Abstract
Gene expression profiling studies of people exposed to chronic threat have identified a Conserved Transcriptional Response to Adversity (CTRA) in circulating immune cells. This physiological pattern is characterized by up-regulated expression of genes involved in inflammation and down-regulated expression of genes involved in Type I interferon responses. The CTRA is mediated by beta-adrenergic signaling pathways that transduce sympathetic nervous system activity into changes in transcription factor activity and hematopoietic output of myeloid lineage immune cells (monocytes, neutrophils, and dendritic cells). Recent research has begun to identify the CNS processes that regulate peripheral CTRA activity, define its implications for disease, and explore the role of positive psychosocial factors in buffering such effects. The CTRA provides a genomic framework for understanding PNI relationships and connecting macro-level psychosocial processes to the micro-level biology of health and disease.
Introduction
Beginning in 2007 [1], a series of RNA profiling studies found that human beings who were exposed to various adverse environmental conditions for extended periods of time showed a recurrent pattern of differences in immune cell gene expression profiles [2]. This pattern was characterized by increased expression of genes involved in inflammation (e.g., IL1B, IL6, IL8/CXCL8, COX2/PTGS2, and TNF) and decreased expression of genes involved in Type I interferon-mediated innate antiviral responses (e.g., IFI-, MX-, and OAS- family genes). This same broad pattern was observed across a diverse array of adverse conditions (e.g., loneliness, poverty, bereavement, chronic stress) and in subsequent experimental animal models [3–9]. The broad consistency of these effects across species and across different forms of adversity led to its description as a Conserved Transcriptional Response to Adversity (CTRA). This article reviews the CTRA’s discovery and theoretical conceptualization, early laboratory analyses mapping its biological mechanisms, and more recent studies assessing its implications for disease and the development of interventions to block its detrimental impact on health. It also surveys some key issues in CTRA measurement and research questions currently under analysis.
A Conserved Transcriptional Response to Adversity
Epidemiologic studies have long documented social gradients in disease, but the molecular mechanisms of these effects have only recently become a topic of significant scientific attention. In the late 1990s and early 2000s, the MacArthur Foundation convened a network of behavioral and biological scientists to analyze the pathways by which social environmental risk factors influenced host resistance to disease. Research on human genome function was surging in parallel with the completion of the human genome sequence and the development of massively parallel microarray assays that could quantify variations in the activity of all ~20,000 human genes simultaneously. Previous research in simple genomic systems such as viruses and in animal models had also shown that the RNA expression of some genes could vary as a function of environmental conditions. These two strands of research intersected in the MacArthur network meetings, with behavioral scientists reasoning that there must be a molecular manifestation of the social gradients in disease they had observed, and biological scientists recognizing that recent advances in genomics had greatly enhanced the feasibility of identifying such mechanisms. These considerations motivated a small pilot study examining the genome-wide RNA correlates in immune cells of one of the best established psychosocial risk factors for disease – perceived social isolation, or loneliness [1]. Despite the limited statistical power available in this proof-of-concept study, a clear pattern of biological differences emerged: immune cells from chronically lonely people showed relative up-regulation of multiple genes involved in inflammation, and relative down-regulation of multiple genes involved in Type I interferon responses and antibody synthesis. These results paralleled earlier observations in mouse models of repeated social defeat (RSD), which showed a similar pro-inflammatory bias, and monkey models of social stress, which showed a similar decrement in innate antiviral gene expression. These molecular differences also provided a clear explanation for the profile of disease risks associated with loneliness in epidemiological studies, with increased inflammation plausibly contributing to the elevated risk of cardiovascular and neoplastic diseases, and decreased antiviral response contributing to increased risk of viral infections and impaired cellular immune responses. Bioinformatic analyses inferred alterations in transcription factor activity that could account for those molecular patterns through increased signaling from the sympathetic nervous system (SNS) and reduced signaling from glucocorticoid hormones that would normally inhibit inflammatory gene expression (paralleling observations of acquired glucocorticoid insensitivity in the RSD mouse model)
Biobehavioral health researchers quickly applied the analytic framework of the “lonely genes” study to other socio-environmental risk factors ranging from chronic stress [10,11] and poverty [12,13] to bereavement [14] and post-traumatic stress disorder [15,16]. As is typical in genomics studies, only a small fraction of the specific individual genes that were differentially expressed in the loneliness study showed similar differential expression in analyses of other types of adversity. However, higher-order bioinformatics analyses identified two recurrent functional themes across studies: genes that were empirically up-regulated in the context of adversity tended to be enriched for transcripts involved in inflammation, and genes that were empirically down-regulated tended to be enriched for transcripts involved in Type I interferon responses. Bioinformatic analyses of transcription factor activity repeatedly indicated up-regulated activity of SNS-responsive signaling pathways (e.g., the CREB family of transcription factors) and pro-inflammatory factors (e.g., NF-κB and AP-1), and down-regulated activity of interferon response factors (IRFs) and, more variably, the glucocorticoid receptor (GR). In light of these common functional characteristics of gene expression differences observed across distinct types of adversity and across species ranging from fish to primates, this pattern was characterized as a conserved transcriptional response to adversity (CTRA). Individual studies also identified varying patterns of unique transcriptomic effects. However, uniqueness is the norm in genomics findings, and so it was remarkable to observe the recurrence a few consistent biological themes regarding gene function and upstream transcriptional control pathways across different species and risk factors.
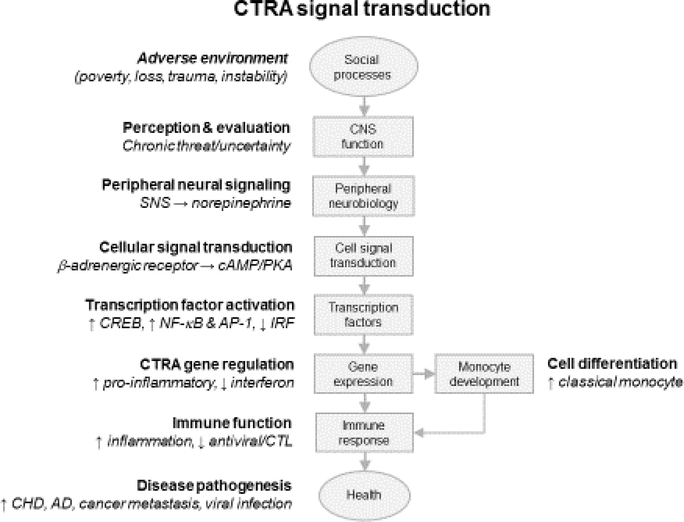
The conservation of the CTRA molecular profile motivated a series of studies to identify the biological mechanisms involved and define their adaptive value in the context of evolutionary theory. Mechanistic studies in experimental cellular and animal models confirmed bioinformatic indications that β-adrenergic signaling played a key role in generating the CTRA, both by altering gene transcription profiles in existing cells and by altering bone marrow production of monocytes and dendritic cells. Transient changes in the prevalence of these short-lived “myeloid lineage” immune cells resulted in a pro-inflammatory / interferon-impaired bias in the population structure of the circulating white blood cell pool (Figure 1). Teleologic analyses suggested that the CTRA’s adaptive significance lay in its ability to pivot the basal anti-microbial stance of the immune system away from its default bias toward resisting viral infections and other intracellular pathogens (mediated by Type I interferons and cellular immune responses) and toward a more pro-inflammatory stance that would provide an optimal defense against bacterial infections and tissue damage associated with wounding injury [2]. Under ancestral conditions, when threat experiences were acute but transient, the induction of such a molecular defense program by SNS responses to perceived or potential threats provided a mechanism for anticipating changing microbial exposures and preempting their impact. Under contemporary conditions of more chronic low-grade threat or anxiety, this physiological programming instead promotes chronic low-grade inflammation and would thus contribute to the development of inflammation-related cardiovascular, neurodegenerative, and neoplastic (cancer) diseases, while chronically undermining cellular immune responses and antiviral defenses.
Figure 1 – CTRA signal transduction.
The “social signal transduction” pathway that drives CTRA gene expression involves extended exposure to adverse environmental conditions, which results in activation of evolutionarily conserved threat response systems in the central nervous system (CNS), resulting in activation of fight-or-flight stress responses from the sympathetic nervous system (SNS) and release of the nerurotransmitter, norepinephrine, from sympathetic nerve terminals. These signals are transduced by leukocyte beta-adrenergic receptors into activation of intracellular second messenger systems such as the cyclic-3’−5’-adenosinemonophosphate / protein kinase A (cAMP/PKA) pathway, which exert diverse effects on multiple transcription control pathways such as increased activity of the cAMP response element binding factor (CREB) family, increased activity of the pro-inflammatory NF-κB/Rel and activator protein 1 (AP-1) transcription factor families, and decreased activity of interferon response factors (IRF). Differential activation of these transcription factor families results in up-regulated transcription of pro-inflammatory genes and down-regulated transcription of Type I interferon antiviral genes, resulting in downstream alterations in inflammatory and antiviral immune responses (including cytotoxic T lymphocytes; CTL) and consequent alterations in the risk of inflammation-related diseases such as coronary heart disease (CHD), Alzheimer’s Disease (AD), tumor development and metastasis, and viral infection. In addition to direct regulation of gene transcription in existing cells, SNS nerve fiber activation in the bone marrow also results in increased production of myeloid lineage immune cells (particularly classical monocytes), resulting in a pro-inflammatory bias in the circulating leukocyte pool.
Health implications
The CTRA pattern was initially recognized due to the well-defined role of inflammation in the pathogenesis of multiple chronic diseases and the key role of cellular immune responses in viral infections. During the basic definition of CTRA mechanisms, experimental animal models confirmed the relevance of the pro-inflammatory / anti-interferon pattern for disease pathogenesis in the context of cancer [17], cardiovascular disease [6], and viral infections [8]. More recent epidemiologic studies have empirically confirmed that the CTRA is associated with increased risk or severity of multiple cardiovascular, metabolic, and neoplastic diseases [13] and clinical studies have linked the CTRA molecular profile to increased risk of cancer relapse [18,19] and poorer response to hematopoietic cell transplant in the context of cancer [19]. Several studies have also linked symptoms of chronic fatigue and depression to the pro-inflammatory and/or anti-interferon components of the CTRA [20–23]. Transcriptome profiling assays were recently introduced into several large population health cohort studies and will provide a basis for assessing the CTRA’s predictive relationship to other disease outcomes as they accumulate over follow-up.
Interventions
The strong public policy interest in reducing social gradients in disease has motivated the search for effective interventions to block CTRA development and its health consequences. One approach involves deploying pharmacologic β-adrenergic antagonists to block SNS transmission of CTRA-inducing β-adrenergic signals to cells of the immune system [24] and diseased tissues [24,25]. Ben-Eliyahu recently reported the first of these studies and documented favorable changes in intra-tumoral gene expression (e.g., reduced expression of pro-metastatic genes and related reductions in mesenchymal and macrophage-related genes) and circulating leukocyte profiles (e.g., reduced expression of inflammatory genes and increased expression of genes supporting cellular immune response) in 38 patients with early-stage breast cancer. Other studies of colorectal, breast, and prostate cancer and multiple myeloma have just been completed and are currently under analysis in the US, Israel, and Australia. Several other larger Phase II studies are examining the impact of beta-adrenergic antagonists in the context of malignant melanoma and other cancers. Pre-clinical studies indicate that CTRA transcriptional alterations are mediated in large part by β2-adrenergic receptors, so it will be important to interpret results from these trials in light of the distinction between selective β-antagonists (which predominately target β1 receptors and may therefore not appreciably impact the CTRA) and non-selective β-antagonists (which are more likely to impact the CTRA due to their coverage of β2-adrenergic receptors).
There is also great interest in blocking CTRA effects upstream of the SNS at the level of CNS processes involved in driving autonomic activity and its downstream impact on gene regulation. Several studies have shown that wellness practices such as meditation [26,27], yoga [28], tai chi [29], and cognitive-behavioral stress management [18,30] can down-regulate CTRA gene expression profiles under basal conditions and in highly threatening conditions such as exposure to warfare or diagnosis with cancer. Recent results also suggest that lifestyle modifications that extend beyond stress reduction to promote positive psychological processes such as value-engagement and prosocial behavior (eudaimonic well-being) may also favorably impact CTRA profiles [31].
A wide variety of different lifestyle modifications could potentially inhibit the CTRA, and which are the most impactful may vary across individuals depending on their specific genetic background, life history, psychological make-up, and socio-environmental conditions. It is unlikely that we will be able to catalogue all of those modifying factors and assess them comprehensively enough to make specific personalized recommendations regarding their relative impact. However, it may be possible to reduce CTRA gene expression empirically, using machine learning analyses of intra-individual variations in gene expression assessed repeatedly over time. Development of highly automated RNA sequencing and analysis platforms that permit near-real-time assessment of targeted gene sets raises the potential for a kind of “genomic biofeedback” in which an array of lifestyle parameters can be sifted for spontaneous association with CTRA activity in a given individual, and the most promising parameters can then be intentionally varied in personalized quasi-experiments to drive further feedback optimization [2]. This type of molecular biofeedback has the potential help motivate preventive changes in lifestyle, behavior, and social conditions decades before they manifest in the form of overt (and often irreversible) disease. Providing individuals with timely insight into their own molecular well-being represents one of the most striking implications of our growing ability to map the functional genomic pathways by which psychological and social processes impact human physiology and health.
Measurement
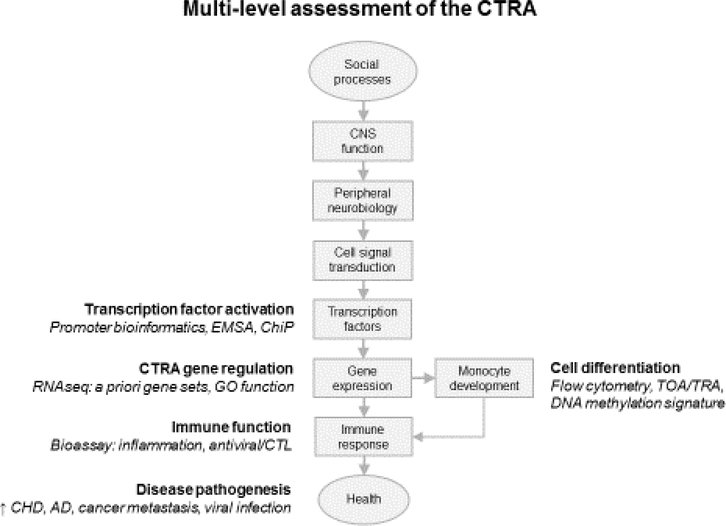
Given the scientific and health relevance of the CTRA, there is growing interest in measuring this pattern in research and clinical settings. As shown in Figure 2, the CTRA can be measured at multiple levels of analysis including: “Gene Ontology” functional tagging of empirical differences in gene expression (i.e., testing for up-regulation of inflammation-related gene annotations and down-regulation of interferon-relate gene annotations) [1]; assessment of transcription factors driving empirical differences in gene expression (i.e., testing for up-regulation of pro-inflammatory mediators such as NF-κB and AP-1 and down-regulation of interferon-responsive IRFs) [1]; quantifying myeloid cell population dynamics (i.e., testing for up-regulation of classical monocyte-related gene transcripts and down-regulation of non-classical monocyte-related transcripts [5,32], or DNA methylation profiles characteristic of myeloid cell up-regulation [13]); and assessing a priori-defined sets of canonical inflammatory and interferon-related genes (e.g., the 53-gene CTRA “contrast score” used in several high-profile biobehavioral studies [31,33–37]). The CTRA could also be assessed at the level of its downstream impact on immune function (e.g., antimicrobial responses to a controlled bacterial or viral challenge, or a relevant molecular mimetic) [6,8,9] or protein-based assessments of cell prevalence (e.g., flow cytometric enumeration of classical and non-classical monocytes) [5,6,8]. None of these approaches is perfect, and each comes with advantages and disadvantages in terms of cost, sensitivity, measurement reliability, and interpretive relevance for health. Most tap only some aspects of the CTRA. For example, analyses of myeloid cell prevalence miss the impact of β-adrenergic signaling on existing cells; analyses of a priori-defined gene sets miss effects on genes outside the pre-specified set; analyses of RNA or methylation profiles do not guarantee an impact on protein profiles or cellular function; functional assays do not capture the transcriptomic phenomenon originally identified and are often noisy and insensitive relative to molecular assays; functional bioassays and protein biomarkers do not enjoy the statistical advantages of RNA and methylation profiling that can efficiently assess 100s or 1000s of parameters and thus benefit from “law of large numbers” statistical smoothing; etc. The optimal approach for any given study depends on research objectives and technical feasibility, and each of the approaches listed above could be considered a valid indicator of the CTRA concept.
Figure 2 – Multi-level assessment of the CTRA.
The CTRA immunoregulatory pattern can be assessed at multiple levels of the “social signal transduction” cascade outlined in Figure 1, including up-regulated activity of pro-inflammatory transcription factors and down-regulated activity of antiviral transcription factors (e.g., through bioinformatic analyses of gene regulation, electrophoretic mobility-shift assays/EMSA, or chromatin immunoprecipitation/ChIP assays); up-regulated expression of pro-inflammatory effector genes (e.g., IL1B, IL6, IL8, TNF) and down-regulated expression of interferon response genes (e.g., IFI-, MX-, and OAS-family genes) as assessed by RNA sequencing (RNAseq) and Gene Ontology (GO) analyses; up-regulated production of classical monocytes and down-regulated prevalence of non-classical monocytes (e.g., as assessed by flow cytometry, bioinformatic analysis of leukocyte transcriptome profiles by Transcript Origin Analysis/TOA or Transcriptome Representation Analysis/TRA, or cell type-specific DNA methylation signatures); up-regulated functional bioassays of inflammation and/or down-regulated antiviral or cytotoxic T lymphocyte (CTL) responses; or epidemiologic increases in CTRA-related diseases such as coronary heart disease (CHD), Alzheimer’s Disease (AD), metastatic cancer, and viral infections.
It is important to note that the CTRA is a physiological pattern and is not equivalent to any specific method of measuring that pattern. Each of the approaches mentioned above represents one way of measuring the CTRA, but the CTRA is not equivalent to (or defined by) any one of those metrics. It has sometimes been assumed that the CTRA is defined by the 53-gene contrast used in several high-visibility studies [33,34]. However, that contrast represents only one way of measuring the CTRA, and analytic and empirical analyses suggest that it may not be the most sensitive or reliable one. (That prize generally goes to bioinformatic inferences of transcription factor activity derived from genome-wide transcriptome differences.) However, the 53-gene contrast score has the advantage of being easy to specify and compute, and so is often used due to its simplicity.
Pragmatic constraints on tissue sampling also influence CTRA measurement. Laboratory studies have verified that CTRA gene expression dynamics are mediated predominately in myeloid lineage immune cells (i.e., blood monocytes, tissue macrophages, and dendritic cells in both compartments) [5,8,10,11,32], which comprise ~2%−10% of circulating leukocytes. CTRA-diagnostic genes are only weakly expressed in non-myeloid blood cells, which makes it feasible to assess the CTRA in samples of whole blood (e.g., as captured by PAXgene or Tempus tubes, or in dried blood spots) and peripheral blood mononuclear cells (e.g., derived from Ficoll density gradient centrifugation) despite the presence of numerous other “contaminating” cell types (which simply lack any appreciable expression of the target mRNAs). It is also important to note that the CTRA is fundamentally a property of circulating myeloid lineage leukocytes, and would not be expected to appear in its canonical form in other types of tissue. However, myeloid cells regulate a wide variety of immunologic and inflammatory processes in other tissues, and so it is not uncommon to find distinct molecular profiles in other tissues that reflect the downstream impact of CTRA activation in circulating myeloid cells [6,17,38,39].
Prospects
The first decade of research on the CTRA yielded rapid progress in characterizing its scope and nature, the cellular and molecular mechanisms involved, its adaptive value under ancestral conditions, and its implications for health and disease under contemporary conditions. Translational studies have begun to identify behavioral and pharmacologic strategies for mitigating the CTRA. Entering its second decade, some major themes of ongoing CTRA research involve the role of positive psychological processes and socio-environmental resources in conferring resistance to CTRA development [16,31,33,34,36]; neuroimaging analyses of the CNS pathways involved in CTRA production (particularly the interplay between threat response circuits involving the amygdala and reward circuits involving the ventral striatum and ventral tegmental area) [40,41]; behavioral metrics to help monitor the activity of CTRA-regulating threat-response systems [37]; and the CTRA’s potential use as a prospective biomarker of risk for a broad range of diseases involving inflammation and interferon responses. In combination with new technical and analytic approaches for measuring CTRA activity, these developments will help deliver on PNI’s conceptual promise of a more integrated understanding of the mind-body information flow that connects macro-level socioenvironmental processes to the micro-level molecular mechanisms of human physiology, development, and health.
Highlights:
Chronic stress activates a conserved transcriptional response to adversity (CTRA)
The CTRA involves increased inflammation and decreased antiviral activity
The CTRA can be assessed at the level of mRNA, transcription factors, and cells
Positive psychological processes can inhibit the CTRA
The CTRA is associated with cancer and cardiovascular disease
Acknowledgements
Preparation of this article was supported by NIH grants R01-AG033590, R01-AG043404, and P30-AG017265. This article is dedicated to the memory of John Cacioppo, who did so much to highlight the importance of social connection to human health, and in the process helped ignite the field of human social genomics with his enthusiasm for new analytic approaches.
Footnotes
Conflict of interest
The author declares no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cole SW, Hawkley LC, Arevalo JM, Sung CY, Rose RM, Cacioppo JT: Social regulation of gene expression in human leukocytes. Genome Biol. 2007, 8:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cole SW: Human social genomics. PLoS Genet 2014, 10:e1004601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cole SW, Arevalo JM, Ruggerio AM, Heckman JJ, Suomi S: Transcriptional modulation of the developing immune system by early life social adversity. Proc Natl Acad Sci U S A 2012, 109:20578–20583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tung J, Barreiro LB, Johnson ZP, Hansen KD, Michopoulos V, Toufexis D, Michelini K, Wilson ME, Gilad Y: Social environment is associated with gene regulatory variation in the rhesus macaque immune system. Proc Natl Acad Sci U S A 2012, 109:6490–6495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Powell ND, Sloan EK, Bailey MT, Arevalo JM, Miller GE, Chen E, Kobor MS, Reader BF, Sheridan JF, Cole SW: Social stress up-regulates inflammatory gene expression in the leukocyte transcriptome via beta-adrenergic induction of myelopoiesis. Proc Natl Acad Sci U S A 2013, 110:16574–16579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heidt T, Sager HB, Courties G, Dutta P, Iwamoto Y, Zaltsman A, von Zur Muhlen C, Bode C, Fricchione GL, Denninger J, et al. : Chronic variable stress activates hematopoietic stem cells. Nat Med 2014, 20:754–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Korytar T, Nipkow M, Altmann S, Goldammer T, Kollner B, Rebl A: Adverse Husbandry of Maraena Whitefish Directs the Immune System to Increase Mobilization of Myeloid Cells and Proinflammatory Responses. Front Immunol 2016, 7:631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cole SW, Capitanio JP, Chun K, Arevalo JM, Ma J, Cacioppo JT: Myeloid differentiation architecture of leukocyte transcriptome dynamics in perceived social isolation. Proc Natl Acad Sci U S A 2015, 112:15142–15147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Snyder-Mackler N, Sanz J, Kohn JN, Brinkworth JF, Morrow S, Shaver AO, Grenier JC, Pique-Regi R, Johnson ZP, Wilson ME, et al. : Social status alters immune regulation and response to infection in macaques. Science 2016, 354:1041–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miller GE, Chen E, Sze J, Marin T, Arevalo JM, Doll R, Ma R, Cole SW: A functional genomic fingerprint of chronic stress in humans: blunted glucocorticoid and increased NF-kappaB signaling. Biol Psychiatry. 2008, 64:266–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miller GE, Murphy MLM, Cashman R, Ma R, Arevalo JMG, Kobor MS, Cole SW: Greater inflammatory activity and blunted glucocorticoid signaling in monocytes of chronically stressed caregivers. Brain, Behavior, and Immunity 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miller GE, Chen E, Fok AK, Walker H, Lim A, Nicholls EF, Cole S, Kobor MS: Low early-life social class leaves a biological residue manifested by decreased glucocorticoid and increased proinflammatory signaling. Proc Natl Acad Sci U S A. 2009, 106:14716–14721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Simons RL, Lei MK, Beach SRH, Barr AB, Cutrona CE, Gibbons FX, Philibert RA: An index of the ratio of inflammatory to antiviral cell types mediates the effects of social adversity and age on chronic illness. Soc Sci Med 2017, 185:158–165.** Using a novel DNA methylation measure of cellular profiles, this paper finds that the CTRA mediates relationships between low SES and the severity of several chronic diseases.
- 14.O’Connor MF, Schultze-Florey CR, Irwin MR, Arevalo JM, Cole SW: Divergent gene expression responses to complicated grief and non-complicated grief. Brain Behav Immun 2014, 37:78–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O’Donovan A, Sun B, Cole S, Rempel H, Lenoci M, Pulliam L, Neylan T: Transcriptional control of monocyte gene expression in post-traumatic stress disorder. Dis Markers 2011, 30:123–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kohrt BA, Worthman CM, Adhikari RP, Luitel NP, Arevalo JM, Ma J, McCreath H, Seeman TE, Crimmins EM, Cole SW: Psychological resilience and the gene regulatory impact of posttraumatic stress in Nepali child soldiers. Proc Natl Acad Sci U S A 2016, 113:8156–8161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sloan EK, Priceman SJ, Cox BF, Yu S, Pimentel MA, Tangkanangnukul V, Arevalo JM, Morizono K, Karanikolas BD, Wu L, et al. : The sympathetic nervous system induces a metastatic switch in primary breast cancer. Cancer Res 2010, 70:7042–7052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Antoni MH, Bouchard LC, Jacobs JM, Lechner SC, Jutagir DR, Gudenkauf LM, Carver CS, Lutgendorf S, Cole SW, Lippman M, et al. : Stress management, leukocyte transcriptional changes and breast cancer recurrence in a randomized trial: An exploratory analysis. Psychoneuroendocrinology 2016, 74:269–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Knight JM, Rizzo JD, Logan BR, Wang T, Arevalo JM, Ma J, Cole SW: Low Socioeconomic Status, Adverse Gene Expression Profiles, and Clinical Outcomes in Hematopoietic Stem Cell Transplant Recipients. Clin Cancer Res 2016, 22:69–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bower JE, Ganz PA, Irwin MR, Arevalo JM, Cole SW: Fatigue and gene expression in human leukocytes: Increased NF-kappaB and decreased glucocorticoid signaling in breast cancer survivors with persistent fatigue. Brain Behav Immun 2011, 25:147–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Black DS, Cole SW, Christodoulou G, Figueiredo JC: Genomic mechanisms of fatigue in survivors of colorectal cancer. Cancer 2018, 124:2637–2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xiao C, Beitler JJ, Higgins KA, Wommack EC, Saba NF, Shin DM, Bruner DW, Miller AH, Cole S: Differential regulation of NF-kB and IRF target genes as they relate to fatigue in patients with head and neck cancer. Brain Behav Immun 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mellon SH, Wolkowitz OM, Schonemann MD, Epel ES, Rosser R, Burke HB, Mahan L, Reus VI, Stamatiou D, Liew CC, et al. : Alterations in leukocyte transcriptional control pathway activity associated with major depressive disorder and antidepressant treatment. Transl Psychiatry 2016, 1:e821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haldar R, Shaashua L, Lavon H, Lyons YA, Zmora O, Sharon E, Birnbaum Y, Allweis T, Sood AK, Barshack I, et al. : Perioperative inhibition of beta-adrenergic and COX2 signaling in a clinical trial in breast cancer patients improves tumor Ki-67 expression, serum cytokine levels, and PBMCs transcriptome. Brain Behav Immun 2018. [DOI] [PubMed] [Google Scholar]
- 25.Shaashua L, Shabat-Simon M, Haldar R, Matzner P, Zmora O, Shabtai M, Sharon E, Allweis T, Barshack I, Hayman L, et al. : Perioperative COX-2 and beta-Adrenergic Blockade Improves Metastatic Biomarkers in Breast Cancer Patients in a Phase-II Randomized Trial. Clin Cancer Res 2017, 23:4651–4661.** Results from the first randomized controlled trial of a pharmacologic intervention to reduce CTRA impact in cancer.
- 26.Black DS, Cole SW, Irwin MR, Breen E, St Cyr NM, Nazarian N, Khalsa DS, Lavretsky H: Yogic meditation reverses NF-kappaB and IRF-related transcriptome dynamics in leukocytes of family dementia caregivers in a randomized controlled trial. Psychoneuroendocrinology 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Creswell JD, Irwin MR, Burklund LJ, Lieberman MD, Arevalo JM, Ma J, Breen EC, Cole SW: Mindfulness-Based Stress Reduction training reduces loneliness and pro-inflammatory gene expression in older adults: A small randomized controlled trial. Brain Behav Immun 2012, 26:1095–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bower JE, Greendale G, Crosswell AD, Garet D, Sternlieb B, Ganz PA, Irwin MR, Olmstead R, Arevalo J, Cole SW: Yoga reduces inflammatory signaling in fatigued breast cancer survivors: A randomized controlled trial. Psychoneuroendocrinology 2014, 43:20–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Irwin M, Olmstead R, Breen E, Witarama T, Carrillo C, Sadeghi N, Arevalo JMG, Ma J, Nicassio P, Ganz PA, et al. : Tai Chi Chih reduces cellular and genomic markers of inflammation in breast cancer survivors with insomnia. Journal of the National Cancer Institute 2014, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Antoni MH, Lutgendorf SK, Blomberg B, Stagl J, Carver CS, Lechner S, Diaz A, Arevalo JMG, Cole SW: Transcriptional modulation of human leukocytes by cognitive-behavioral stress management in women undergoing treatment for breast cancer. Biological Psychiatry 2012, 71:366–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nelson-Coffey SK, Fritz MM, Lyubomirsky S, Cole SW: Kindness in the blood: A randomized controlled trial of the gene regulatory impact of prosocial behavior. Psychoneuroendocrinology 2017, 81:8–13.** A randomized controlled experiment showing that pro-social behavior can reduce CTRA gene expression.
- 32.Cole SW, Hawkley LC, Arevalo JM, Cacioppo JT: Transcript origin analysis identifies antigen-presenting cells as primary targets of socially regulated gene expression in leukocytes. Proc Natl Acad Sci U S A 2011, 108:3080–3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fredrickson BL, Grewen KM, Algoe SB, Firestine AM, Arevalo JMG, Ma J, Cole SW: Psychological well-being and the human conserved transcriptional response to adversity. PLoS One 2015, 10:e0121839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fredrickson BL, Grewen KM, Coffey KA, Algoe SB, Firestine AM, Arevalo JM, Ma J, Cole SW: A functional genomic perspective on human well-being. Proc Natl Acad Sci U S A 2013, 110:13684–13689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cole SW, Levine ME, Arevalo JM, Ma J, Weir DR, Crimmins EM: Loneliness, eudaimonia, and the human conserved transcriptional response to adversity. Psychoneuroendocrinology 2015, 62:11–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kitayama S, Akutsu S, Uchida Y, Cole SW: Work, meaning, and gene regulation: Findings from a Japanese information technology firm. Psychoneuroendocrinology 2016, 72:175–181. [DOI] [PubMed] [Google Scholar]
- 37.Mehl MR, Raison CL, Pace TWW, Arevalo JMG, Cole SW: Natural language indicators of differential gene regulation in the human immune system. Proc Natl Acad Sci U S A 2017, 114:12554–12559.* Documents a surprising relationship between CTRA gene expression and patterns of natural language use in everyday life.
- 38.Chun K, Capitanio JP, Lamkin DM, Sloan EK, Arevalo JM, Cole SW: Social regulation of the lymph node transcriptome in rhesus macaques (Macaca mulatta). Psychoneuroendocrinology 2017, 76:107–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lutgendorf SK, Degeest K, Sung CY, Arevalo JM, Penedo F, Lucci J 3rd, Goodheart M, Lubaroff D, Farley DM, Sood AK, et al. : Depression, social support, and beta-adrenergic transcription control in human ovarian cancer. Brain Behav Immun 2009, 23:176–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ben-Shaanan TL, Schiller M, Azulay-Debby H, Korin B, Boshnak N, Koren T, Krot M, Shakya J, Rahat MA, Hakim F, et al. : Modulation of anti-tumor immunity by the brain’s reward system. Nat Commun 2018,* An innovative basic science study mapping the pathways by which the brain’s reward system can alter myeloid cell development and enhance anti-tumor immune responses.
- 41.Eisenberger NI, Cole SW: Social neuroscience and health: neurophysiological mechanisms linking social ties with physical health. Nat Neurosci 2012, 15:669–674. [DOI] [PubMed] [Google Scholar]