10.1084/jem.20210518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kimita W., Petrov M.S. Iron metabolism and the exocrine pancreas. Clin Chim Acta. 2020;511:167–176. doi: 10.1016/j.cca.2020.10.013. [DOI] [PubMed] [Google Scholar]
- 30.Ma D., Li C., Jiang P., Jiang Y., Wang J., Zhang D. Inhibition of ferroptosis attenuates acute kidney injury in rats with severe acute pancreatitis. Dig Dis Sci. 2020;66:483–492. doi: 10.1007/s10620-020-06225-2. [DOI] [PubMed] [Google Scholar]
- 31.Liu Y., Wang Y., Liu J., Kang R., Tang D. The circadian clock protects against ferroptosis-induced sterile inflammation. Biochem Biophys Res Commun. 2020;525:620–625. doi: 10.1016/j.bbrc.2020.02.142. [DOI] [PubMed] [Google Scholar]
- 32.Dai E., Han L., Liu J., Xie Y., Zeh H.J., Kang R., Bai L., Tang D. Ferroptotic damage promotes pancreatic tumorigenesis through a TMEM173/STING-dependent DNA sensor pathway. Nat Commun. 2020;11:6339. doi: 10.1038/s41467-020-20154-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meher S., Mishra T.S., Sasmal P.K., Rath S., Sharma R., Rout B., Sahu M.K. Role of biomarkers in diagnosis and prognostic evaluation of acute pancreatitis. J Biomark. 2015;2015:519534. doi: 10.1155/2015/519534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Artigas J.M., Garcia M.E., Faure M.R., Gimeno A.M. Serum trypsin levels in acute pancreatic and non-pancreatic abdominal conditions. Postgrad Med J. 1981;57:219–222. doi: 10.1136/pgmj.57.666.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cristina Oliveira de Lima V., Piuvezam G., Leal Lima Maciel B., Heloneida de Araujo Morais A. Trypsin inhibitors: promising candidate satietogenic proteins as complementary treatment for obesity and metabolic disorders? J Enzyme Inhib Med Chem. 2019;34:405–419. doi: 10.1080/14756366.2018.1542387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Draper H.H., Hadley M. Malondialdehyde determination as index of lipid peroxidation. Methods Enzymol. 1990;186:421–431. doi: 10.1016/0076-6879(90)86135-i. [DOI] [PubMed] [Google Scholar]
- 37.Dixon S.J., Lemberg K.M., Lamprecht M.R., Skouta R., Zaitsev E.M., Gleason C.E., Patel D.N., Bauer A.J., Cantley A.M., Yang W.S., Morrison B., 3rd, Stockwell B.R. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149:1060–1072. doi: 10.1016/j.cell.2012.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Malsy M., Bitzinger D., Graf B., Bundscherer A. Staurosporine induces apoptosis in pancreatic carcinoma cells PaTu 8988t and Panc-1 via the intrinsic signaling pathway. Eur J Med Res. 2019;24:5. doi: 10.1186/s40001-019-0365-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xie Y., Zhu S., Zhong M., Yang M., Sun X., Liu J., Kroemer G., Lotze M., Zeh H.J., 3rd, Kang R., Tang D. Inhibition of aurora kinase a induces necroptosis in pancreatic carcinoma. Gastroenterology. 2017;153:1429–1443 e5. doi: 10.1053/j.gastro.2017.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang Y., Luo M., Zhang K., Zhang J., Gao T., Connell D.O., Yao F., Mu C., Cai B., Shang Y., Chen W. Nedd4 ubiquitylates VDAC2/3 to suppress erastin-induced ferroptosis in melanoma. Nat Commun. 2020;11:433. doi: 10.1038/s41467-020-14324-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang Y., Liu Y., Liu J., Kang R., Tang D. NEDD4L-mediated LTF protein degradation limits ferroptosis. Biochem Biophys Res Commun. 2020;531:581–587. doi: 10.1016/j.bbrc.2020.07.032. [DOI] [PubMed] [Google Scholar]
- 42.Yang L., Chen X., Yang Q., Chen J., Huang Q., Yao L., Yan D., Wu J., Zhang P., Tang D., Zhong N., Liu J. Broad spectrum deubiquitinase inhibition induces both apoptosis and ferroptosis in cancer cells. Front Oncol. 2020;10:949. doi: 10.3389/fonc.2020.00949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen X., Yu C., Kang R., Kroemer G., Tang D. Cellular degradation systems in ferroptosis. Cell Death Differ. 2021;28:1135–1148. doi: 10.1038/s41418-020-00728-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lin Z., Liu J., Kang R., Yang M., Tang D. Lipid metabolism in ferroptosis. Adv Biol (Weinh) 2021;5 doi: 10.1002/adbi.202100396. [DOI] [PubMed] [Google Scholar]
- 45.Kagan V.E., Mao G., Qu F., Angeli J.P., Doll S., Croix C.S., Dar H.H., Liu B., Tyurin V.A., Ritov V.B., Kapralov A.A., Amoscato A.A., Jiang J., Anthonymuthu T., Mohammadyani D., Yang Q., Proneth B., Klein-Seetharaman J., Watkins S., Bahar I., Greenberger J., Mallampalli R.K., Stockwell B.R., Tyurina Y.Y., Conrad M., Bayir H. Oxidized arachidonic and adrenic PEs navigate cells to ferroptosis. Nat Chem Biol. 2017;13:81–90. doi: 10.1038/nchembio.2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang W.S., SriRamaratnam R., Welsch M.E., Shimada K., Skouta R., Viswanathan V.S., Cheah J.H., Clemons P.A., Shamji A.F., Clish C.B., Brown L.M., Girotti A.W., Cornish V.W., Schreiber S.L., Stockwell B.R. Regulation of ferroptotic cancer cell death by GPX4. Cell. 2014;156:317–331. doi: 10.1016/j.cell.2013.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xie Y., Hou W., Song X., Yu Y., Huang J., Sun X., Kang R., Tang D. Ferroptosis: process and function. Cell Death Differ. 2016;23:369–379. doi: 10.1038/cdd.2015.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wu Z., Geng Y., Lu X., Shi Y., Wu G., Zhang M., Shan B., Pan H., Yuan J. Chaperone-mediated autophagy is involved in the execution of ferroptosis. Proc Natl Acad Sci U S A. 2019;116:2996–3005. doi: 10.1073/pnas.1819728116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hou W., Xie Y., Song X., Sun X., Lotze M.T., Zeh H.J., 3rd, Kang R., Tang D. Autophagy promotes ferroptosis by degradation of ferritin. Autophagy. 2016;12:1425–1428. doi: 10.1080/15548627.2016.1187366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang M., Chen P., Liu J., Zhu S., Kroemer G., Klionsky D.J., Lotze M.T., Zeh H.J., Kang R., Tang D. Clockophagy is a novel selective autophagy process favoring ferroptosis. Sci Adv. 2019;5 doi: 10.1126/sciadv.aaw2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li J., Liu J., Xu Y., et al. Tumor heterogeneity in autophagy-dependent ferroptosis. Autophagy. 2021 doi: 10.1080/15548627.2021.1872241. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu J., Kuang F., Kroemer G., Klionsky D.J., Kang R., Tang D. Autophagy-dependent ferroptosis: machinery and regulation. Cell Chem Biol. 2020;27:420–435. doi: 10.1016/j.chembiol.2020.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Doll S., Freitas F.P., Shah R., Aldrovandi M., da Silva M.C., Ingold I., Grocin A.G., Xavier da Silva T.N., Panzilius E., Scheel C.H., Mourao A., Buday K., Sato M., Wanninger J., Vignane T., Mohana V., Rehberg M., Flatley A., Schepers A., Kurz A., White D., Sauer M., Sattler M., Tate E.W., Schmitz W., Schulze A., O'Donnell V., Proneth B., Popowicz G.M., Pratt D.A., Angeli J.P.F., Conrad M. FSP1 is a glutathione-independent ferroptosis suppressor. Nature. 2019;575:693–698. doi: 10.1038/s41586-019-1707-0. [DOI] [PubMed] [Google Scholar]
- 54.Bersuker K., Hendricks J.M., Li Z., Magtanong L., Ford B., Tang P.H., Roberts M.A., Tong B., Maimone T.J., Zoncu R., Bassik M.C., Nomura D.K., Dixon S.J., Olzmann J.A. The CoQ oxidoreductase FSP1 acts parallel to GPX4 to inhibit ferroptosis. Nature. 2019;575:688–692. doi: 10.1038/s41586-019-1705-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dai E., Zhang W., Cong D., Kang R., Wang J., Tang D. AIFM2 blocks ferroptosis independent of ubiquinol metabolism. Biochem Biophys Res Commun. 2020;523:966–971. doi: 10.1016/j.bbrc.2020.01.066. [DOI] [PubMed] [Google Scholar]
- 56.Chen X., Kang R., Kroemer G., Tang D. Broadening horizons: the role of ferroptosis in cancer. Nat Rev Clin Oncol. 2021;18:280–296. doi: 10.1038/s41571-020-00462-0. [DOI] [PubMed] [Google Scholar]
- 57.Yuan H., Li X., Zhang X., Kang R., Tang D. Identification of ACSL4 as a biomarker and contributor of ferroptosis. Biochem Biophys Res Commun. 2016;478:1338–1343. doi: 10.1016/j.bbrc.2016.08.124. [DOI] [PubMed] [Google Scholar]
- 58.Doll S., Proneth B., Tyurina Y.Y., Panzilius E., Kobayashi S., Ingold I., Irmler M., Beckers J., Aichler M., Walch A., Prokisch H., Trumbach D., Mao G., Qu F., Bayir H., Fullekrug J., Scheel C.H., Wurst W., Schick J.A., Kagan V.E., Angeli J.P., Conrad M. ACSL4 dictates ferroptosis sensitivity by shaping cellular lipid composition. Nat Chem Biol. 2017;13:91–98. doi: 10.1038/nchembio.2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Irving H.M., Samokhvalov A.V., Rehm J. Alcohol as a risk factor for pancreatitis. A systematic review and meta-analysis. JOP. 2009;10:387–392. [PMC free article] [PubMed] [Google Scholar]
- 60.Hirota M., Ohmuraya M., Baba H. The role of trypsin, trypsin inhibitor, and trypsin receptor in the onset and aggravation of pancreatitis. J Gastroenterol. 2006;41:832–836. doi: 10.1007/s00535-006-1874-2. [DOI] [PubMed] [Google Scholar]
- 61.Halangk W., Lerch M.M., Brandt-Nedelev B., Roth W., Ruthenbuerger M., Reinheckel T., Domschke W., Lippert H., Peters C., Deussing J. Role of cathepsin B in intracellular trypsinogen activation and the onset of acute pancreatitis. J Clin Invest. 2000;106:773–781. doi: 10.1172/JCI9411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sendler M., Weiss F.U., Golchert J., Homuth G., van den Brandt C., Mahajan U.M., Partecke L.I., Doring P., Gukovsky I., Gukovskaya A.S., Wagh P.R., Lerch M.M., Mayerle J. Cathepsin B-mediated activation of trypsinogen in endocytosing macrophages increases severity of pancreatitis in mice. Gastroenterology. 2018;154:704–718 e10. doi: 10.1053/j.gastro.2017.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kuang F., Liu J., Li C., Kang R., Tang D. Cathepsin B is a mediator of organelle-specific initiation of ferroptosis. Biochem Biophys Res Commun. 2020;533:1464–1469. doi: 10.1016/j.bbrc.2020.10.035. [DOI] [PubMed] [Google Scholar]
- 64.Gao H., Bai Y., Jia Y., Zhao Y., Kang R., Tang D., Dai E. Ferroptosis is a lysosomal cell death process. Biochem Biophys Res Commun. 2018;503:1550–1556. doi: 10.1016/j.bbrc.2018.07.078. [DOI] [PubMed] [Google Scholar]
- 65.Archer H., Jura N., Keller J., Jacobson M., Bar-Sagi D. A mouse model of hereditary pancreatitis generated by transgenic expression of R122H trypsinogen. Gastroenterology. 2006;131:1844–1855. doi: 10.1053/j.gastro.2006.09.049. [DOI] [PubMed] [Google Scholar]
- 66.Geisz A., Sahin-Toth M. A preclinical model of chronic pancreatitis driven by trypsinogen autoactivation. Nat Commun. 2018;9:5033. doi: 10.1038/s41467-018-07347-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Selig L., Sack U., Gaiser S., Kloppel G., Savkovic V., Mossner J., Keim V., Bodeker H. Characterisation of a transgenic mouse expressing R122H human cationic trypsinogen. BMC Gastroenterol. 2006;6:30. doi: 10.1186/1471-230X-6-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kuang F., Liu J., Tang D., Kang R. Oxidative damage and antioxidant defense in ferroptosis. Front Cell Dev Biol. 2020;8 doi: 10.3389/fcell.2020.586578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tang D., Chen X., Kang R., Kroemer G. Ferroptosis: molecular mechanisms and health implications. Cell Res. 2021;31:107–125. doi: 10.1038/s41422-020-00441-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chen X., Comish P.B., Tang D., Kang R. Characteristics and biomarkers of ferroptosis. Front Cell Dev Biol. 2021;9:637162. doi: 10.3389/fcell.2021.637162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kang R., Zhang Q., Hou W., Yan Z., Chen R., Bonaroti J., Bansal P., Billiar T.R., Tsung A., Wang Q., Bartlett D.L., Whitcomb D.C., Chang E.B., Zhu X., Wang H., Lu B., Tracey K.J., Cao L., Fan X.G., Lotze M.T., Zeh H.J., 3rd, Tang D. Intracellular Hmgb1 inhibits inflammatory nucleosome release and limits acute pancreatitis in mice. Gastroenterology. 2014;146:1097–1107. doi: 10.1053/j.gastro.2013.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Friedmann Angeli J.P., Schneider M., Proneth B., Tyurina Y.Y., Tyurin V.A., Hammond V.J., Herbach N., Aichler M., Walch A., Eggenhofer E., Basavarajappa D., Radmark O., Kobayashi S., Seibt T., Beck H., Neff F., Esposito I., Wanke R., Forster H., Yefremova O., Heinrichmeyer M., Bornkamm G.W., Geissler E.K., Thomas S.B., Stockwell B.R., O'Donnell V.B., Kagan V.E., Schick J.A., Conrad M. Inactivation of the ferroptosis regulator Gpx4 triggers acute renal failure in mice. Nat Cell Biol. 2014;16:1180–1191. doi: 10.1038/ncb3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Matsushita M., Freigang S., Schneider C., Conrad M., Bornkamm G.W., Kopf M. T cell lipid peroxidation induces ferroptosis and prevents immunity to infection. J Exp Med. 2015;212:555–568. doi: 10.1084/jem.20140857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mayr L., Grabherr F., Schwarzler J., Reitmeier I., Sommer F., Gehmacher T., Niederreiter L., He G.W., Ruder B., Kunz K.T.R., Tymoszuk P., Hilbe R., Haschka D., Feistritzer C., Gerner R.R., Enrich B., Przysiecki N., Seifert M., Keller M.A., Oberhuber G., Sprung S., Ran Q., Koch R., Effenberger M., Tancevski I., Zoller H., Moschen A.R., Weiss G., Becker C., Rosenstiel P., Kaser A., Tilg H., Adolph T.E. Dietary lipids fuel GPX4-restricted enteritis resembling Crohn's disease. Nat Commun. 2020;11:1775. doi: 10.1038/s41467-020-15646-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Liu Y., Wang Y., Liu J., Kang R., Tang D. Interplay between MTOR and GPX4 signaling modulates autophagy-dependent ferroptotic cancer cell death. Cancer Gene Ther. 2021;28:55–63. doi: 10.1038/s41417-020-0182-y. [DOI] [PubMed] [Google Scholar]
- 76.Letoha T., Somlai C., Takacs T., Szabolcs A., Rakonczay Z., Jr., Jarmay K., Szalontai T., Varga I., Kaszaki J., Boros I., Duda E., Hackler L., Kurucz I., Penke B. The proteasome inhibitor MG132 protects against acute pancreatitis. Free Radic Biol Med. 2005;39:1142–1151. doi: 10.1016/j.freeradbiomed.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 77.Letoha T., Feher L.Z., Pecze L., Somlai C., Varga I., Kaszaki J., Toth G., Vizler C., Tiszlavicz L., Takacs T. Therapeutic proteasome inhibition in experimental acute pancreatitis. World J Gastroenterol. 2007;13:4452–4457. doi: 10.3748/wjg.v13.i33.4452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kang R., Zeng L., Zhu S., Xie Y., Liu J., Wen Q., Cao L., Xie M., Ran Q., Kroemer G., Wang H., Billiar T.R., Jiang J., Tang D. Lipid peroxidation drives gasdermin D-mediated pyroptosis in lethal polymicrobial sepsis. Cell Host Microbe. 2018;24:97–108 e4. doi: 10.1016/j.chom.2018.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Canli O., Alankus Y.B., Grootjans S., Vegi N., Hultner L., Hoppe P.S., Schroeder T., Vandenabeele P., Bornkamm G.W., Greten F.R. Glutathione peroxidase 4 prevents necroptosis in mouse erythroid precursors. Blood. 2016;127:139–148. doi: 10.1182/blood-2015-06-654194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ran Q., Gu M., Van Remmen H., Strong R., Roberts J.L., Richardson A. Glutathione peroxidase 4 protects cortical neurons from oxidative injury and amyloid toxicity. J Neurosci Res. 2006;84:202–208. doi: 10.1002/jnr.20868. [DOI] [PubMed] [Google Scholar]
- 81.Buckens H., Pirenne S., Achouri Y., Baldan J., Dahou H., Bouwens L., Lemaigre F.P., Jacquemin P., Assi M. Peroxiredoxin-I sustains inflammation during pancreatitis. Cell Mol Gastroenterol Hepatol. 2021;12:741–743. doi: 10.1016/j.jcmgh.2021.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Meftah A.M., Deckler E., Citrome L., Kantrowitz J.T. New discoveries for an old drug: a review of recent olanzapine research. Postgrad Med. 2020;132:80–90. doi: 10.1080/00325481.2019.1701823. [DOI] [PubMed] [Google Scholar]
- 83.Mareninova O.A., Hermann K., French S.W., O'Konski M.S., Pandol S.J., Webster P., Erickson A.H., Katunuma N., Gorelick F.S., Gukovsky I., Gukovskaya A.S. Impaired autophagic flux mediates acinar cell vacuole formation and trypsinogen activation in rodent models of acute pancreatitis. J Clin Invest. 2009;119:3340–3355. doi: 10.1172/JCI38674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cope M.B., Nagy T.R., Fernandez J.R., Geary N., Casey D.E., Allison D.B. Antipsychotic drug-induced weight gain: development of an animal model. Int J Obes (Lond) 2005;29:607–614. doi: 10.1038/sj.ijo.0802928. [DOI] [PubMed] [Google Scholar]
- 85.Orekhova A., Geisz A., Sahin-Toth M. Ethanol feeding accelerates pancreatitis progression in CPA1 N256K mutant mice. Am J Physiol Gastrointest Liver Physiol. 2020;318:G694–G704. doi: 10.1152/ajpgi.00007.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Schmidt J., Rattner D.W., Lewandrowski K., Compton C.C., Mandavilli U., Knoefel W.T., Warshaw A.L. A better model of acute pancreatitis for evaluating therapy. Ann Surg. 1992;215:44–56. doi: 10.1097/00000658-199201000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tang D., Kang R., Livesey K.M., Cheh C.W., Farkas A., Loughran P., Hoppe G., Bianchi M.E., Tracey K.J., Zeh H.J., 3rd, Lotze M.T. Endogenous HMGB1 regulates autophagy. J Cell Biol. 2010;190:881–892. doi: 10.1083/jcb.200911078. [DOI] [PMC free article] [PubMed] [Google Scholar]