Abstract
Background
The antiviral efficacy of remdesivir against SARS-CoV-2 is still controversial. We aimed to evaluate the clinical efficacy of remdesivir plus standard of care compared with standard of care alone in patients admitted to hospital with COVID-19, with indication of oxygen or ventilator support.
Methods
DisCoVeRy was a phase 3, open-label, adaptive, multicentre, randomised, controlled trial conducted in 48 sites in Europe (France, Belgium, Austria, Portugal, Luxembourg). Adult patients (aged ≥18 years) admitted to hospital with laboratory-confirmed SARS-CoV-2 infection and illness of any duration were eligible if they had clinical evidence of hypoxaemic pneumonia, or required oxygen supplementation. Exclusion criteria included elevated liver enzymes, severe chronic kidney disease, any contraindication to one of the studied treatments or their use in the 29 days before random assignment, or use of ribavirin, as well as pregnancy or breastfeeding. Participants were randomly assigned (1:1:1:1:1) to receive standard of care alone or in combination with remdesivir, lopinavir–ritonavir, lopinavir–ritonavir and interferon beta-1a, or hydroxychloroquine. Randomisation used computer-generated blocks of various sizes; it was stratified on severity of disease at inclusion and on European administrative region. Remdesivir was administered as 200 mg intravenous infusion on day 1, followed by once daily, 1-h infusions of 100 mg up to 9 days, for a total duration of 10 days. It could be stopped after 5 days if the participant was discharged. The primary outcome was the clinical status at day 15 measured by the WHO seven-point ordinal scale, assessed in the intention-to-treat population. Safety was assessed in the modified intention-to-treat population and was one of the secondary outcomes. This trial is registered with the European Clinical Trials Database, EudraCT2020-000936-23, and ClinicalTrials.gov, NCT04315948.
Findings
Between March 22, 2020, and Jan 21, 2021, 857 participants were enrolled and randomly assigned to remdesivir plus standard of care (n=429) or standard of care only (n=428). 15 participants were excluded from analysis in the remdesivir group, and ten in the control group. At day 15, the distribution of the WHO ordinal scale was: (1) not hospitalised, no limitations on activities (61 [15%] of 414 in the remdesivir group vs 73 [17%] of 418 in the control group); (2) not hospitalised, limitation on activities (129 [31%] vs 132 [32%]); (3) hospitalised, not requiring supplemental oxygen (50 [12%] vs 29 [7%]); (4) hospitalised, requiring supplemental oxygen (76 [18%] vs 67 [16%]); (5) hospitalised, on non-invasive ventilation or high flow oxygen devices (15 [4%] vs 14 [3%]); (6) hospitalised, on invasive mechanical ventilation or extracorporeal membrane oxygenation (62 [15%] vs 79 [19%]); (7) death (21 [5%] vs 24 [6%]). The difference between treatment groups was not significant (odds ratio 0·98 [95% CI 0·77–1·25]; p=0·85). There was no significant difference in the occurrence of serious adverse events between treatment groups (remdesivir, 135 [33%] of 406 vs control, 130 [31%] of 418; p=0·48). Three deaths (acute respiratory distress syndrome, bacterial infection, and hepatorenal syndrome) were considered related to remdesivir by the investigators, but only one by the sponsor's safety team (hepatorenal syndrome).
Interpretation
No clinical benefit was observed from the use of remdesivir in patients who were admitted to hospital for COVID-19, were symptomatic for more than 7 days, and required oxygen support.
Funding
European Union Commission, French Ministry of Health, Domaine d'intérêt majeur One Health Île-de-France, REACTing, Fonds Erasme-COVID-Université Libre de Bruxelles, Belgian Health Care Knowledge Centre, Austrian Group Medical Tumor, European Regional Development Fund, Portugal Ministry of Health, Portugal Agency for Clinical Research and Biomedical Innovation.
Translation
For the French translation of the abstract see Supplementary Materials section.
Research in context.
Evidence before this study
We searched Medline using MESH terms on July 22, 2021, for randomised controlled trials with the terms (“remdesivir” OR “remdesivir triphosphate” OR “GS-441524”) AND (“SARS-CoV-2” OR “COVID-19”) AND (“randomised controlled trial”) for completed clinical trials evaluating the effectiveness of remdesivir in patients hospitalised with COVID-19, with no language restrictions. Our search retrieved ten articles, among which only four compared remdesivir with placebo (n=2) or standard of care (n=2). Two of these studies reported a faster time to recovery in patients treated with remdesivir, although no difference in mortality was observed overall. In the two studies comparing remdesivir with placebo, the occurrence of adverse events was similar in remdesivir and placebo groups.
Added value of this study
DisCoVeRy is a multicentre, open-label, randomised, controlled trial evaluating the safety and efficacy of repurposed drugs on the clinical status of adult patients admitted to hospital with COVID-19. We found no significant difference in the clinical status at days 15 and 29, time to hospital discharge, 28-day all-cause mortality, or SARS-CoV-2 viral kinetics in participants receiving standard of care alone compared with standard of care plus remdesivir. No significant difference in the occurrence of serious adverse events was observed between groups.
Implications of all the available evidence
The faster time to recovery previously reported was not observed in the DisCoVeRy trial. Together with previous evidence, results from the DisCoVeRy trial do not support the use of remdesivir in hospitalised patients with COVID-19 in a population with symptoms for more than a week and requiring oxygen support.
Introduction
Repurposed drugs for SARS-CoV-2-associated COVID-19 have been evaluated in several large-scale, randomised clinical trials. Among them, the DisCoVeRy trial has investigated the efficacy and the safety of lopinavir–ritonavir, lopinavir–ritonavir plus interferon beta-1a, hydroxychloroquine, and remdesivir compared with standard of care in adults admitted to hospital with COVID-19.1 Results for lopinavir–ritonavir, lopinavir–ritonavir plus interferon beta-1a, and hydroxychloroquine have been reported elsewhere.2
Remdesivir is a small molecule that is formulated with sulfobutylether B-cyclodextrin sodium for injection, is dialysable, is known to penetrate well into deep compartments, and is devoid of drug interactions via CYP450.3, 4 Remdesivir is a nucleotide analogue prodrug that is intracellularly metabolised to an analogue of ATP, which inhibits RNA polymerase activity in some pathogenic coronaviruses.5 Remdesivir has shown evidence of antiviral activity against SARS-CoV-2 in preclinical models, both in vitro and in vivo,6, 7 supporting its evaluation in COVID-19. In a double-blind, randomised clinical trial in China including 237 patients with COVID-19, remdesivir was associated with a shorter time to clinical improvement in patients who started treatment within 10 days of symptom onset.8 In the Adaptive Covid-19 Treatment Trial (ACTT 1), which included 1062 patients, remdesivir was associated with a shorter time to recovery (10 days vs 15 days with placebo), but was not associated with a decrease in mortality,9 resulting in emergency use authorisation. Similarly, the international Solidarity Consortium trial sponsored by WHO, which included 2750 patients on remdesivir, found no benefit of remdesivir for in-hospital mortality across various health-care settings.10 Overall, these mixed results have not led so far to a consensus on the use of remdesivir for patients with COVID-19.
As an add-on trial, DisCoVeRy shared patients' baseline characteristics with the WHO Solidarity Consortium, as well as the dates of hospital discharge and eventual need for oxygen therapy either through standard device, high flow device, non-invasive ventilation, mechanical ventilation or extracorporeal membrane oxygenation (ECMO), or death.10 Among the participants included in the Solidarity trial, 219 (8·0%) of 2750 participants who were randomly assigned to receive remdesivir and 221 (5·4%) of 4088 randomly assigned to standard of care were shared by the DisCoVeRy trial. DisCoVeRy was designed to further document clinical outcomes, virological kinetics, treatment pharmacokinetics, and related safety data, and the preliminary analyses are reported here for remdesivir compared with control.
Methods
Study design
DisCoVeRy is a phase 3, open-label, adaptive, multicentre, randomised, controlled trial for evaluating the efficacy and safety of repurposed drugs in adults admitted to hospital for COVID-19.1 The trial was done across 48 sites in France (39 centres), Belgium (three), Austria (three), Portugal (two), and Luxembourg (one). The trial was approved by the Ethics Committee (CPP Ile-de-France-III, approval #20.03.06.51744), is sponsored by the Institut National de la Santé et de la Recherche Médicale (INSERM, France), and was done in accordance with the Declaration of Helsinki. This analysis is based on protocol version 11.0 of Dec 12, 2020.
Participants
Patients aged 18 years or older who were admitted to hospital with laboratory-confirmed SARS-CoV-2 infection and illness of any duration could be enrolled if they presented at least one of the following: clinical assessment (evidence of rales or crackles on examination) and oxygen saturation (SpO2) of 94% or less on room air; or requirement of supplemental oxygen, high-flow oxygen devices, non-invasive ventilation, or mechanical ventilation. Participants of childbearing potential agreed to use at least one primary form of contraception for the duration of the study. Participants were excluded if they had liver enzymes (alanine aminotransferase or aspartate aminotransferase) more than five times the upper limit of normal, a stage 4 severe chronic kidney disease or requiring dialysis (estimated glomerular filtration rate less than 30 mL/min), or if a transfer within 72 h to another hospital that was not a study site was anticipated. Participants were also excluded if they were pregnant or breastfeeding, if they had contraindication to any study medication including allergy, if they were treated with one of the evaluated antiviral drugs in the past 29 days, or if they had used ribavirin either in the past 29 days or concomitantly to random assignment. The criterion of laboratory-confirmed SARS-CoV-2 infection was initially restricted to 72 h before random assignment, but was extended to 9 days before random assignment in protocol version 10.0 on Oct 1, 2020. Written, informed consent was obtained from all participants or from their legal representative if they were unable to provide consent.
Randomisation and masking
Participants were randomly assigned 1:1:1:1:1 when five groups were initially implemented, and were then assigned 1:1 to receive either standard of care or standard of care plus remdesivir, once the other three treatment groups had been stopped for futility.2 Participants allocated to standard of care alone or in combination with remdesivir were recruited contemporaneously.
Randomisation was done in the electronic case report form to ensure appropriate allocation concealment and used computer-generated blocks of various sizes; it was stratified on severity of disease at inclusion and on European administrative region. Disease was defined as moderate in participants not receiving supplemental oxygen or requiring supplemental oxygen through face mask or nasal prongs (ie, ordinal scale value of 3 or 4); it was defined as severe in participants requiring non-invasive ventilation, a high-flow oxygen device, invasive mechanical ventilation, or ECMO (ie, ordinal scale value of 5 or 6). Allocated treatment was not masked to participants nor study investigator.
Procedures
Remdesivir was administered intravenously at a loading dose of 200 mg on day 1 followed by a 100 mg, 1-h infusion once-daily for a total duration of 10 days. Its cessation was allowed after 5 days if the participant was discharged from the hospital.
Corticosteroids and anticoagulants were added to the standard of care on Oct 1, 2020 (protocol version 10.0). The suggested corticosteroids regimen was dexamethasone 6 mg once daily for 10 days or until discharge.11, 12 In participants who were critically ill with acute respiratory distress syndrome requiring intensive care unit admission, a standard acute respiratory distress syndrome dexamethasone regimen could be proposed at the clinician's discretion (dexamethasone 20 mg once daily for 5 days, followed by 10 mg once daily for 5 days).13 Dosage regimens of anticoagulation were administered according to local protocols for venous thromboembolism prophylaxis or therapy.14, 15 Other supportive treatments, such as immunomodulatory agents, were allowed in all groups and left to the investigator's discretion. No participant received a SARS-CoV-2 vaccine during the course of the trial.
Participants were assessed daily while hospitalised, and at days 3, 5, 8, 11, 15 (plus or minus 2), and 29 (plus or minus 3) if discharged. Clinical data, concomitant medications, adverse events, blood cell counts, and levels of serum creatinine and liver aminotransferases were collected. Nasopharyngeal swab specimens were collected for SARS-CoV-2 real-time (rt)RT-PCR at days 3, 5, 8, 11, 15 (plus or minus 2), and 29 (plus or minus 3). Blood samples were collected at the discretion of the investigator in charge for measurement of remdesivir and its metabolite GS-441524 in plasma post-infusion (up to 30 min after completion of first infusion) and at trough (up to 4 h before infusion on days 2, 5, and 8).
Systematic determination of the normalised viral load blinded to treatment group was done on nasopharyngeal swab specimens by RNA extraction on the EMAG platform (bioMerieux, Marcy-l'Étoile, France) following manufacturer's instructions. The SARS-CoV-2 load was measured by quantitative rtRT-PCR, according to a scale of calibrated in-house plasmid, using the rtRT-PCR RdRp-IP4 developed by the Institut Pasteur (Paris, France).16 The amplification protocol was developed using QuantStudio 5 rtRT-PCR Systems (Thermo Fisher Scientific, Waltham, MA, USA). The number of cells in sample (quality criteria for nasopharyngeal swabs and normalisation tool or viral load determination) was checked using the CELL Control r-gene kit (Argene-BioMérieux, Marcy-l'Étoile, France). If cell quantification was less than 500 cells per reaction, the quality of the sample was considered too low to be measured. We computed a normalised SARS-CoV-2 load (in log10 of RNA copies per 10 000 cells) by dividing the viral load by the number of cells. All viral loads strictly less than 1 log10 RNA copies per 10 000 cells were considered less than the limit of detection and were reported as a negative result. Any point of kinetics corresponding to a rebound of SARS-CoV-2 detection was tested again for confirmation.
Concentrations of remdesivir and its metabolite GS-441524 were determined in plasma using an UPLC-MS/MS (Xevo TQ-D, Waters, Milford, MA, USA) method after precipitation of plasma proteins.17 The active triphosphorylated metabolite GS-443902 was not determined in the peripheral blood mononuclear cells, as this represented a heavy workload for centres in the context of the pandemic. The lower limit of quantification of both remdesivir and GS-441524 was 1 ng/mL.
Outcomes
The primary outcome measure was the clinical status at day 15 as measured on the seven-point ordinal scale of the WHO Master Protocol (version 3.0, March 3, 2020): (1) not hospitalised, no limitation on activities; (2) not hospitalised, limitation on activities; (3) hospitalised, not requiring supplemental oxygen; (4) hospitalised, requiring supplemental oxygen; (5) hospitalised, on non-invasive ventilation or high flow oxygen devices; (6) hospitalised, on invasive mechanical ventilation or ECMO; and (7) dead.
Secondary efficacy outcome measures were: clinical status and change from baseline of the clinical status at days 3, 5, 8, 11, and 29; time to an improvement of one and two categories as measured on the seven-point ordinal scale or hospital discharge until day 29; change from baseline of the National Early Warning Score 2 (NEWS-2) at days 3, 5, 8, 11, 15, and 29; time to NEWS-2 of 2 or lower or hospital discharge until day 29; time to hospital discharge until day 29 and duration of hospitalisation; time to new mechanical ventilation, ECMO, or death until day 29; oxygenation and ventilator free days until day 29; and in-hospital mortality and mortality at days 28 and 90. Exploratory outcome measures included the proportion of patients with SARS-CoV-2 detectable in nasopharyngeal swabs at six timepoints from baseline to day 29; the decrease of the normalised SARS-CoV-2 viral load in nasopharyngeal swabs from baseline to day 15; the post-infusion plasma concentration of remdesivir and GS-441524 at day 1 and the trough at days 2, 5, and 8.
Safety outcomes were the cumulative incidence of any grade 3 or 4 adverse events or of any serious adverse event and the grade changes in the biological and inflammatory patterns of participants over time, coded using the medical dictionary for regulatory affairs, version 23.0 and graded according to the Division of AIDS (DAIDS) table for grading the severity of adult and paediatric adverse events, version 2.1, July, 2017.
The analyses of duration of hospitalisation, in-hospital mortality, 90-day mortality, and grade changes in the biological and inflammatory patterns of participants over time will be available after final database lock.
Statistical analysis
The sample size was determined assuming the following scenario under standard of care for each item of the ordinal scale at day 15: item 1, 42%; item 2, 38%; item 3, 8%; item 4, 7%; item 5, 2%; item 6, 1%; item 7, 2%. At the time of the trial design (March, 2020), there was a substantial uncertainty with these assumptions. We powered the study for an odds ratio (OR) of 1·5 (an OR greater than 1 indicates superiority of the experimental treatment over the control for each ordinal scale category), with 90% power and using an overall one-sided type I error rate of 0·05. This size effect appeared statistically relevant, meaning that 52% of patients would be discharged with no limitation of activity at day 15 in the remdesivir group, instead of 42% of patients in the control group. We determined that the inclusion of 450 participants in each treatment group was required; this number was increased to 475 participants per group to account for unevaluable participants.
An independent data safety and monitoring board (DSMB) externally reviewed the trial data at regular intervals regarding treatment efficacy, safety, and futility. Following cessation of hydroxychloroquine on June 17, 2020, and of both groups being treated with lopinavir–ritonavir on June 27, 2020, the trial continued the evaluation of remdesivir. On Jan 13, 2021, the DisCoVeRy DSMB recommended to suspend participant recruitment on the basis of the evaluation of an interim report of 842 randomly assigned participants, of whom 776 participants had been evaluated at day 15 (389 on remdesivir and 387 on standard of care). Calculating conditional power on the basis of the intended recruitment of 900 participants (ie, an additional 124 evaluable participants), the DSMB estimated the chances of reaching 5% significance on the originally hypothesised OR of 1·5 to be 0·02% at the end of the trial. They also found no evidence of efficacy on the WHO scale at day 29, nor on mortality at day 29, and noticed the low recruitment rate in the trial over the past 6 weeks. The decision was endorsed by the DisCoVeRy steering committee on Jan 19, 2021, with subsequent cessation of participant recruitment on Jan 21, 2021. Since April 28, 2021, participants enrolled in the trial are randomly assigned (1:1) to receive either AZ7442 (a combination of two long-acting antibodies derived from convalescent patients) or placebo.
The intention-to-treat population included all randomly assigned participants with a positive SARS-CoV-2 PCR result obtained in the past 9 days, for whom a valid consent form was obtained and who did not receive any investigational treatment in the past 29 days.
The modified intention-to-treat population included participants from the intention-to-treat population who received at least one dose of the treatment allocated by random assignment.
Efficacy analyses were done in the intention-to-treat population. Safety analyses were done in the modified intention-to-treat population. Analyses were stratified by baseline severity but not by region of inclusion due to a small number of inclusions in some regions; all tests were two-sided with a type I error of 0·05. When an endpoint was statistically significant, we did a non-prespecified subgroup analysis according to baseline severity of COVID-19.
For the seven-point ordinal scale, missing data were imputed using the last observation carried forward method, except in the case of known death or hospital discharge, in which case the ordinal scale was imputed to the value of 7 (death) or 2 (not hospitalised, limitation of activities), respectively. For NEWS-2 oxygenation and mechanical ventilation outcomes, missing data were treated using the last observation carried forward method, except on the day of death, in which case participants were imputed to the worst NEWS-2 value or considered to require oxygen or mechanical ventilation. For time-to-event analyses, participants were censored at day 29, at their date of loss of follow-up, or of study withdrawal, whichever occurred first. For outcomes in which death was not included, participants who died before day 29 were censored at day 29. Missing SARS-CoV-2 viral loads were not imputed. For the analysis of viral load by mixed models, undetectable viral load values (ie, values <1 log10 copies per 10 000 cells) were imputed to half the limit of detection (0·7 log10 copies per 10 000 cells). In case of several consecutive undetectable values, only the first value was replaced, and the subsequent values discarded (until the next detectable value if values were available afterwards).
For the seven-point ordinal scale, data were analysed using a proportional odds model. Time-to-event data were analysed using a Cox proportional hazards model. An analysis of covariance was done for the comparison of oxygenation and ventilator free days between groups; in-hospital mortality, 28-day mortality, and the number of participants with detectable SARS-CoV-2 in respiratory tract specimens at each timepoint were analysed using a Cochran-Mantel-Haenszel test. For safety endpoints, the number of participants with at least one adverse event, with at least one grade 3 or 4 adverse event, and with at least one serious adverse event were compared between groups using a Cochran-Mantel-Haenszel test. Prespecified subgroup analyses for the primary outcome were done using proportional odds models across the following subgroups: age (<50 years, 50–69 years, ≥70 years); sex (female, male); duration of symptoms before random assignment (≤7 days, 8–14 days, >14 days); disease severity (moderate, severe); and country. The evolution of the viral load since random assignment was analysed using a mixed-effects linear model with a test of treatment effect on the slope, and a non-prespecified subgroup analysis was done across duration of symptoms before random assignment (≤7 days, 8–14 days, >14 days) and disease severity at random assignment.
All analyses were done using SAS version 9.4. This trial is registered with the European Clinical Trials Database, EudraCT2020-000936-23, and ClinicalTrials.gov, NCT04315948.
Role of the funding source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
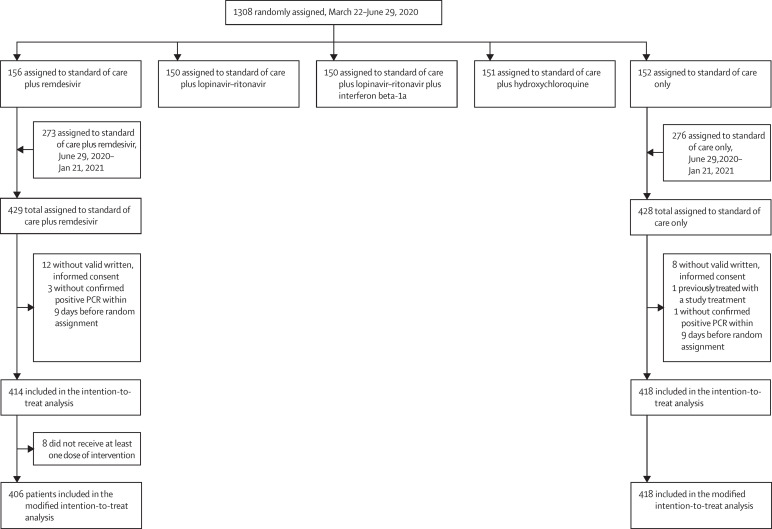
Between March 22, 2020, and Jan 21, 2021, 857 participants were enrolled and randomly assigned to remdesivir plus standard of care (n=429) or standard of care only (n=428) in France (n=724), Belgium (n=51), Portugal (n=36), Austria (n=31), and Luxembourg (n=15). 414 participants in the remdesivir group and 418 in the control group were included in the intention-to-treat analysis (figure 1 ). Among participants in the remdesivir group, the median duration of treatment was 9 days (IQR 5–10).
Figure 1.
Trial profile
Participants' baseline characteristics are shown in table 1 and appendix 2 pp 4–6 and concomitant treatments are shown in appendix 2 p 7. Overall, systemic corticosteroids were more often administered to participants included after July 1, 2020 (appendix 2 p 8).
Table 1.
Baseline characteristics of participants included in the intention-to-treat population of the DisCoVeRy trial
| Overall (n=832) | Remdesivir group (n=414) | Control group (n=418) | ||
|---|---|---|---|---|
| Median age, years | 64 (54–73) | 63 (55–73) | 64 (54–72) | |
| Sex | ||||
| Female | 253 (30%) | 123 (30%) | 130 (31%) | |
| Male | 579 (70%) | 291 (70%) | 288 (69%) | |
| Ethnicity* | ||||
| White | 499 (69%) | 244 (68%) | 255 (70%) | |
| North African | 110 (15%) | 49 (14%) | 61 (17%) | |
| Sub-Saharan African | 47 (7%) | 30 (8%) | 17 (5%) | |
| Other | 68 (9%) | 37 (10%) | 31 (9%) | |
| Number of coexisting conditions* | ||||
| 0 | 219 (27%) | 109 (27%) | 110 (26%) | |
| 1 | 276 (34%) | 142 (35%) | 134 (32%) | |
| 2 | 194 (24%) | 97 (24%) | 97 (23%) | |
| >2 | 135 (16%) | 60 (15%) | 75 (18%) | |
| Coexisting condition* | ||||
| Obesity | 278 (34%) | 138 (34%) | 140 (34%) | |
| Chronic cardiac disease | 229 (28%) | 111 (27%) | 118 (28%) | |
| Diabetes | 217 (26%) | 104 (26%) | 113 (27%) | |
| Chronic pulmonary disease | 146 (18%) | 71 (17%) | 75 (18%) | |
| Chronic kidney disease stage 1 to 3 | 51 (6%) | 19 (5%) | 32 (8%) | |
| Auto-inflammatory disease | 41 (5%) | 17 (4%) | 24 (6%) | |
| Malignant haemopathy | 35 (5%) | 16 (4%) | 19 (5%) | |
| Chronic neurological disorder including dementia | 34 (4%) | 18 (4%) | 16 (4%) | |
| Mild liver disease | 30 (4%) | 15 (4%) | 15 (4%) | |
| Active malignant neoplasm | 28 (3%) | 13 (3%) | 15 (4%) | |
| Transplantation | 11 (1%) | 2 (<1%) | 9 (2%) | |
| Asplenia | 4 (<1%) | 1 (<1%) | 3 (1%) | |
| AIDS/HIV not on ART | 2 (<1%) | 0 | 2 (<1%) | |
| Current or former smoker | 141 (18%) | 73 (19%) | 68 (17%) | |
| Current smoker | 32 (4%) | 15 (4%) | 17 (4%) | |
| Median days from symptoms onset to random assignment* | 9·0 (7·0–12·0) | 9·0 (7·0–11·0) | 9·0 (7·0–12·0) | |
| Severity of COVID-19 at random assignment | ||||
| Moderate | 504 (61%) | 253 (61%) | 251 (60%) | |
| Severe | 328 (39%) | 161 (39%) | 167 (40%) | |
| Ventilatory support at random assignment | ||||
| Room air | 12 (1%) | 6 (1%) | 6 (1%) | |
| Oxygen support with nasal canula or face mask | 492 (59%) | 247 (60%) | 245 (59%) | |
| High-flow oxygen device | 148 (18%) | 71 (17%) | 77 (18%) | |
| Non-invasive ventilation | 31 (4%) | 15 (4%) | 16 (4%) | |
| Invasive mechanical ventilation | 147 (18%) | 75 (18%) | 72 (17%) | |
| ECMO | 2 (<1%) | 0 | 2 (<1%) | |
| NEWS-2* | 9·0 (7·0–11·0) | 9·0 (6·0–11·0) | 9·0 (7·0–11·0) | |
| 7-point ordinal scale at baseline | ||||
| 3: hospitalised, not requiring supplemental oxygen | 16 (2%) | 8 (2%) | 8 (2%) | |
| 4: hospitalised, requiring supplemental oxygen | 485 (58%) | 241 (58%) | 244 (58%) | |
| 5: hospitalised, on non-invasive ventilation or high flow oxygen devices | 183 (22%) | 90 (22%) | 93 (22%) | |
| 6: hospitalised, on invasive mechanical ventilation or ECMO | 148 (18%) | 75 (18%) | 73 (18%) | |
| Randomisation site* | ||||
| Intensive care unit | 365 (44%) | 182 (45%) | 183 (44%) | |
| Conventional unit | 460 (56%) | 227 (56%) | 233 (56%) | |
| Median viral load on nasopharyngeal swab at baseline, log10 copies per 10 000 cells* | 3·2 (1·8–4·5) | 3·2 (1·7–4·5) | 3·2 (1·9–4·5) | |
| Biological data at baseline* | ||||
| Minimal lymphocyte count, 109 cells per L | 0·8 (0·6–1·2) | 0·8 (0·6–1·1) | 0·8 (0·6–1·2) | |
| Maximal neutrophil count, 109 cells per L | 5·8 (3·9–8·3) | 6·0 (4·0–8·4) | 5·6 (3·8–8·0) | |
| Maximal platelet count, 109 cells per L | 222·5 (170·0–296·0) | 223·0 (172·5–304·0) | 219·5 (165·0–291·0) | |
| Maximal urea, mmol/L | 6·0 (5·0–9·0) | 6·0 (5·0–9·0) | 6·0 (5·0–9·0) | |
| Maximal creatininaemia, μmol/L | 74·0 (61·0–92·5) | 74·0 (60·0–92·0) | 75·0 (61·0–93·0) | |
| Maximal aspartate aminotransferase, U/L | 46·0 (33·0–67·0) | 46·0 (33·0–67·0) | 46·0 (32·0–67·0) | |
| Maximal alanine aminotransferase, U/L | 37·0 (23·0–59·0) | 36·0 (23·0–55·0) | 38·0 (24·0–62·0) | |
| Maximal total bilirubin, μmol/L | 8·6 (6·0–12·0) | 8·6 (6·0–12·0) | 9·0 (6·0–13·0) | |
| Maximal international normalised ratio | 1·1 (1·0–1·2) | 1·1 (1·0–1·2) | 1·1 (1·0–1·2) | |
| Maximal C-reactive protein, mg/L | 106·0 (55·0–168·0) | 102·0 (53·0–160·0) | 109·0 (56·0–174·0) | |
| Maximal D-dimers, μg/L | 930·0 (580·0–1659·0) | 900·0 (573·0–1520·0) | 990·0 (593·0–1820·0) | |
| Maximal procalcitonin, g/mL | 0·2 (0·1–0·8) | 0·2 (0·1–0·7) | 0·3 (0·1–1·0) | |
| Maximal ferritin, mg/L | 812·0 (365·0–1596·0) | 885·5 (427·0–1703·0) | 791·0 (229·0–1454·0) | |
Data are median (IQR) or n (%). ART=antiretroviral therapy. ECMO=extracorporeal membrane oxygenation. NEWS-2=National Early Warning Score 2.
The following numbers of participants had missing data for these variables: ferritin (remdesivir: n=276, control: n=277); procalcitonin (remdesivir: n=270, control: n=272); international normalised ratio (remdesivir: n=207, control: n=211); viral loads measured on nasopharyngeal swabs (remdesivir: n=186, control: n=217); D-dimers (remdesivir: n=184, control: n=209); neutrophil count (remdesivir: n=103, control: n=118); C-reactive protein (remdesivir: n=91, control: n=79); platelet count (remdesivir: n=66, control: n=76); lymphocyte count (remdesivir: n=65, control: n=75); total bilirubin (remdesivir: n=66, control: n=65); NEWS-2 (remdesivir: n=61, control: n=59); ethnicity (remdesivir: n=54, control: n=54); urea (remdesivir: n=47, control: n=51); malignant haemopathy (remdesivir: n=50, control: n=43); aspartate aminotransferase (remdesivir: n=42, control: n=36); alanine aminotransferase (remdesivir: n=39, control: n=35); current smoking status (remdesivir: n=26, control: n=22); current or former smoking status (remdesivir: n=25, control: n=22); creatinine (remdesivir: n=14, control: n=22); time from symptoms onset to random assignment (remdesivir: n=12, control: n=8); obesity (remdesivir: n=12, control: n=4); auto-inflammatory disease (remdesivir: n=9, control: n=2); AIDS/HIV not on ART (remdesivir: n=8, control: n=3); asplenia (remdesivir: n=8, control: n=3); mild liver disease (remdesivir: n=8, control: n=2); chronic neurological disorder including dementia (remdesivir: n=8, control: n=2); active malignant neoplasm (remdesivir: n=8, control: n=2); transplantation (remdesivir: n=8, control: n=2); chronic cardiac disease (remdesivir: n=7, control: n=2); chronic pulmonary disease (n=9, remdesivir: n=7, control: n=2); chronic kidney disease stage 1 to 3 (n=9, remdesivir: n=7, control: n=2); diabetes (n=8, remdesivir: n=6, control: n=2); randomisation site (n=7, remdesivir: n=5, control: n=2).
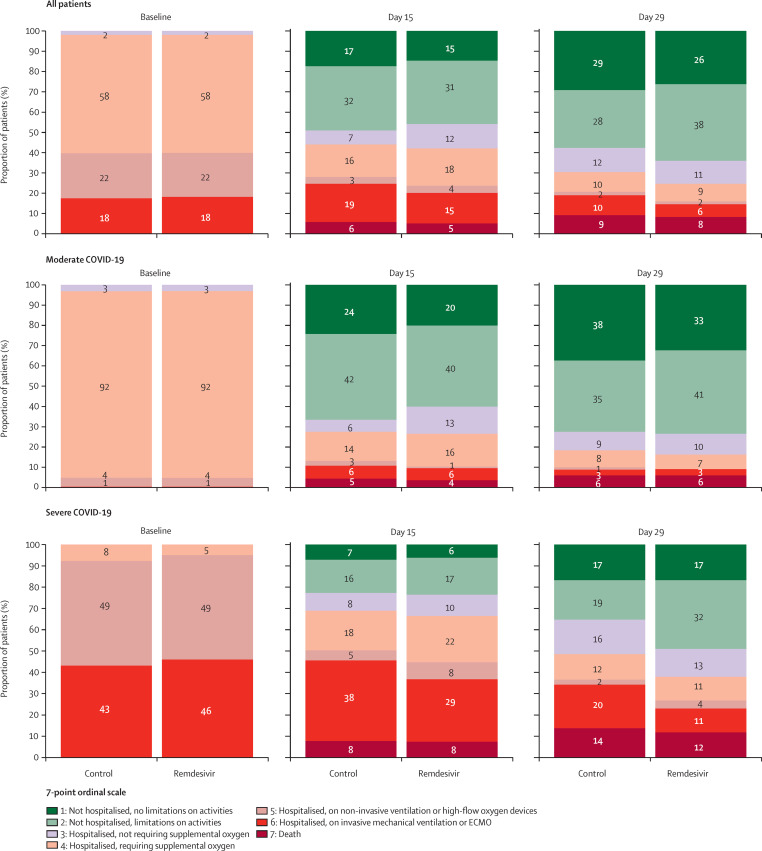
Clinical status of patients in the remdesivir group and the control group according to the WHO ordinal scale at day 15 are shown in table 2 . Ordinal scale data were missing from 18 (4%) participants in the remdesivir group and 20 (5%) in the control group at day 15 and from 29 (7%) participants in the remdesivir group and 29 (7%) in the control group at day 29. There was no significant difference in the distribution of the seven-point ordinal scale at day 15 between the remdesivir and control groups (figure 2 ; table 2; appendix 2 p 20). No significant difference was observed between the remdesivir and control groups in subgroup analyses according to age, sex, duration of symptoms before random assignment, disease severity, or country of randomisation (appendix 2 p 21).
Table 2.
Primary and secondary outcomes in the intention-to-treat population of the DisCoVeRy trial, overall, according to treatment group and COVID-19 severity at random assignment
|
Overall (n=832) |
Moderate COVID-19 (n=504) |
Severe COVID-19 (n=328) |
Remdesivir vs control, effect measure (95% CI); p value | |||||
|---|---|---|---|---|---|---|---|---|
| Remdesivir group (n=414) | Control group (n=418) | Remdesivir group (n=253) | Control group (n=251) | Remdesivir group (n=161) | Control group (n=167) | |||
| 7-point ordinal scale at day 15 | .. | .. | .. | .. | .. | .. | OR 0·98 (0·77 to 1·25); p=0·85 | |
| 1: not hospitalised, no limitations on activities | 61 (15%) | 73 (18%) | 51 (20%) | 61 (24%) | 10 (6%) | 12 (7%) | .. | |
| 2: not hospitalised, limitation on activities | 129 (31%) | 132 (32%) | 101 (40%) | 106 (42%) | 28 (17%) | 26 (16%) | .. | |
| 3: hospitalised, not requiring supplemental oxygen | 50 (12%) | 29 (7%) | 34 (13%) | 15 (6%) | 16 (10%) | 14 (8%) | .. | |
| 4: hospitalised, requiring supplemental oxygen | 76 (18%) | 67 (16%) | 41 (16%) | 36 (14%) | 35 (22%) | 31 (18%) | .. | |
| 5: hospitalised, on non-invasive ventilation or high flow oxygen devices | 15 (4%) | 14 (3%) | 2 (1%) | 6 (3%) | 13 (8%) | 8 (5%) | .. | |
| 6: hospitalised, on invasive mechanical ventilation or ECMO | 62 (15%) | 79 (19%) | 15 (6%) | 16 (6%) | 47 (29%) | 63 (38%) | .. | |
| 7: death | 21 (5%) | 24 (6%) | 9 (4%) | 11 (5%) | 12 (8%) | 13 (8%) | .. | |
| 7-point ordinal scale at day 29 | .. | .. | .. | .. | .. | .. | OR 1·11 (0·87 to 1·42); p=0·39 | |
| 1: not hospitalised, no limitations on activities | 109 (26%) | 122 (29%) | 82 (33%) | 94 (38%) | 27 (17%) | 28 (17%) | .. | |
| 2: not hospitalised, limitation on activities | 156 (38%) | 119 (28%) | 104 (41%) | 88 (35%) | 52 (32%) | 31 (19%) | .. | |
| 3: hospitalised, not requiring supplemental oxygen | 47 (11%) | 50 (12%) | 26 (10%) | 23 (9%) | 21 (13%) | 27 (16%) | .. | |
| 4: hospitalised, requiring supplemental oxygen | 36 (9%) | 41 (10%) | 18 (7%) | 21 (8%) | 18 (11%) | 20 (12%) | .. | |
| 5: hospitalised, on non-invasive ventilation or high flow oxygen devices | 6 (2%) | 7 (2%) | 0 (0%) | 3 (1%) | 6 (4%) | 4 (2%) | .. | |
| 6: hospitalised, on invasive mechanical ventilation or ECMO | 26 (6%) | 41 (10%) | 8 (3%) | 7 (3%) | 18 (11%) | 34 (20%) | .. | |
| 7: death | 34 (8%) | 38 (9%) | 15 (6%) | 15 (6%) | 19 (12%) | 23 (14%) | .. | |
| Days to improvement of two categories of the 7-point ordinal scale or hospital discharge within day 29 | 12 (8 to 24) | 11 (7 to 26) | 11 (8 to 20) | 9 (6 to 15) | 16 (10 to 29) | 17 (10 to 29) | HR 0·92 (0·79 to 1·08); p=0·30 | |
| Change from baseline in NEWS-2 to day 3 | 0 (−2 to 1) | 0 (−2 to 2) | −1 (−2 to 1) | 0 (−2 to 1) | 0 (−2 to 2) | 0 (−2 to 2) | LSMD 0·09 (−0·36 to 0·55); p=0·69 | |
| Change from baseline in NEWS-2 to day 8 | −2 (−4 to 1) | −1 (−4 to 1) | −2 (−5 to 0) | −2 (−4 to 0) | 0 (−3 to 1) | 0 (−3 to 2) | LSMD −0·12 (−0·71 to 0·47); p=0·70 | |
| Days to NEWS-2 ≤2 or hospital discharge within 29 days | 11 (7 to 24) | 11 (6 to 29) | 9 (5 to 14) | 8 (5 to 13) | 20 (12 to 29) | 26 (12 to 29) | HR 1·03 (0·88 to 1·21); p=0·74 | |
| Days to hospital discharge within 29 days | 15 (10 to 29) | 13 (8 to 29) | 11 (8 to 25) | 10 (7 to 22) | 24 (13 to 29) | 29 (13 to 29) | HR 0·94 (0·80 to 1·11); p=0·49 | |
| New mechanical ventilation, ECMO, or death within 29 days* | 60/339 (18%) | 87/344 (25%) | 35/253 (14%) | 40/251 (16%) | 25/86 (29%) | 47/93 (51%) | HR 0·66 (0·47 to 0·91); p=0·010 | |
| Oxygenation-free days until day 29 | 17 (2 to 22) | 17 (0 to 23) | 21 (14 to 24) | 21 (11 to 25) | 10 (0 to 17) | 5 (0 to 18) | LSMD 0·35 (−0·90 to 1·60); p=0·59 | |
| Ventilator-free days until day 29 | 29 (20 to 29) | 29 (16 to 29) | 29 (29 to 29) | 29 (29 to 29) | 21 (6 to 29) | 17 (2 to 29) | LSMD 1·08 (−0·15 to 2·30); p=0·080 | |
| Death within 28 days | 34 (8%) | 37 (9%) | 15 (6%) | 15 (6%) | 19 (12%) | 22 (13%) | OR 0·93 (0·57 to 1·52); p=0·77 | |
Data are n (%), median (IQR), or n/N (%), except where otherwise stated. Analyses were stratified on the disease severity at random assignment and adjusted effect measures are reported. For the ordinal scale results, an OR greater than 1 is in the direction of remdesivir conferring benefit over standard of care alone. For time to new mechanical ventilation, ECMO, or death within 29 days, an HR less than 1 is in the direction of remdesivir conferring benefit over standard of care alone. For other time to event analyses, an HR greater than 1 is in the direction of remdesivir conferring benefit over standard of care alone. ECMO=extracorporeal membrane oxygenation. HR=hazard ratio. LSMD=least-square mean difference. OR=odds ratio.
This outcome was evaluated only in participants not under mechanical ventilation or ECMO at random assignment; among the 147 participants with occurrence of new mechanical ventilation, ECMO, or death within 29 days, 49 died (24 [7%] of 339 in the remdesivir group, 25 [7%] of 344 in the control group), all of whom were mechanically ventilated before death; because incidence of new mechanical ventilation, ECMO, or death was less than 50%, incidences are reported instead of median times.
Figure 2.
Clinical status at baseline, day 15, and day 29 in the intention-to-treat population, according to treatment group and COVID-19 severity at random assignment
24 participants (12 in each group) were assessed as having moderate disease at random assignment but had their disease severity revised to severe at the baseline evaluation. 21 participants (eight in the remdesivir group, 13 in the control group) were assessed as having severe disease at random assignment but had their disease severity revised to moderate at the baseline evaluation. ECMO=extracorporeal membrane oxygenation.
There was no significant difference between the remdesivir and control groups in the distribution of the seven-point ordinal scale at day 29 (figure 2; table 2; appendix 2 p 20). The proportion of deaths at day 28 was not significantly different between the remdesivir and control groups (table 2). In participants without mechanical ventilation or ECMO at random assignment, the time to the composite endpoint of new mechanical ventilation, ECMO, or death was significantly longer in the remdesivir group than in the control group (table 2; appendix 2 p 22). In this subset of participants, 24 (7%) of 339 in the remdesivir group and 25 (7%) of 344 in the control group had died at day 29. In non-prespecified analyses, this effect was significant in participants with severe disease at random assignment, but not in participants with moderate disease (appendix 2 p 22).
No significant difference between the groups was observed for any other secondary outcomes (table 2; appendix 2 pp 9–11, 23–28).
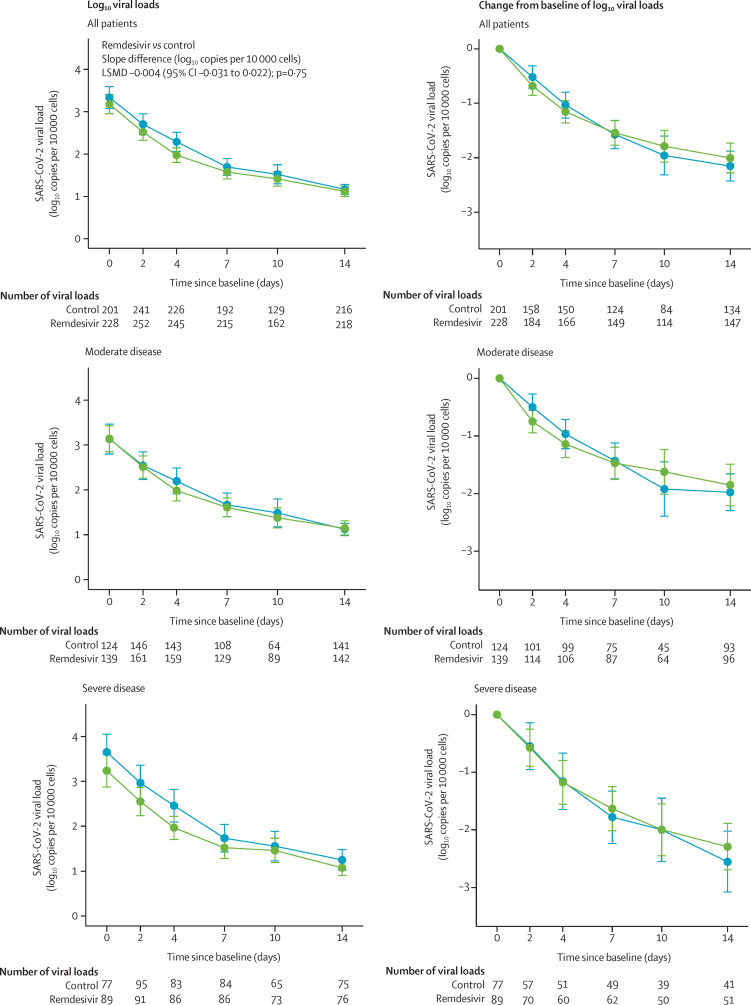
2852 nasopharyngeal swabs were analysed from 677 participants. The median normalised viral loads in the remdesivir and control groups at baseline are shown in table 1. The median decrease in viral loads between baseline and day 3 was similar in the remdesivir and control groups (appendix 2 pp 12–13). There was no significant effect of remdesivir on the viral kinetics (figure 3 ; appendix 2 pp 14, 29). Similar results were obtained in subgroup analyses according to COVID-19 severity at random assignment or duration of symptoms (appendix 2 p 15). Accordingly, there was no significant difference between the groups in the proportion of participants with detectable viral loads at each sampling time (appendix 2 pp 12–13).
Figure 3.
Normalised SARS-CoV-2 viral loads in nasopharyngeal swabs in the intention-to-treat population at each timepoint and as change from baseline, according to treatment group and COVID-19 severity at random assignment
Data are mean (95% CI). Green lines show the remdesivir group. Blue lines show the control group. LSMD=least-square mean difference.
Median post-infusion concentrations of remdesivir and GS-441524 at day 1 and post-infusion concentrations of remdesivir and GS-441524 according to COVID-19 disease severity at random assignment for a subset of 52 participants are shown in the appendix 2 (p 16). Trough plasma concentrations of remdesivir were below the limit of quantification for all participants, while median trough plasma concentrations of GS-441524 were stable at days 2, 5, and 8 (appendix 2 p 16).
824 participants were included in the safety analysis (remdesivir, n=406; control, n=418). Safety outcomes are shown in table 3 and appendix 2 (pp 17–19). Among the 1800 reported adverse events, 556 (255 in the remdesivir group, 301 in the control group) were graded 3 or 4 adverse events, affecting 128 (32%) of 406 participants in the remdesivir group and 130 (31%) of 418 in the control group (p=0·84; table 3). 488 (239 in the remdesivir group, 249 in the control group) serious adverse events were reported, affecting 135 (33%) participants in the remdesivir group and 130 (31%) in the control group. Three deaths (acute respiratory distress syndrome, bacterial infection, and hepatorenal syndrome) were considered related to remdesivir by the investigators, but only one by the sponsor's safety team (hepatorenal syndrome). The most frequently reported serious adverse events in both groups were acute respiratory failure, acute respiratory distress syndrome, and acute kidney injury (table 3).
Table 3.
Summary of adverse events in the modified intention-to-treat population of the DisCoVeRy trial, overall and according to treatment group
| Remdesivir (n=406) | Control (n=418) | Remdesivir vs control, OR (95% CI); p value | ||
|---|---|---|---|---|
| Any adverse events | 241 (59%) | 236 (57%) | 1·14 (0·86–1·50); p=0·37 | |
| Any grade 3 and 4 adverse events | 128 (32%) | 130 (31%) | 1·03 (0·76–1·39); p=0·84 | |
| Any serious adverse events | 135 (33%) | 130 (31%) | 1·11 (0·83–1·50); p=0·48 | |
| Most frequent serious adverse event | ||||
| Acute kidney injury* | 12 (3%) | 15 (4%) | .. | |
| Acute renal failure based on the RIFLE classification | 3 (1%) | 5 (1%) | .. | |
| Acute respiratory distress syndrome | 35 (9%) | 37 (9%) | .. | |
| Acute respiratory failure | 30 (7%) | 47 (11%) | .. | |
| Sepsis | 6 (1%) | 6 (1%) | .. | |
| Arrhythmia | 13 (3%) | 6 (1%) | .. | |
| Transaminases increased | 11 (3%) | 3 (1%) | .. | |
| Pulmonary embolism | 8 (2%) | 11 (3%) | .. | |
| Cholestasis | 0 (0%) | 0 (0%) | .. | |
Some patients had more than a single serious adverse event. Analyses were done in the modified Intention-to-treat population. OR=odds ratio. RIFLE=risk, injury, failure, loss of kidney function, end-stage kidney disease.
Excluding acute renal failures defined based on the RIFLE classification.
Discussion
Here we report the results of the DisCoVeRy trial comparing remdesivir to control in hospitalised patients with COVID-19. Remdesivir administration was well tolerated but was neither associated with a better clinical outcome at day 15 and 29 nor with a faster viral clearance.
Regarding day-15 clinical status, the discrepancy observed between the present results and those from the ACTT-19 (which contributed to obtaining emergency use authorisation) might be explained by the differences in study populations. In ACTT-1, a smaller proportion of participants required oxygen support at baseline (87% in ACTT-1 vs 99% in DisCoVeRy, which might be due to differences in participants' inclusion criteria and disease severity at inclusion) and fewer received corticosteroids (23% received corticosteroids in ACTT-1 vs 40% in DisCoVeRy). In DisCoVeRy, among the subset of participants without mechanical ventilation or ECMO at randomisation, remdesivir significantly delayed the need for new mechanical ventilation or ECMO, or death, consistent with what was reported in ACTT-1.9 This finding suggests that remdesivir could delay the worsening of respiratory disorders in patients with COVID-19. Nevertheless, the decision to implement mechanical ventilation or ECMO can vary based on investigator's judgement and centre practices. In addition, this effect was not observed for other secondary outcomes of respiratory status, such as the NEWS-2 score, and it did not translate into a reduced mortality at day 28, similar to the findings of the Solidarity trial for in-hospital mortality.10 In the meta-analysis of four trials that compared remdesivir with control, the conclusion was that remdesivir might have little or no effect on mortality.10
In DisCoVeRy, SARS-CoV-2 kinetic assessments were centralised and normalised to ensure consistency throughout centres. There was no effect of remdesivir on SARS-CoV-2 viral kinetics, consistent with previous results.8, 18 This finding could be due to a genuine absence of effect, but could also reflect that treatment was administered too late to be effective (median 9 days after onset of symptoms). Modelling studies of SARS-CoV-2 infection19, 20 have suggested that antiviral efficacy depends on early administration, before attaining the peak viral load.21 Consistently, clinical studies on influenza have shown that the administration of oseltamivir within 48 h after the onset of symptoms is required to ensure decreased viral shedding.22 Recent results obtained with an alternative antiviral approach through infusion of anti-SARS-CoV-2 antibodies confirm this need of early treatment to ensure effectiveness.23, 24, 25, 26 However, when restricting the viral kinetics analysis to participants who initiated treatment within 7 days after onset of symptoms, still no effect of remdesivir on SARS-CoV-2 clearance was observed in this study.
Post-infusion plasma concentrations of remdesivir were consistent with those reported in healthy volunteers,27 and trough plasma concentrations after day 1 were undetectable in all participants, consistent with the estimated 1-h elimination half-life.7 This is probably due to rapid entry of remdesivir into cells. GS-441524 is one intracellular remdesivir metabolite able to cross cellular membranes and whose levels can be measured in plasma. It is renally eliminated unchanged (27-h half-life). Over the study period, trough concentrations of GS-441524 were consistent with those previously reported.28 Although we were not able to measure the active triphosphorylated compound, the lack of viral efficacy is not likely to be attributable to inappropriate drug concentrations.
This trial has some limitations. It was open-label and not placebo-controlled. Indeed, several treatments were concomitantly evaluated at the beginning of the trial, and masking was thus impossible due to the different modes of administration (intravenous, subcutaneous, or oral) of the different treatment groups. This might have introduced bias in the follow-up and management of patients, and in the evaluation of endpoints whose assessment includes elements of subjectivity: the decision to begin corticosteroids in patient management or to begin mechanical ventilation might have been influenced by the knowledge of the treatment group, even unconsciously. However, this risk of bias is mitigated by the viral load, which was analysed blindly from treatment group. Next, no viral load assessment was available at any timepoint for 18% of participants (and nearly 50% of participants had no viral load available at baseline). However, the proportions of participants with available viral loads at each sampling time were similar in both experimental groups, suggesting that nasopharyngeal sampling was not guided by the allocated treatment. Finally, plasma concentrations of the prodrug remdesivir and GS-441524 were assessed in only 10% of participants and the concentrations of its intracellular active metabolite were not measured. Although the trial was not designed as a pharmacokinetic study, it provides data on remdesivir exposure in patients admitted to hospital with COVID-19, which are currently lacking.
In this randomised controlled trial, the use of remdesivir for the treatment of hospitalised patients with COVID-19 was not associated with clinical improvement at day 15 or day 29, nor with a reduction in mortality, nor with a reduction in SARS-CoV-2 RNA.
Data sharing
With publication, deidentified, individual participant data that underlie this Article, along with a data dictionary describing variables in the dataset, will be made available to researchers whose proposed purpose of use is approved by the DisCoVeRy Steering Committee. To request the dataset, please address directly to the corresponding author (florence.ader@chu-lyon.fr) or to the sponsor's representative (helene.esperou@inserm.fr) to obtain a data access form. All requests will be evaluated by the Trial Management Team and the DisCoVeRy Steering Committee. For accepted requests, data will be shared after signing a data transfer agreement with the study sponsor. Data will be shared directly or through access on the INSERM repository. Related documents, such as the study protocol, statistical analysis plan, and informed consent form, will be made available (with publication) on request to the corresponding author or to the sponsor's representative. The data will be open access for the informed consent form, protocol, and statistical analysis plan.
Declaration of interests
DC reports grants and lecture fees from Janssen and lecture fees from Gilead, outside the submitted work. FM reports grants and consulting fees from Da Volterra, grants from Sanofi, and consulting fees from Ipsen, outside the submitted work. MH reports grants from The Belgian Center for Knowledge (KCE), the Fonds Erasme-COVID-Université Libre de Bruxelles and the EU-Horizon programme, for the submitted work; and has received support for attending meetings from Pfizer; support for participation on an advisory board for therapeutics on COVID-19; and support for leadership for the Belgian guidelines on therapeutics for COVID-19 and acting as a treasurer for the Belgian Society of Clinical Microbiology and Infectious Diseases. JP reports lecture fees from Gilead; support for attending meetings from Gilead, Eumedica, Merck Sharp & Dohme, outside the submitted work. GP reports grants or contracts from Gilead Sciences, Merck France, Takeda, TheraTechnologies, and ViiV Healthcare; consulting fees from Gilead Sciences, Merck France, Takeda, TheraTechnologies, and ViiV Healthcare; lecture fees from Gilead Sciences, Merck France, and ViiV Healthcare; support for attending meetings from Gilead Sciences; and participation in a Data Safety and Monitoring Board for Gilead Sciences, Merck France, and ViiV Healthcare, outside the submitted work. CB reports participation in a Data Safety and Monitoring Board for 4Living Biotech; and consulting fees from Da Volterra and Mylan Pharmaceuticals, outside the submitted work. RG reports consulting fees from Celgene, Novartis, Roche, Bristol Myers Squibb, Takeda, Abbvie, AstraZeneca, Janssen, Merck Sharp & Dohme, Merck, Gilead, and Daiichi Sankvo; lecture fees from Celgene, Roche, Merck, Takeda, AstraZeneca, Novartis, Amgen, Bristol Myers Squibb, Merck Sharp & Dohme, Sandoz, Abbvie, Gilead, and Daiichi Sankvo; support for attending meetings from Roche, Amgen, Janssen, AstraZeneca, Novartis, Merck Sharp & Dohme, Celgene, Gilead, Bristol Myers Squibb, Abbvie, and Daiichi Sankvo; participation in a Data Safety and Monitoring Board for Celgene, Novartis, Roche, Bristol Myers Squibb, Takeda, Abbvie, AstraZeneca, Janssen, Merck Sharp & Dohme, Merck, Gilead, and Daiichi Sankyo; research grants from Celgene, Roche, Merck, Takeda, AstraZeneca, Novartis, Amgen, Bristol Myers Squibb, Merck Sharp & Dohme, Sandoz, Abbvie, Gilead, and Daiichi Sankyo. J-AP reports consulting fees from Pfizer, Merck Sharp & Dohme, and Janssen-Cilag; lecture fees from Pfizer; and support for attending meetings from Pfizer. All other authors decalre no competing interests.
Acknowledgments
Acknowledgments
This work received funding from several sources: European Union's Horizon 2020 research and innovation programme (Europe); Austrian Group Medical Tumor (Austria); Belgian Health Care Knowledge Centre (Belgium); Fonds Erasme-COVID-Université Libre de Bruxelles (Belgium); REACTing, a French multi-disciplinary collaborative network working on emerging infectious diseases (France); Ministry of Health (France); Domaine d'intérêt majeur One Health Île-de-France (France); European Regional Development Fund (Luxembourg); Ministry of Health (Portugal); Agency for Clinical Research and Biomedical Innovation (Portugal). We thank all participants who consented to enrol in the trial, as well as all study and site staff whose indispensable assistance made the conduct of the DisCoVeRy trial possible (all listed in the appendix 2 pp 35–47).
Contributors
FA, NP-S, JP, MB-D, GP, TS, RG, J-AP, DC, YY, CB, and FM were involved in the design, establishment, and day-to-day management and implementation of the trial. FA, MH, TS, RG, J-AP, DC, YY, and FM obtained funding for the trial. FA, MH, NP-S, JP, TS, RG, and J-AP included participants in the trial. MB-D was responsible for the virological analyses. M-PL and GP were responsible for the pharmacological analyses. FA, MB-D, DB, AD, M-PL, GP, CB, and FM were in charge of data curation and accessed and verified the data. DB, JG, DC, CB, and FM were involved in the statistical analyses. FA, MB-D, DB, AD, MH, JG, CB, and FM wrote the original draft of the manuscript, which was reviewed and edited by NP-S, JP, MPL, GP, DC, and YY. All authors contributed to refinement of and approved this manuscript. All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Contributor Information
DisCoVeRy Study Group:
Alexander Egle, Richard Greil, Michael Joannidis, Bernd Lamprecht, Antoine Altdorfer, Leila Belkhir, Vincent Fraipont, Maya Hites, Gil Verschelden, Jérôme Aboab, Florence Ader, Hafid Ait-Oufella, Claire Andrejak, Pascal Andreu, Laurent Argaud, Firouzé Bani-Sadr, François Benezit, Mathieu Blot, Elisabeth Botelho-Nevers, Lila Bouadma, Olivier Bouchaud, David Bougon, Kevin Bouiller, Fanny Bounes-Vardon, David Boutoille, Alexandre Boyer, Cédric Bruel, André Cabié, Emmanuel Canet, Charles Cazanave, Cyrille Chabartier, Catherine Chirouze, Raphaël Clere-Jehl, Johan Courjon, Flora Crockett, François Danion, Agathe Delbove, Jean Dellamonica, Félix Djossou, Clément Dubost, Alexandre Duvignaud, Olivier Epaulard, Loïc Epelboin, Murielle Fartoukh, Karine Faure, Emmanuel Faure, Tristan Ferry, Cécile Ficko, Samy Figueiredo, Benjamin Gaborit, Rostane Gaci, Amandine Gagneux-Brunon, Sébastien Gallien, Denis Garot, Guillaume Geri, Sébastien Gibot, François Goehringer, Marie Gousseff, Didier Gruson, Yves Hansmann, Olivier Hinschberger, Stéphane Jaureguiberry, Vanessa Jeanmichel, Solen Kerneis, Antoine Kimmoun, Kada Klouche, Marie Lachâtre, Karine Lacombe, Fabrice Laine, Jean-Philippe Lanoix, Odile Launay, Bruno Laviolle, Vincent Le Moing, Jérôme Le Pavec, Yves Le Tulzo, Paul Le Turnier, David Lebeaux, Benjamin Lefevre, Sylvie Leroy, François-Xavier Lescure, Henry Lessire, Benjamin Leveau, Paul Loubet, Alain Makinson, Denis Malvy, Charles-Hugo Marquette, Guillaume Martin-Blondel, Martin Martinot, Julien Mayaux, Armand Mekontso-Dessap, Ferhat Meziani, Jean-Paul Mira, Jean-Michel Molina, Xavier Monnet, Joy Mootien, Bruno Mourvillier, Marlène Murris-Espin, Jean-Christophe Navellou, Saad Nseir, Walid Oulehri, Nathan Peiffer-Smadja, Thomas Perpoint, Gilles Pialoux, Benoît Pilmis, Vincent Piriou, Lionel Piroth, Julien Poissy, Valérie Pourcher, Jean-Pierre Quenot, François Raffi, Jean Reignier, Matthieu Revest, Jean-Christophe Richard, Béatrice Riu-Poulenc, Céline Robert, Pierre-Alexandre Roger, Claire Roger, Elisabeth Rouveix-Nordon, Yvon Ruch, Nadia Saidani, Naomi Sayre, Eric Senneville, Albert Sotto, Francois Stefan, Charles Tacquard, Nicolas Terzi, Julien Textoris, Guillaume Thiery, Jean-François Timsit, Violaine Tolsma, Jean-Marie Turmel, Florent Valour, Florent Wallet, Guilhem Wattecamps, Yazdan Yazdanpanah, Yoann Zerbib, Marc Berna, Jean Reuter, Thérèse Staub, Sandra Braz, Joao-Miguel Ferreira Ribeiro, José-Artur Paiva, Roberto Roncon-Albuquerque, Maude Bouscambert-Duchamp, Alexandre Gaymard, Minh-Patrick Lê, Bruno Lina, Gilles Peytavin, Sarah Tubiana, Sandrine Couffin-Cadièrgues, Hélène Esperou, Drifa Belhadi, Charles Burdet, Dominique Costagliola, Aline Dechanet, Christelle Delmas, Alpha Diallo, Claire Fougerou, Jérémie Guedj, France Mentré, Noémie Mercier, Marion Noret, Juliette Saillard, and Priyanka Velou
Supplementary Materials
References
- 1.Ader F. Protocol for the DisCoVeRy trial: multicentre, adaptive, randomised trial of the safety and efficacy of treatments for COVID-19 in hospitalised adults. BMJ Open. 2020;10 doi: 10.1136/bmjopen-2020-041437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ader F, Peiffer-Smadja N, Poissy J, et al. An open-label randomized controlled trial of the effect of lopinavir/ritonavir, lopinavir/ritonavir plus IFN-β-1a and hydroxychloroquine in hospitalized patients with COVID-19. Clin Microbiol Infect. 2021 doi: 10.1016/j.cmi.2021.05.020. published online May 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lê MP, Le Hingrat Q, Jaquet P, et al. Removal of remdesivir's metabolite GS-441524 by hemodialysis in a double lung transplant recipient with COVID-19. Antimicrob Agents Chemother. 2020;64:e01521–e01530. doi: 10.1128/AAC.01521-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.European Medicines Agency Summary on compassionate use: remdesivir Gilead. April 3, 2020. https://www.ema.europa.eu/en/documents/other/summary-compassionate-use-remdesivir-gilead_en.pdf
- 5.Warren TK, Jordan R, Lo MK, et al. Therapeutic efficacy of the small molecule GS-5734 against Ebola virus in rhesus monkeys. Nature. 2016;531:381–385. doi: 10.1038/nature17180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang M, Cao R, Zhang L, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Williamson BN, Feldmann F, Schwarz B, et al. Clinical benefit of remdesivir in rhesus macaques infected with SARS-CoV-2. Nature. 2020;585:273–276. doi: 10.1038/s41586-020-2423-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang Y, Zhang D, Du G, et al. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020;395:1569–1578. doi: 10.1016/S0140-6736(20)31022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of COVID-19—final report. N Engl J Med. 2020;383:1813–1826. doi: 10.1056/NEJMoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pan H, Peto R, Henao-Restrepo AM, et al. Repurposed antiviral drugs for COVID-19—interim WHO Solidarity trial results. N Engl J Med. 2021;384:497–511. doi: 10.1056/NEJMoa2023184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Horby P, Lim WS, Emberson JR, et al. Dexamethasone in hospitalized patients with COVID-19. N Engl J Med. 2021;384:693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sterne JAC, Murthy S, Diaz JV, et al. Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: a meta-analysis. JAMA. 2020;324:1330–1341. doi: 10.1001/jama.2020.17023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Villar J, Ferrando C, Martínez D, et al. Dexamethasone treatment for the acute respiratory distress syndrome: a multicentre, randomised controlled trial. Lancet Respir Med. 2020;8:267–276. doi: 10.1016/S2213-2600(19)30417-5. [DOI] [PubMed] [Google Scholar]
- 14.Tang N, Bai H, Chen X, Gong J, Li D, Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020;18:1094–1099. doi: 10.1111/jth.14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Paranjpe I, Fuster V, Lala A, et al. Association of treatment dose anticoagulation with in-hospital survival among hospitalized patients with COVID-19. J Am Coll Cardiol. 2020;76:122–124. doi: 10.1016/j.jacc.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Etievant S, Bal A, Escuret V, et al. Performance assessment of SARS-CoV-2 PCR assays developed by WHO referral laboratories. J Clin Med. 2020;9 doi: 10.3390/jcm9061871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Avataneo V, de Nicolò A, Cusato J, et al. Development and validation of a UHPLC-MS/MS method for quantification of the prodrug remdesivir and its metabolite GS-441524: a tool for clinical pharmacokinetics of SARS-CoV-2/COVID-19 and Ebola virus disease. J Antimicrob Chemother. 2020;75:1772–1777. doi: 10.1093/jac/dkaa152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barratt-Due A, Olsen IC, Nezvalova-Henriksen K, et al. Evaluation of the effects of remdesivir and hydroxychloroquine on viral clearance in COVID-19: a randomized trial. Ann Intern Med. 2021 doi: 10.7326/M21-0653. published online July 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gonçalves A, Bertrand J, Ke R, et al. Timing of antiviral treatment initiation is critical to reduce SARS-CoV-2 viral load. CPT Pharmacometrics Syst Pharmacol. 2020;9:509–514. doi: 10.1002/psp4.12543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Néant N, Lingas G, Le Hingrat Q, et al. Modeling SARS-CoV-2 viral kinetics and association with mortality in hospitalized patients from the French COVID cohort. Proc Natl Acad Sci USA. 2021;118 doi: 10.1073/pnas.2017962118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rasmussen AL, Popescu SV. SARS-CoV-2 transmission without symptoms. Science. 2021;371:1206–1207. doi: 10.1126/science.abf9569. [DOI] [PubMed] [Google Scholar]
- 22.Nicholson KG, Aoki FY, Osterhaus AD, et al. Efficacy and safety of oseltamivir in treatment of acute influenza: a randomised controlled trial. Lancet. 2000;355:1845–1850. doi: 10.1016/s0140-6736(00)02288-1. [DOI] [PubMed] [Google Scholar]
- 23.Gottlieb RL, Nirula A, Chen P, et al. Effect of bamlanivimab as monotherapy or in combination with etesevimab on viral load in patients with mild to moderate COVID-19: a randomized clinical trial. JAMA. 2021;325:632–644. doi: 10.1001/jama.2021.0202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Libster R, Pérez Marc G, Wappner D, et al. Early high-titer plasma therapy to prevent severe COVID-19 in older adults. N Engl J Med. 2021;384:610–618. doi: 10.1056/NEJMoa2033700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dougan M, Nirula A, Azizad M, et al. Bamlanivimab plus etesevimab in mild or moderate COVID-19. N Engl J Med. 2021 doi: 10.1056/NEJMoa2102685. published online July 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weinreich DM, Sivapalasingam S, Norton T, et al. REGN-COV2, a neutralizing antibody cocktail, in outpatients with COVID-19. N Engl J Med. 2021;384:238–251. doi: 10.1056/NEJMoa2035002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Humeniuk R, Mathias A, Cao H, et al. Safety, tolerability, and pharmacokinetics of remdesivir, an antiviral for treatment of COVID-19, in healthy subjects. Clin Transl Sci. 2020;13:896–906. doi: 10.1111/cts.12840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Humeniuk R, Mathias A, Kirby BJ, et al. Pharmacokinetic, pharmacodynamic, and drug-interaction profile of remdesivir, a SARS-CoV-2 replication inhibitor. Clin Pharmacokinet. 2021;60:569–583. doi: 10.1007/s40262-021-00984-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
With publication, deidentified, individual participant data that underlie this Article, along with a data dictionary describing variables in the dataset, will be made available to researchers whose proposed purpose of use is approved by the DisCoVeRy Steering Committee. To request the dataset, please address directly to the corresponding author (florence.ader@chu-lyon.fr) or to the sponsor's representative (helene.esperou@inserm.fr) to obtain a data access form. All requests will be evaluated by the Trial Management Team and the DisCoVeRy Steering Committee. For accepted requests, data will be shared after signing a data transfer agreement with the study sponsor. Data will be shared directly or through access on the INSERM repository. Related documents, such as the study protocol, statistical analysis plan, and informed consent form, will be made available (with publication) on request to the corresponding author or to the sponsor's representative. The data will be open access for the informed consent form, protocol, and statistical analysis plan.