Significance
Perceived social isolation (PSI) (loneliness) is linked to increased risk of chronic disease and mortality, and previous research has implicated up-regulated inflammation and down-regulated antiviral gene expression (the conserved transcriptional response to adversity; CTRA) as a potential mechanism for such effects. The present studies used integrative analyses of transcriptome regulation in high-PSI humans and rhesus macaques to define the basis for such effects in neuroendocrine-related alterations in myeloid immune cell population dynamics. CTRA up-regulation also preceded increases in PSI, suggesting a reciprocal mechanism by which CTRA gene expression may both propagate PSI and contribute to its related disease risks.
Keywords: social genomics, loneliness, inflammation, health
Abstract
To define the cellular mechanisms of up-regulated inflammatory gene expression and down-regulated antiviral response in people experiencing perceived social isolation (loneliness), we conducted integrative analyses of leukocyte gene regulation in humans and rhesus macaques. Five longitudinal leukocyte transcriptome surveys in 141 older adults showed up-regulation of the sympathetic nervous system (SNS), monocyte population expansion, and up-regulation of the leukocyte conserved transcriptional response to adversity (CTRA). Mechanistic analyses in a macaque model of perceived social isolation confirmed CTRA activation and identified selective up-regulation of the CD14++/CD16− classical monocyte transcriptome, functional glucocorticoid desensitization, down-regulation of Type I and II interferons, and impaired response to infection by simian immunodeficiency virus (SIV). These analyses identify neuroendocrine-related alterations in myeloid cell population dynamics as a key mediator of CTRA transcriptome skewing, which may both propagate perceived social isolation and contribute to its associated health risks.
Perceived social isolation (PSI) (loneliness in humans) is a risk factor for chronic illness and all-cause mortality (1, 2), but the molecular mechanisms of its health effects remain poorly understood. PSI represents a discrepancy between an animal’s preferred and actual social relations and triggers phylogenetically conserved neuroendocrine responses (3–5). In humans, PSI involves an implicit hypervigilance for social threat (4–6). In animal models, threat-related signaling from the sympathetic nervous system (SNS) to the bone marrow hematopoietic niche can stimulate the development of immature, glucocorticoid-resistant monocytes and neutrophils (myeloid lineage immune cells) via β-adrenergic up-regulation of the myelopoietic growth factor, granulocyte-macrophage colony-stimulating factor (GM-CSF) (7, 8). Up-regulated myeloid cell populations may subsequently contribute to the development of inflammation-related chronic diseases (9). Here, we show that individuals chronically high in PSI manifest an SNS-related alteration in myeloid cell population dynamics that mediates a previously observed conserved transcriptional response to adversity (CTRA) involving up-regulation of proinflammatory genes and down-regulation of genes involved in type I interferon (IFN) responses. CTRA gene expression also predicts subsequent PSI, suggesting a reciprocal interaction between inflammatory biology and social perception. Mechanistic analyses in a macaque model of PSI (10) confirm CTRA activation, identify the CD14++/CD16− classical monocyte subpopulation as the cellular origin of that transcriptome shift, and document functional immunologic consequences, including reduced cellular sensitivity to glucocorticoid regulation, down-regulation of type I and II IFNs, and impaired response to infection with the simian immunodeficiency virus (SIV).
Results
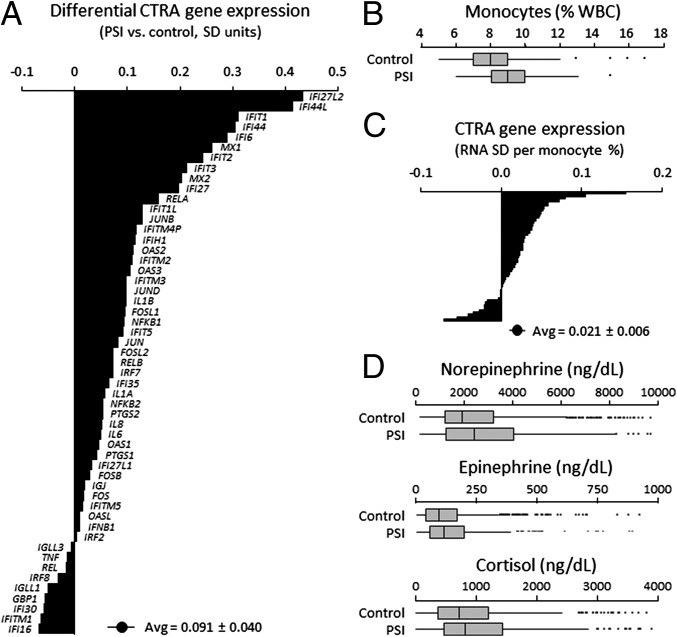
To assess the role of myeloid lineage gene expression in PSI-related CTRA up-regulation (11–13), we analyzed 412 genome-wide transcriptome surveys of peripheral blood mononuclear cells (PBMCs) collected longitudinally from 141 participants in the Chicago Health, Aging, and Social Relations Study (CHASRS) (14) during study years 5, 7, 8, 9, and 10 (Table S1). This subsample was representative of the overall CHASRS cohort (14), and 26% were classified as chronically high in PSI based on a previously established criterion of UCLA Loneliness Scale scores ≥41 in at least 60% of study years 1–5 (12). (Trait PSI was defined before gene expression profiling to rule out reverse causation.) Participants chronically high in PSI did not differ from those intermediate or low in PSI on any measured demographic or health behavior risk factor (all Ps > 0.05). However, they did show greater levels of perceived stress and depressive symptoms and lower levels of social support (Table S1), as previously observed (15–17). In mixed effect linear model analyses relating chronically high PSI to a CTRA indicator gene contrast reflecting up-regulated inflammation and down-regulated type I IFN activity and antibody synthesis, results showed an average 6.5% elevation in the CTRA gene expression profile [adjusting for covariates: study year, age, sex, race/ethnicity, body mass index (BMI), smoking, alcohol consumption, and household income] (Table 1, row A and Fig. 1A). The CTRA profile was up-regulated by 12.2% after additional adjustments for stress, depression, and social support (Table 1, row B) whereas none of the latter three psychological risk factors was associated with increased CTRA expression (Table 1, row B).
Table S1.
CHASRS gene expression sample characteristics
| Variable | Mean (SD) or % | Association with chronic high PSI (P value)* | Association with continuous PSI (P value)† |
| Age, y | 57.3 (4.4) | r = −0.15 (0.078) | r = −0.12 (0.141) |
| Sex (female) | 54% | r = 0.02 (0.853) | r = 0.00 (0.969) |
| Race/ethnicity | n/a (0.598) | n/a (0.261) | |
| White | 39% | ||
| Black | 35% | ||
| Hispanic | 25% | ||
| Married (current) | 64% | r = −0.02 (0.780) | r = −0.05 (0.544) |
| Household income, $/y | 10.8 (1.0) | r = −0.08 (0.342) | r = −0.06 (0.487) |
| BMI, kg/m2 | 31.6 (6.5) | r = −0.02 (0.810) | r = −0.10 (0.241) |
| Smoking history (present) | 61% | r = 0.16 (0.054) | r = 0.14 (0.091) |
| Alcohol history (present) | 76% | r = 0.02 (0.782) | r = −0.07 (0.416) |
| Stress (PSS) | 11.1 (5.9) | r = 0.40 (<0.001) | r = 0.57 (<0.001) |
| Depressive symptoms (CESD) | 9.0 (8.3) | r = 0.52 (<0.001) | r = 0.42 (<0.001) |
| Social support (ISEL) | 13.1 (2.1) | r = −0.49 (<0.001) | r = −0.66 (<0.001) |
All values pertain to first year of gene expression profiling (study year 5).
r, Point-biserial or tetrachoric; P, ANOVA or χ2.
r, Pearson or point-biserial; P, ANOVA.
Table 1.
Perceived social isolation (PSI), monocyte percentages, and CTRA gene expression
| Outcome | Association b ± SE* | Test statistic | P value |
| A. CTRA gene expression† | |||
| Chronic PSI‡ | 0.091 ± 0.040 | F(1,2E4) = 5.22 | 0.0239 |
| B. CTRA gene expression† | |||
| Chronic PSI‡ | 0.166 ± 0.044 | F(1,2E4) = 14.44 | 0.0002 |
| Depressive symptoms (CESD) | −0.003 ± 0.002 | F(1,2E4) = 2.01 | 0.1559 |
| Stress (PSS) | −0.005 ± 0.002 | F(1,2E4) = 6.57 | 0.0104 |
| Social support (ISEL) | 0.011 ± 0.007 | F(1,2E4) = 2.51 | 0.1134 |
| C. CTRA gene expression† | |||
| Concurrent continuous PSI§ | 0.013 ± 0.002 | F(1,1E4) = 28.81 | 0.0001 |
| 1-y antecedent continuous PSI§ | 0.007 ± 0.002 | F(1,1E4) = 11.24 | 0.0008 |
| D. CTRA gene expression† | |||
| Chronic PSI‡ | −0.232 ± 0.127 | F(1,1E4) = 3.33 | 0.0705 |
| Concurrent continuous PSI§ | 0.014 ± 0.002 | F(1,1E4) = 31.43 | < 0.0001 |
| 1-y antecedent continuous PSI§ | 0.008 ± 0.002 | F(1,1E4) = 13.19 | 0.0003 |
| E. PSI§ | |||
| Concurrent CTRA gene expression† | 0.137 ± 0.036 | F(1,9E3) = 14.53 | 0.0001 |
| 1-y antecedent CTRA gene expression† | 0.202 ± 0.034 | F(1,9E3) = 35.16 | < 0.0001 |
| F. Monocyte %¶ | |||
| Chronic PSI‡ | 0.833 ± 0.423 | F(1,76) = 3.87 | 0.0520 |
| Concurrent continuous PSI§ | 0.057 ± 0.022 | F(1,76) = 6.58 | 0.0123 |
| G. CTRA gene expression† | |||
| Monocyte %¶ | 0.021 ± 0.006 | F(1,9E3) = 11.57 | 0.0007 |
| H. CTRA gene expression† | |||
| Leukocyte subset marker mRNA# | n/a | F(8,2E4) = 7.88 | < 0.0001 |
| I. CTRA gene expression† | |||
| Chronic PSI|leukocyte subset mRNA# | 0.102 ± 0.041 | F(1,2E4) = 6.19 | 0.0141 |
| Leukocyte subset mRNA#|chronic PSI¶ | n/a | F(8,2E4) = 8.00 | < 0.0001 |
CESD, Center for Epidemiological Studies Depression; ISEL, Interpersonal Support Evaluation List; PSS, Perceived Stress Scale.
Partial regression coefficients relating listed predictor variables to listed outcomes, with additional control for age, sex, race (white, black, Hispanic), marital status, (log) household income, BMI, smoking, and alcohol consumption. n/a, not applicable.
Standardized expression values (mean = 0, SD = 1) for 53 CTRA indicator genes.
1/0 indicator.
Continuous UCLA Loneliness Scale scores: mean = 36.5, SD = 8.9. Association parameter represents change in expected UCLA Loneliness Scale score per log2 unit change in average CTRA gene expression level.
Monocyte %: mean = 8.5, SD = 2.1.
Standardized expression values (mean = 0, SD = 1) for mRNAs encoding CD14, CD3D, CD3E, CD4, CD8, CD19, CD16/FCGR3A, and CD56/NCAM1.
Fig. 1.
CTRA gene expression, monocyte prevalence, and neuroendocrine parameters in individuals chronically high in PSI. (A) Differential expression of 53 CTRA indicator transcripts in five yearly longitudinal PBMC samples from individuals chronically high in PSI (n = 36) vs. those intermediate or low in PSI (n = 105). Point estimates and SE come from repeated measures mixed effect linear models (P = 0.024, as in Table 1, row A). (B) Monocyte percentages in two longitudinal whole blood samples from individuals chronically high versus intermediate or low in PSI (association with categorical chronically high PSI, P = 0.052; association with continuously varying PSI as measured by UCLA Loneliness Scale scores, P = 0.012). Boxes, 25th, 50th, and 75th percentiles; whiskers, first and 99th percentiles. (C) CTRA gene expression as a function of monocyte percentage (association, P < 0.001 by repeated measures mixed linear model). (D) PSI-related differences in urinary metabolite concentrations for norepinephrine (association with categorical chronic high PSI, b = 332.1 ± SE 194.7 ng/dL, P = 0.090; association with continuous PSI, b = 23.0 ± 7.4 ng/dL, P = 0.002), epinephrine (categorical, b = 16.0 ± 12.2 ng/dL, P = 0.192; continuous, b = 0.4 ± 0.5 ng/dL, P = 0.401), and cortisol (categorical, b = 197.5 ± 83.6 ng/dL, P = 0.018; continuous, b = 5.4 ± 3.6 ng/dL, P = 0.134).
To determine whether high PSI might constitute an antecedent influence on CTRA expression, analyses examined relationships between longitudinal variations in continuous Loneliness Scale scores measured concurrently and 1 y before observed gene expression values in the entire study cohort (again controlling for covariates and stress, depression, and social support). CTRA gene expression was up-regulated in proportion to both concurrent and 1-y-antecedent PSI (Table 1, row C), and these effects emerged above and beyond individual differences in chronic (trait) high PSI (Table 1, row D).
Inflammation may potentially influence PSI (18–20) so we also examined the reciprocal effects of concurrent and 1-y-antecedent CTRA expression on continuous PSI measures (Table 1, row E). PSI was elevated in proportion to both concurrent and 1-y-antecedent CTRA expression, suggesting a reciprocal relationship between social cognition and immune system gene regulation (19).
Monocyte Population Dynamics.
Chronic social threat perception may up-regulate CTRA gene expression via SNS stimulation of bone marrow myelopoiesis and consequent up-regulation of immature, proinflammatory classical monocytes (CD14++/CD16−) in circulating PBMCs (7, 8). Both categorical chronic PSI and longitudinal variations in state PSI were associated with increased circulating monocyte percentages (again controlling for covariates; cell percentage is the hematologic parameter relevant to dynamics of a fixed-mass RNA transcriptome) (Fig. 1B and Table 1, row F). In turn, increased circulating monocyte percentages were associated with CTRA up-regulation (Fig. 1C and Table 1, row G), as were canonical mRNA markers of major leukocyte subsets including the monocyte marker CD14 (Table 1, row H). However, control for leukocyte subset indicators could not account for the entire relationship between chronically high PSI and CTRA up-regulation (Table 1, row I). These results suggest that CTRA up-regulation may be mediated not by expansion of the total CD14+ monocyte population, but rather by selective expansion of the immature CD14++/CD16− classical monocyte subset (7, 8).
Consistent with neuronal SNS regulation of myelopoiesis (7, 8), longitudinal variations in state PSI were associated with concurrent elevations in urinary norepinephrine metabolites (indicative of neuronal SNS activity) (Fig. 1D) but not with epinephrine (indicative of adrenal SNS activity) (Fig. 1D). Cortisol levels were also up-regulated in chronic high PSI (Fig. 1D), as previously observed (4).
Macaque Model.
To facilitate mechanistic analyses of PSI-related immunologic and behavioral processes, we established a rhesus macaque model that distinguished behaviorally between animals high in PSI versus controls (i.e., animals low or intermediate in PSI) based on (i) high vs. low rates of spontaneous social interaction and (ii) subdivision of low-social interaction animals into social threat-sensitive and -insensitive groups based on patterns of threat discrimination in spontaneous social behavior (i.e., a bias to approach safe social targets such as juveniles or adult females vs. risky adult males, and inability to convert social initiations into complex social interactions such as grooming), as described (10). The resulting classification yields categories of low PSI (high sociability), intermediate PSI (low sociability, low social threat sensitivity), and high PSI (low sociability, high social threat sensitivity). Macaque PSI classification was relatively stable over time (1 y test–retest, κ = 0.51 in 30 animals) and similar to PSI stability in humans (mean 1 y test–retest reliability of UCLA Loneliness score of ≥41, κ = 0.56). Behavioral homology of the macaque model to human PSI was verified by assessing individual differences in behavioral sensitivity to social threat in 27 animals (high PSI, n = 7; intermediate PSI, n = 10; low PSI, n = 10). High-PSI macaques produced higher rates of preference-indicating behavior in response to video-presented affiliative behaviors generated by novel safe (juvenile) vs. risky (adult) male social partners whereas low-PSI animals showed no such target-based response discrimination (Fig. 2A). Social threat-sensitive behavioral response biases habituated over three daily exposure sessions, confirming that response biases stemmed from social novelty rather than an inherent preference for juveniles per se (Fig. 2A). In analyses of archival data on urinary catecholamine metabolites from a previous study of 18 adult male macaques (21), high-PSI macaques were also homologous to human PSI in showing elevated norepinephrine but no differences in epinephrine (Fig. 2B).
Fig. 2.
Perceived social isolation, social threat, and SNS activity. (A) Behavioral indicators of preference (gaze frequency and duration) were assessed in 27 adult macaques classified as high PSI (n = 7), intermediate PSI (n = 10), and low PSI (n = 10) as they viewed video presentations of affiliative behavior directed at them by a novel safe juvenile male or risky adult male. Data represent mean ± SE threat discrimination ratios (juvenile target/adult target) on the first day of video exposure (novel social situation) and the third day (habituated) in high-PSI animals vs. intermediate- and low-PSI controls; P values from mixed effects linear model analysis of log-ratios. (B) Average urinary catecholamine metabolite concentrations in 18 adult male macaques classified as high PSI (n = 4) vs. intermediate- (n = 6) or low-PSI (n = 8) controls. Data come from archival analysis of newly derived PSI classifications in a previously published study (21) and represent mean ± SE of up to six assessments over 20 wk; P values indicate group main effect for each metabolite.
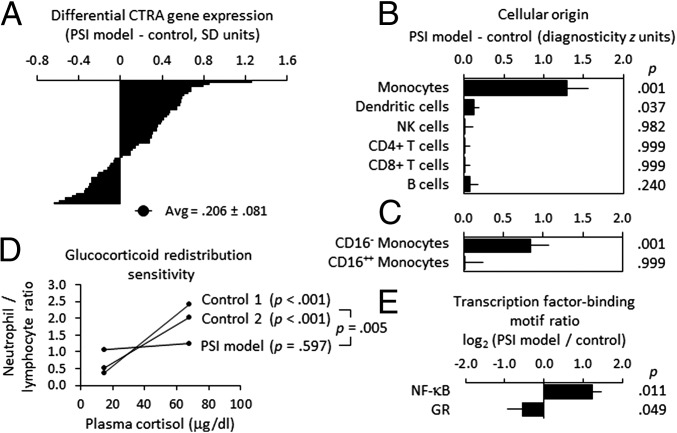
In analyses of genome-wide transcriptional profiles from a new sample of 49 macaques (high PSI, n = 8; intermediate PSI, n = 19; low PSI, n = 22), mixed effect linear model analyses found high-PSI macaques to parallel high-PSI humans in showing up-regulated CTRA expression within the PBMC pool (Fig. 3A) (contrast with low- and intermediate-PSI animals, b = 0.206 ± 0.081 standardized RNA expression units, P = 0.0147). Transcript origin analyses (TOA) (12) of all genes showing ≥1.2-fold differential expression in high- vs. low- and intermediate-PSI animals confirmed that PBMC transcriptome differences originated predominantly from monocytes (Fig. 3B). Additional TOAs using isolated CD14++/CD16− and CD14+/CD16+ monocyte samples as dimensional reference points confirmed that PBMC transcriptome differences originated specifically from the CD14++/CD16− classical monocyte subset (Fig. 3C).
Fig. 3.
Differential gene expression, monocyte transcriptome representation, and glucocorticoid regulation in the rhesus macaque model of PSI. (A) Differential expression of 53 CTRA indicator transcripts in PBMC samples from 49 rhesus macaques classified by behavioral observations as low or intermediate PSI (n = 22 and 19, respectively) vs. high PSI (n = 8) as in Fig. 1A (P = 0.017, controlling for age). (B) Transcript origin analyses (12) identifying major leukocyte subset origins of the 229 gene transcripts that showed ≥1.2-fold differential expression in high-PSI vs. intermediate- and low-PSI animals (70 up-regulated transcripts and 159 down-regulated transcripts are listed in Table S1). (C) Transcript origin analyses assessing the extent to which CD14++/CD16− classical and CD14+/CD16+ nonclassical monocyte transcriptomes contributed to differential gene expression. (D) Functional glucocorticoid sensitivity of myeloid lineage immune cells (neutrophils) was assessed using an in vivo hematological redistribution assay that quantified the extent to which circulating neutrophil numbers (normalized to lymphocyte numbers to control for variations in total white blood cell count) varied as a function of endogenous physiological variations in cortisol levels (22, 24). Log-transformed neutrophil/lymphocyte ratios were subject to mixed effect linear model analysis of four repeated measurements collected at 9:00 AM and 3:00 PM on 2 d from low-PSI animals (n = 172 observations on 27 animals, 16 of whom were also resampled 1 y later), intermediate-PSI animals (n = 132 observations on 21 animals, 12 of whom were resampled 1 y later), and high-PSI animals (n = 32 observations on 6 animals, 2 of whom were resampled 1 y later). Average values are displayed in raw ratio units to facilitate interpretation, and lines span the observed range of cortisol values. (E) In vivo transcriptional activity of glucocorticoid receptor (GR) and NF-κB transcription factors as assessed by TELiS bioinformatic analysis (50) of transcription factor-binding motif prevalence in promoter DNA sequences of genes showing ≥1.2-fold differential gene expression in high-PSI animals vs. low- and intermediate-PSI controls.
Consistent with previous analyses of high-PSI humans (11, 22) and socially stressed mice (7, 23–26), circulating immune cells from high-PSI macaques also showed reduced glucocorticoid receptor (GR) functional activity, including blunted neutrophil redistribution response to endogenous diurnal variation in cortisol levels (Fig. 3D) and bioinformatic indications of down-regulated GR transcriptional activity and reciprocal up-regulation of NF-κB (which is generally inhibited by GR signaling) (Fig. 3E).
To determine whether chronically experienced social threat (a central mechanism of human chronic PSI) (4–6) can causally impact monocyte population dynamics, we analyzed archival hematologic data from a previous study in which 21 individually housed adult male macaques were randomized to socialize for 100 min/d on 3–5 d/wk with either a continually varying array of novel social partners or a stable group of social partners (21). Relative to the familiar contact control condition, animals exposed to chronic social novelty over 5 wk showed up-regulated prevalence of circulating monocytes in general (Fig. 4A) and CD14++/CD16− classical monocytes in particular (Fig. 4B). CD14+/CD16++ nonclassical monocytes were not significantly expanded (Fig. 4B).
Fig. 4.
Social threat and monocyte population dynamics. Effect of chronic novelty-related social threat on circulating monocyte populations was assessed in 21 adult male macaques randomized to socialize for 100 min/d on 3–5 d/wk with either a continually varying array of novel social partners (n = 11) or a stable group of social partners (n = 10) (21). Data represent mean ± SE change from presocialization baseline to 5-wk follow-up in total monocytes (A) and classical and nonclassical monocyte subsets (B); P values from mixed effect linear model analysis.
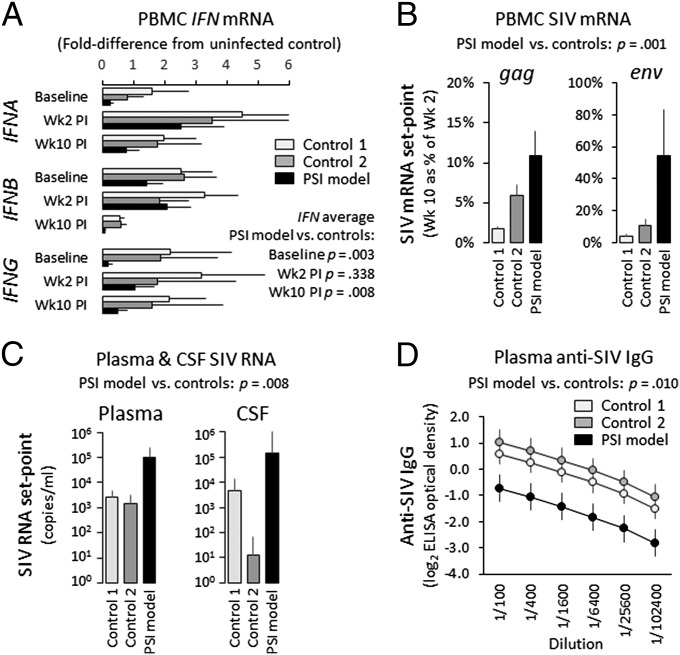
To assess the functional significance of PSI-related alterations in myeloid transcriptome regulation, we surveyed expression of type I and II IFNs in an additional sample of 17 macaques under basal physiologic conditions and after experimental infection with SIV. At baseline (2 wk preinfection), high-PSI animals showed up-regulated circulating monocyte frequencies (high PSI, mean ± SE, 544 ± 103 cells per mm3; low and intermediate PSI controls, 295 ± 46; P = 0.0465) and percentages (high PSI, 4.5 ± 0.9%; controls, 2.5 ± 0.4%; P = 0.0540). Within the monocyte population, high-PSI animals also showed up-regulation of CD14++/CD16− classical monocytes (413 ± 71 cells per mm3 blood vs. 230 ± 29 in controls; P = 0.0310) but no difference in CD14+/CD16++ nonclassical monocytes (71 ± 71 cells per mm3 vs. 22 ± 29; P = 0.5393). At baseline, high-PSI animals showed PBMC mRNA levels of IFNA, IFNB, and IFNG that were down-regulated by an average of 82%, 48%, and 92%, respectively, compared with controls (Fig. 5A). At 2 wk postinfection (peak of acute viral replication), IFN gene expression was significantly up-regulated across all groups (average > fourfold across all 3 IFNs; P < 0.0001) and did not differ significantly in high-PSI animals (Fig. 5A). At 10 wk postinfection, after the establishment of a long-term viral replication set point, high-PSI animals again showed reduced IFN gene expression (IFNA down-regulated by 64%, IFNB by 86%, and IFNG by 77%) (Fig. 5A). Presumably as a consequence of this impaired antiviral response, high-PSI animals also showed poorer suppression of PBMC SIV gene expression from the 2-wk peak of acute viral replication to the long-term viral replication set point at 10 wk postinfection (Fig. 5B), as well as elevated SIV viral load set point in plasma and cerebrospinal fluid (Fig. 5C) and reduced anti-SIV IgG antibody titers (Fig. 5D).
Fig. 5.
IFN gene expression and response to SIV infection in the rhesus macaque model of PSI. (A) mRNA encoding type I (IFNA, IFNB) and type II (IFNG) interferons was assessed in PBMCs from 12 adult male macaques experimentally infected with simian immunodeficiency virus (SIV) (Control 1, 6 low PSI; Control 2, 4 intermediate PSI; PSI model: 2 high PSI) at preinfection baseline, 2 wk postinfection (peak of acute viral replication), and 10 wk postinfection (long-term viral replication set point). Data represent mean ± SE fold-difference from five SIV-uninfected controls; P values contrast high-PSI vs. controls pooling across IFNs. (B) Immunologic response to SIV was assessed by quantifying suppression of SIV gag and env mRNA levels in PBMC from peak viral replication (wk 2 postinfection) to long-term set point (wk 10). Data represent mean ± SE % reduction from wk 2 to wk 10; P value contrasts high-PSI vs. controls pooling across SIV transcripts. Long-term control of viral replication was assessed by plasma SIV viral load (C) and anti-SIV IgG titers (D) at set point (wk 10). Data represent mean ± SE log10 SIV RNA copies per milliliter of plasma or log2 optical density of IgG ELISA over fourfold plasma dilutions; P values contrast high-PSI vs. controls.
Discussion
These results show that the CTRA gene expression profile observed in PBMCs from chronically lonely individuals (11–13) is mediated by selective up-regulation of the CD14++/CD16− classical monocyte transcriptome. Lonely people showed elevated SNS and hypothalamus-pituitary-adrenal–axis activity, relative expansion of the circulating monocyte pool, and monocyte-related activation of the CTRA transcriptome profile marked by up-regulated expression of proinflammatory genes and down-regulated expression of type I IFN- and antibody-related genes (4, 7, 19, 27). However, CTRA transcriptome skewing was not mediated by expansion of the total monocyte pool. Instead, analyses of gene regulation and cellular function in the macaque model of PSI (10) identified selective expansion of the CD14++/CD16− classical monocyte subset as the primary mechanism of CTRA up-regulation. High-PSI macaques also showed heightened sensitivity to social threat, elevated SNS activity, down-regulated type I and II IFNs under basal conditions, impaired response to infectious challenge with SIV, and reduced cellular sensitivity to regulation by endogenous glucocorticoids (i.e., blunted redistribution responses to endogenous cortisol variations; reduced GR target gene expression; and reciprocal up-regulation of NF-κB target genes, which are normally inhibited by GR activation). Experimental studies in macaques also confirmed that CD14++/CD16− classical monocytes can be causally up-regulated by chronic exposure to novelty-related social threat, which constitutes a central psychological mechanism of human chronic PSI (4–6). Collectively, these results support a mechanistic model in which lonely individuals’ chronic perception of social threat (6) results in SNS-mediated up-regulation of myelopoiesis (7, 8) and a consequent increase in the prevalence of immature, inflammation-primed, IFN-impaired, and glucocorticoid-insensitive myeloid-lineage immune cells within the circulating leukocyte pool. The associated cell-intrinsic glucocorticoid insensitivity may explain how lonely individuals can show up-regulated proinflammatory gene expression despite concurrent elevations in systemic cortisol levels (which generally exert antiinflammatory effects) (28–32); reduced GR signaling activity may leave the myeloid lineage immune cells of lonely individuals relatively insensitive to the antiinflammatory effects of endogenous glucocorticoids.
The present longitudinal associations also suggest that CTRA gene expression may reciprocally influence PSI. Up-regulation of circulating immature monocytes provides a plausible mechanism for such effects through the known ability of these cells to traffic into the brain where they act to promote anxiety and alter social behavior (33). Proinflammatory cytokines can also circulate to the brain to activate “sickness behaviors” (19, 34) that include affective, motivational, and perceptual processes that may amplify PSI and reduce social engagement (18–20). Inflammation-mediated reciprocal regulation could act to amplify or prolong socially originated PSI (35). However, the fact that these reciprocal associations emerge above and beyond the general effects of chronic (trait) PSI also implies that behavioral interventions to reduce PSI may trigger concomitant biological changes that help propagate social integration (as well as reduced CTRA gene expression) (13).
These findings are limited by the correlational design of the human longitudinal study and the macaque PSI model (PSI may constitute both a cause and an effect of CTRA gene expression, and both may be commonly regulated by neural, endocrine, or epigenetic dynamics). This study was not designed to discover individual genes associated with PSI, and PSI may relate to other transcriptome dynamics that remain to be identified in future studies (e.g., blood informative transcript axes) (36). These results are consistent with epidemiologic data linking PSI to disease risk (1, 2, 37, 38). However, experimental manipulations of PSI, SNS activity, and myelopoiesis will be required to more fully define the molecular mechanisms and health significance of these effects. In addition to myelopoiesis (7, 8), the SNS may also regulate CTRA expression via per-cell transcriptional alterations (e.g., β-adrenergic inhibition of IFN gene transcription) (39, 40) or transient mobilization of nonclassical monocytes from the vascular margin (41). However, the latter dynamic cannot account for the present effects because acute SNS activation mobilizes nonclassical monocytes (41) whereas classical monocytes were up-regulated here. It is also important to note that the present analysis does not hypothesize any change in gene expression within classical vs. nonclassical monocytes (i.e., on a per-cell basis), only an increase in the relative prevalence of the (per-cell fixed) classical monocyte transcriptome within the overall PBMC pool. Future research will be required to confirm the specific neuroimmune interactions mediating these monocyte population dynamics. This study’s molecular, cellular, behavioral, and immunologic validation of a macaque model of human PSI provides a useful experimental system for mapping the causal interactions among social perception, neural activity, immunologic function, and health.
Methods
Human Neuroendocrine, Immune, and Transcriptome Parameters.
Data came from the Chicago Health, Aging, and Social Relations Study (CHASRS) (14, 42). Overnight urine concentrations of cortisol, epinephrine, and norepinephrine were determined by HPLC (14), monocyte prevalence by automated complete blood counts (CBCs), and peripheral blood mononuclear cell (PBMC) transcriptome profiles by Illumina bead array assays for all 144 participants who provided a blood sample for RNA assessment during CHASRS years 5, 7, 8, 9, or 10 (12). Year 5, 7, and 8 samples were assayed by Ref8 arrays (Gene Expression Omnibus Series GSE65213, GSE65298, and GSE65317), year 9–10 samples were assayed by HT-12 BeadArrays (GSE65341 and GSE65403), and CTRA gene expression was analyzed using mixed effect linear models (43) testing association between average expression of 53 CTRA indicator transcripts (44, 45) and either continuous UCLA Loneliness Scale scores or a 1/0 indicator of chronically high PSI (12) (UCLA Loneliness Scale score of ≥41 in at least 60% of measurements taken during study years 1–5) while controlling for study year, age, sex, race/ethnicity, marital status, household income, BMI, alcohol consumption, smoking history, and gene-specific differences in average expression level. Written informed consent was obtained from all study participants after the nature of this research was explained and all participant questions were addressed, and all procedures were approved by the Institutional Review Board of the University of Chicago. Additional analytic details are available in SI Methods.
Macaque Neuroendocrine, Hematologic, and Transcriptome Parameters.
Animals were classified as high, intermediate, or low PSI based on (i) high (>0.5 SD above mean) and low (>0.5 SD below mean) levels of ethologically assessed sociability and (ii) subdivision of low-sociable animals based on social threat discrimination in approach behavior (i.e., preferential approach to safe juveniles or females vs. risky adult males), yielding low PSI (high sociability), intermediate PSI (low sociability, low social threat sensitivity), and high PSI classifications (low sociability, high social threat sensitivity) (10). Homology to social threat perception in human chronic PSI (4–6, 10) was assessed by behavioral response to videos depicting a novel safe (juvenile) vs. threatening (adult) male macaque. Urinary catecholamine metabolite levels and hematologic responses to experimentally imposed chronic social novelty were assessed in archival data from two published studies (21) by applying PSI classification to archival social behavior data. For transcriptome analyses, PBMCs from a new sample of 56 PSI-classified macaques were assayed by Illumina bead arrays (46)(GSE65243); the 53-gene CTRA indicator profile was analyzed by mixed effect linear models controlling for age; and bioinformatic analyses of all transcripts showing ≥1.2-fold differential expression between high-PSI and the average of low- and medium-PSI assessed (i) the cellular origins of differential gene expression using transcript origin analysis (12) [mapped to major leukocyte subsets (47, 48) or CD14++/CD16− vs. CD14+/CD16++ monocytes (49)] and (ii) functional glucocorticoid insensitivity using Transcription Element Listening System (TELiS) analysis of GR and NF-κB response element distribution (50). Functional glucocorticoid sensitivity was verified by neutrophil redistribution sensitivity to diurnal cortisol variation (22, 24). Antiviral response was assessed by inoculating macaques with SIVmac251 (or saline control) and assessing PBMC mRNA for IFNA, IFNB, IFNG, and SIV gag and env at baseline and 2 wk and 10 wk postinfection (21, 51), plasma SIV viral load set point (52, 53), and IgG antibody titers (54). All procedures were approved by the Institutional Animal Care and Use Committee of the California National Primate Research Center. SI Methods contains additional details.
SI Methods
Human Neuroendocrine, Immune, and Transcriptome Parameters.
Study design and sample characteristics of the Chicago Health, Aging, and Social Relations Study (CHASRS) have been previously described (14, 42). For the present analyses, overnight urine concentrations of cortisol, epinephrine, and norepinephrine were assayed by HPLC as described (14). In study years 9 and 10, complete blood counts (CBCs) with white blood cell differential were performed on 6-mL venipuncture blood samples using a Sysmex XE-5000 automated hematology analyzer in the Clinical Laboratory Improvement Amendments-certified Barnard Mitchell Hospital Blood Processing Laboratory at the University of Chicago. Genome-wide transcriptional profiling was conducted on peripheral blood mononuclear cells (PBMCs) obtained from all 144 participants who agreed to provide at least one blood sample for RNA assessment during study years 5, 7, 8, 9, or 10, with assays performed using Illumina BeadArrays as previously described (12). Year 5, 7, and 8 samples were assayed by Ref8 BeadArrays (data deposited as Gene Expression Omnibus Series GSE65213, GSE65298, and GSE65317), and year 9–10 samples were assayed by its successor HT-12 BeadArrays (GSE65341 and GSE65403).
Gene expression data were analyzed using mixed effect linear models (43) testing association between average expression of the 53 CTRA indicator transcripts and a 1/0 indicator of chronically high PSI as previously described (12) (UCLA Loneliness Scale score of ≥41 in at least 60% of measurements taken during study years 1–5) while controlling for study year, age, sex, race/ethnicity (white, black, Hispanic), marital status, (ln) household income, BMI, alcohol consumption, smoking history, and gene-specific differences in average expression level. Antecedent effects of PSI on CTRA gene expression were evaluated in models predicting gene expression from concurrent and 1-y-antecedent continuous UCLA Loneliness Scale scores. Reciprocal analyses examined changes in UCLA Loneliness Scale scores as a function of concurrent and 1-y-antecedent CTRA gene expression levels while controlling for the same covariates. Contributions of leukocyte subset population dynamics were assessed in additional mixed models including eight mRNA transcripts indicating the relative prevalence of major leukocyte subsets within the circulating PBMC pool (CD14 for monocytes; CD3D, CD3E, CD4, and CD8A for T lymphocyte subsets; CD19 for B lymphocytes; and CD56/NCAM1 and CD16/FCGR3A for natural killer cells). Quantile-normalized gene expression data were log2-transformed, sign-inverted for negative elements of the CTRA gene set (31 IFN-related genes and 3 antibody-related genes) (44), and analyzed using SAS PROC MIXED, with subjects treated as a random effect and models estimated by maximum likelihood (43, 45). Missing data reduced the 720 possible gene expression samples (5 y × 144 subjects who provided at least 1 blood specimen for RNA) to a final analyzed sample of 412 samples analyzed in 141 subjects (n = 3 subjects missing demographic or behavioral data and n = 308 unavailable blood samples). The Ref8 arrays used to assay samples in years 5, 7, and 8 assessed 50 of the 53 CTRA indicator transcripts, and the HT-12 arrays used for years 9 and 10 assayed all 53 indicator transcripts, yielding a total of 21,155 gene expression values in primary analyses. Similar mixed model analyses also examined relationships between PSI and circulating monocyte percentages as determined by CBC (available in study years 9–10), and urinary concentrations of metabolites of cortisol, epinephrine, and norepinephrine (available in years 1–5 and 7–10). Unstructured covariance matrices were used for mixed model analyses of norepinephrine and epinephrine to accommodate their more complex residual variance structures.
Macaque PSI Model.
As detailed previously (10), individual rhesus macaques were classified as high-, intermediate-, or low-PSI based on (i) high (>0.5 SD above mean) and low (>0.5 SD below mean) levels of observer-rated sociability based on >100 min of ethological focal animal observations and (ii) subsequent subdivision of low-sociable animals into groups based on social threat discrimination in their spontaneous social approach behavior (i.e., preferential approach to safe social targets such as juveniles or adult females vs. risky adult males), yielding three categories of low-PSI (high sociability), intermediate-PSI (low sociability, low social threat sensitivity), and high-PSI classifications (low sociability, high social threat sensitivity).
Homology to human PSI.
To assess stability of PSI classification over time, 30 adult male macaques from the California National Primate Research Center were classified independently on two separate occasions ∼1 y apart. Consistency was assessed by the κ reliability coefficient (55) and compared with the average 1 y test–retest κ for high-PSI classification (UCLA Loneliness Scale score of ≥41) over 5 y in the CHASRS sample.
To assess the homology of macaque high-PSI classification with the social threat perception processes underlying chronic PSI in humans (4–6, 10), 27 of the PSI-classified animals were individually assessed for sensitivity to social threat based on behavioral responses to 10-min digital stimulus videos depicting affiliative behavior directed at the observer monkey by a novel safe (juvenile) vs. potentially more threatening (adult) male macaque. Animals were captured from their outdoor half-acre living cages and transported to an indoor testing room for four sessions, at approximately weekly intervals. Each animal was placed into a viewing cage [2.7 ft (wide) × 2.7 ft (deep) × 3.3 ft (high)] that was situated 5 ft from a Panasonic 42-in monitor. Session 1 was a 90-min period of adaptation in which no stimuli were shown. For the remaining three sessions, each animal observed two 10-min digital stimulus videos per session. Within each session, 1 stimulus video showed a single unfamiliar juvenile male (2–4 y of age) displaying affiliative behavior directed at the viewing monkey, and 1 stimulus video showed a single unfamiliar adult male (5 y or older) also displaying affiliative behavior (order counterbalanced over sessions). Different stimulus animals were used for each playback video across the three sessions. After playback of the two 10-min stimuli, animals were released back into their familiar living cages. Responses of the subjects were video recorded (Panasonic HD AG-AC7 camera) onto DVD (Panasonic DMREZ28 recorder) for later coding by trained behavioral observers who achieved greater than 85% interobserver reliability as previously described (10). Video behavioral records were coded for frequency and duration of looking at the display (indicators of interest). Behavioral sensitivity to stimulus animal threat level was assessed as the ratios of gaze frequency and duration in response to videos depicting low-threat juvenile targets vs. potentially high-threat adult targets within each session. (Note that all video stimuli depicted affiliative behavior directed at the viewer so any threat-related behavioral biases derived from the viewing monkey’s perceptual processes rather than the stimulus monkey’s behavior.) Log2-transformed ratios were analyzed by mixed effect linear models of behavioral response bias on the first video session (novel social situation) vs. the third (habituated social situation) using SAS PROC MIXED with random subject-specific intercepts (43) and fixed effects of PSI classification (high- vs. low- and intermediate-PSI controls) nested within stimulus session.
SNS activity.
PSI-related differences in SNS activity were assessed by RIA of urinary catecholamine metabolite levels in archival data from a previously published study of social influences on pathogenesis of simian immunodeficiency virus (SIV) infection (study 1 of ref. 21). Urine samples were collected on up to six occasions at 4- to 5-wk intervals (three before SIV infection and three beginning at 2 wk postinfection). As described above, 18 animals were classified for PSI level based on archival social behavior data. Creatinine-normalized concentrations of metanephrine (epinephrine metabolite) and normetanephrine (norepinephrine metabolite) were analyzed by mixed effect linear models involving random subject-specific intercepts and fixed effects of PSI group (high-PSI vs. intermediate- and low-PSI controls) nested within metabolite (metanephrine vs. normetanephrine), with repeated measurements pooled over time after finding no significant main effect or interaction involving pre- vs. postinfection time point.
Monocyte response to social threat.
Effects of experimentally imposed chronic social novelty were assessed in analyses of CBC and flow cytometry data from an experimental study of social stress effects on SIV pathogenesis [study 2 of ref. (21)]. Peripheral blood samples were collected from 21 adult male macaques at two prestudy baselines and at 4 and 5 wk after randomization to novel vs. familiar socialization conditions as previously described (21) (all before SIV infection). One week after the initiation of social conditions, approximately half of the animals in each condition began receiving gradually increasing daily i.m. injections of methamphetamine, and the other half received parallel vehicle control injections as described (21). Monocyte counts and % of total white blood cell (WBC) count were determined by automated CBC (Pentra 60C+), CD14++/CD16− vs. CD14+/CD16++ monocyte subsets percentages were determined by flow cytometry on a FACSCalibur instrument (BD Biosciences), and CD14++/CD16− and CD14+/CD16++ monocyte subset frequencies were derived by multiplication of absolute monocyte counts. Log2-transformed monocyte counts and % were analyzed by mixed effect linear models involving random subject-specific intercepts and fixed effects of time period (baseline vs. socialization), social condition (familiar vs. novel socialization), and their interaction. Monocyte subset frequencies were analyzed by a similar model nesting effects within CD14++/CD16− vs. CD14+/CD16++ monocyte subset. All analyses pooled over methamphetamine vs. vehicle control conditions after finding no significant main effect or interaction involving that factor in a time period × social condition × injection condition factorial design.
Transcriptome profiling.
PBMC samples were collected from a new sample of 56 macaques under basal conditions as previously described (21), 49 of which had been classified for PSI status. As in previous studies (46), transcriptome profiles were assayed by Illumina human BeadArrays (GSE65243). Gene expression values were quantile-normalized and log2-transformed for mixed effect linear model analyses relating CTRA gene expression to PSI status while controlling for age and including a heterogeneous compound symmetry covariance matrix to account for additional residual covariance beyond random subject intercepts (43). Additional bioinformatics analyses examined all gene transcripts showing ≥1.2-fold differential expression between high-PSI animals and the average of low- and medium-PSI controls to identify (i) predominate cellular origins of differential gene expression, using Transcript Origin Analysis (12) with reference data defining empirical transcriptome differences among major leukocyte subsets (47, 48) and CD14++/CD16− vs. CD14+/CD16++ monocyte subsets (49) and (ii) functional glucocorticoid insensitivity as indicated by TELiS promoter sequence bioinformatics analyses (50) assessing relative depletion of genes bearing promoter response elements for the GR (V$GR_Q6) and relative enrichment of genes bearing response elements for NF-κB (defined by V$NFKB_C), which is generally inhibited by GR activity. SEs and P values for these analyses were derived by nonparametric bootstrapping of linear model residuals as previously described (56). To prevent overcontrol for targeted outcome variance, linear model estimates for Transcript Origin and TELiS analyses did not control for leukocyte subset indicator transcripts.
Glucocorticoid sensitivity.
For in vivo hematological assessments of glucocorticoid sensitivity, venipuncture whole blood samples were obtained at 9:00 AM and 3:00 PM on 2 d ∼1 wk apart from 54 animals, 30 of whom were also subsequently resampled ∼1 y later. Blood specimens were subject to automated CBC as described above and RIA for plasma cortisol concentration (Siemens DPC Coat-a-Count). Following the analytic approach previously described (22, 24), differences across PSI groups in the quantitative relationship between variations in cortisol levels and variations in log-transformed neutrophil/lymphocyte ratios were estimated using mixed effect linear models that controlled for animal age and heterogeneous correlation among residuals due to repeated measurements (unstructured covariance matrix) (43). Group differences in sensitivity were estimated in models parameterized as PSI group × cortisol concentration (continuous) interaction terms, and group-specific sensitivity slopes were estimated in models nesting cortisol concentration effects within PSI group.
IFN expression and antiviral response.
PSI-related differences in IFN expression and antiviral response were assessed in PBMC samples from 17 adult male macaques participating in a study on the effects of methamphetamine and two pharmacological agents (recombinant human IFN beta and propranolol) on progression of SIV infection. The 17 studied animals came from an experimental cohort of 30 adult male macaques and represent all animals for whom sufficient archival social behavior data were available to allow PSI classification as described above. PBMC samples collected at preinfection baseline, 2 wk postinfection, and 10 wk postinfection were assayed by quantitative real-time RT-PCR for macaque IFNA, IFNB, and IFNG transcripts, and in SIV-infected animals, SIV gag and env mRNA levels, as previously detailed (21, 51). Among the 17 PSI-classified animals, 12 were randomly assigned to i.v. inoculation with 500 50% tissue culture infective dose (TCID50) units of SIVmac251 (6 low, 4 intermediate, and 2 high PSI), and 5 were inoculated in parallel with saline as uninfected controls (3 low and 2 intermediate PSI). Among the 12 SIV-infected animals, 8 were randomized to receive gradually increasing daily i.m. injections of methamphetamine [as detailed in ref. (21)], and the remaining 4 received parallel i.m. injections of vehicle control solution. Among the eight SIV-infected animals randomized to receive methamphetamine, one was randomized to receive 1 mg/kg propranolol (14423-0; Cardinal Health) orally in a solution of Boost (Nestle) twice daily (animals not receiving propranolol received Boost-only) 7 d/wk, beginning at SIV inoculation; and three were randomized to receive 0.5 μg/kg recombinant human IFN beta (11410-9; PBL IFN Source) once daily three times per week (Monday, Wednesday, and Friday) by s.c. injection (animals not receiving recombinant human IFN beta received a parallel s.c. injection of saline), beginning the day after SIV inoculation. Statistical analyses focused on animals randomized to SIV infection conditions and analyzed ACTB-normalized threshold cycle numbers (log2 metric) using mixed effect linear models that included random subject-specific intercepts and fixed effects of gene (two-level repeated measure for SIV gag and env or a three-level repeated measure for IFNA, IFNB, and IFNG), experimental time point (three-level repeated measure), drug exposure (separate indicator variables for methamphetamine, propranolol, and recombinant human IFN beta), PSI classification, and a PSI classification × time interaction assessing group differences in responses to infection. Time point-specific differences in gene expression were quantified by estimation of PSI main effects nested within time. Parallel analyses examined preinfection baseline CBCs and flow cytometric assessments of CD14++/CD16− and CD14+/CD16++ monocyte subsets as described above. Analyses also examined PSI group differences in week 10 plasma viral load (RNA) set point and cerebrospinal fluid (CSF) viral load set point [assessed by RT-PCR as previously detailed (52)] and plasma anti-SIV IgG titers [assessed by ELISA as previously detailed (54)]. RT-PCR determinations of plasma and CSF SIV RNA copy numbers were log10-transformed for analysis in mixed effect linear models that included effects for PSI classification, methamphetamine exposure (no other drug exposure significantly related to viral load or IgG set points; all P > 0.05), and anatomical compartment (plasma vs. CSF, treated as a repeated measure), with a compound symmetry residual covariance matrix specified to accommodate correlation among residuals. Anti-SIV IgG ELISA optical density determinations taken at six fourfold plasma dilutions ranging from 1/100–1/102,400 were log2-transformed for analysis in a mixed effect linear model that included effects of PSI classification, methamphetamine exposure, and (log2) dilution factor (treated as a repeated measure factor), with a heterogeneous compound symmetry residual covariance matrix specified to accommodate correlation among residuals within subject and heteroscedasticity across dilution factors. All animals were necropsied at week 10 to assess tissue viral load and neuropathology so no clinical disease progression data are available. However, week 10 plasma viral load set point is highly predictive of survival time in the macaque SIV model (r > 0.95) (53).
Acknowledgments
We thank Emma Adam, Louise Hawkley, HsiYuan Chen, Erna Tarara, and the University of California, Los Angeles Neuroscience Genomics Core for assistance. This study was supported by National Institutes of Health Grants R37-AG033590, P01-AG18911, R01-AG034052, R01-AG043404, P30-AG017265, P30-AG028748, R01-DA024441, and P51-OD011107; the Mind, Body, Brain and Health Initiative of the John D. and Catherine T. MacArthur Foundation; and the John Templeton Foundation.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The data reported in this paper have been deposited in the Gene Expression Omnibus (GEO) database, www.ncbi.nlm.nih.gov/geo (accession nos. GSE65213, GSE65298, GSE65317, GSE65341, GSE65403, and GSE65243).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1514249112/-/DCSupplemental.
References
- 1.Holt-Lunstad J, Smith TB, Layton JB. Social relationships and mortality risk: A meta-analytic review. PLoS Med. 2010;7(7):e1000316. doi: 10.1371/journal.pmed.1000316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Luo Y, Hawkley LC, Waite LJ, Cacioppo JT. Loneliness, health, and mortality in old age: A national longitudinal study. Soc Sci Med. 2012;74(6):907–914. doi: 10.1016/j.socscimed.2011.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cacioppo S, Capitanio JP, Cacioppo JT. Toward a neurology of loneliness. Psychol Bull. 2014;140(6):1464–1504. doi: 10.1037/a0037618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cacioppo JT, Cacioppo S, Capitanio JP, Cole SW. The neuroendocrinology of social isolation. Annu Rev Psychol. 2015;66:733–767. doi: 10.1146/annurev-psych-010814-015240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cacioppo JT, et al. Loneliness across phylogeny and a call for comparative studies and animal models. Perspect Psychol Sci. 2015;10(2):202–212. doi: 10.1177/1745691614564876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cacioppo JT, Hawkley LC. Perceived social isolation and cognition. Trends Cogn Sci. 2009;13(10):447–454. doi: 10.1016/j.tics.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Powell ND, et al. Social stress up-regulates inflammatory gene expression in the leukocyte transcriptome via β-adrenergic induction of myelopoiesis. Proc Natl Acad Sci USA. 2013;110(41):16574–16579. doi: 10.1073/pnas.1310655110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heidt T, et al. Chronic variable stress activates hematopoietic stem cells. Nat Med. 2014;20(7):754–758. doi: 10.1038/nm.3589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Finch CE. The Biology of Human Longevity: Inflammation, Nutrition, and Aging in the Evolution of Lifespans. Academic; Burlington, MA: 2007. [Google Scholar]
- 10.Capitanio JP, Hawkley LC, Cole SW, Cacioppo JT. A behavioral taxonomy of loneliness in humans and rhesus monkeys (Macaca mulatta) PLoS One. 2014;9(10):e110307. doi: 10.1371/journal.pone.0110307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cole SW, et al. Social regulation of gene expression in human leukocytes. Genome Biol. 2007;8(9):R189. doi: 10.1186/gb-2007-8-9-r189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cole SW, Hawkley LC, Arevalo JM, Cacioppo JT. Transcript origin analysis identifies antigen-presenting cells as primary targets of socially regulated gene expression in leukocytes. Proc Natl Acad Sci USA. 2011;108(7):3080–3085. doi: 10.1073/pnas.1014218108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Creswell JD, et al. Mindfulness-based stress reduction training reduces loneliness and pro-inflammatory gene expression in older adults: A small randomized controlled trial. Brain Behav Immun. 2012;26(7):1095–1101. doi: 10.1016/j.bbi.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hawkley LC, Masi CM, Berry JD, Cacioppo JT. Loneliness is a unique predictor of age-related differences in systolic blood pressure. Psychol Aging. 2006;21(1):152–164. doi: 10.1037/0882-7974.21.1.152. [DOI] [PubMed] [Google Scholar]
- 15.Hawkley LC, Burleson MH, Berntson GG, Cacioppo JT. Loneliness in everyday life: Cardiovascular activity, psychosocial context, and health behaviors. J Pers Soc Psychol. 2003;85(1):105–120. doi: 10.1037/0022-3514.85.1.105. [DOI] [PubMed] [Google Scholar]
- 16.Steptoe A, Owen N, Kunz-Ebrecht SR, Brydon L. Loneliness and neuroendocrine, cardiovascular, and inflammatory stress responses in middle-aged men and women. Psychoneuroendocrinology. 2004;29(5):593–611. doi: 10.1016/S0306-4530(03)00086-6. [DOI] [PubMed] [Google Scholar]
- 17.Cacioppo JT, Hughes ME, Waite LJ, Hawkley LC, Thisted RA. Loneliness as a specific risk factor for depressive symptoms: Cross-sectional and longitudinal analyses. Psychol Aging. 2006;21(1):140–151. doi: 10.1037/0882-7974.21.1.140. [DOI] [PubMed] [Google Scholar]
- 18.Eisenberger NI, Inagaki TK, Mashal NM, Irwin MR. Inflammation and social experience: An inflammatory challenge induces feelings of social disconnection in addition to depressed mood. Brain Behav Immun. 2010;24(4):558–563. doi: 10.1016/j.bbi.2009.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Irwin MR, Cole SW. Reciprocal regulation of the neural and innate immune systems. Nat Rev Immunol. 2011;11(9):625–632. doi: 10.1038/nri3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Inagaki TK, Muscatell KA, Irwin MR, Cole SW, Eisenberger NI. Inflammation selectively enhances amygdala activity to socially threatening images. Neuroimage. 2012;59(4):3222–3226. doi: 10.1016/j.neuroimage.2011.10.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Capitanio JP, Cole SW. Social instability and immunity in rhesus monkeys: the role of the sympathetic nervous system. Philos Trans R Soc Lond B Biol Sci. 2015;370(1669):370. doi: 10.1098/rstb.2014.0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cole SW. Social regulation of leukocyte homeostasis: The role of glucocorticoid sensitivity. Brain Behav Immun. 2008;22(7):1049–1055. doi: 10.1016/j.bbi.2008.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stark JL, et al. Social stress induces glucocorticoid resistance in macrophages. Am J Physiol Regul Integr Comp Physiol. 2001;280(6):R1799–R1805. doi: 10.1152/ajpregu.2001.280.6.R1799. [DOI] [PubMed] [Google Scholar]
- 24.Cole SW, Mendoza SP, Capitanio JP. Social stress desensitizes lymphocytes to regulation by endogenous glucocorticoids: Insights from in vivo cell trafficking dynamics in rhesus macaques. Psychosom Med. 2009;71(6):591–597. doi: 10.1097/PSY.0b013e3181aa95a9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wohleb ES, et al. β-Adrenergic receptor antagonism prevents anxiety-like behavior and microglial reactivity induced by repeated social defeat. J Neurosci. 2011;31(17):6277–6288. doi: 10.1523/JNEUROSCI.0450-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hanke ML, Powell ND, Stiner LM, Bailey MT, Sheridan JF. Beta adrenergic blockade decreases the immunomodulatory effects of social disruption stress. Brain Behav Immun. 2012;26(7):1150–1159. doi: 10.1016/j.bbi.2012.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cole SW. Human social genomics. PLoS Genet. 2014;10(8):e1004601. doi: 10.1371/journal.pgen.1004601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miller GE, Cohen S, Ritchey AK. Chronic psychological stress and the regulation of pro-inflammatory cytokines: A glucocorticoid-resistance model. Health Psychol. 2002;21(6):531–541. doi: 10.1037//0278-6133.21.6.531. [DOI] [PubMed] [Google Scholar]
- 29.Pace TW, Hu F, Miller AH. Cytokine-effects on glucocorticoid receptor function: relevance to glucocorticoid resistance and the pathophysiology and treatment of major depression. Brain Behav Immun. 2007;21(1):9–19. doi: 10.1016/j.bbi.2006.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miller GE, et al. A functional genomic fingerprint of chronic stress in humans: Blunted glucocorticoid and increased NF-kappaB signaling. Biol Psychiatry. 2008;64(4):266–272. doi: 10.1016/j.biopsych.2008.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miller GE, et al. Low early-life social class leaves a biological residue manifested by decreased glucocorticoid and increased proinflammatory signaling. Proc Natl Acad Sci USA. 2009;106(34):14716–14721. doi: 10.1073/pnas.0902971106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miller GE, et al. Greater inflammatory activity and blunted glucocorticoid signaling in monocytes of chronically stressed caregivers. Brain Behav Immun. 2014;41:191–199. doi: 10.1016/j.bbi.2014.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wohleb ES, McKim DB, Sheridan JF, Godbout JP. Monocyte trafficking to the brain with stress and inflammation: A novel axis of immune-to-brain communication that influences mood and behavior. Front Neurosci. 2014;8:447. doi: 10.3389/fnins.2014.00447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: When the immune system subjugates the brain. Nat Rev Neurosci. 2008;9(1):46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hawkley LC, et al. 2008. From social structure factors to perceptions of relationship quality and loneliness: The Chicago Health, Aging, and Social Relations Study. J Gerontol Soc Sci 63B(6):S375–S384.
- 36.Preininger M, et al. Blood-informative transcripts define nine common axes of peripheral blood gene expression. PLoS Genet. 2013;9(3):e1003362. doi: 10.1371/journal.pgen.1003362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Holwerda TJ, et al. Increased risk of mortality associated with social isolation in older men: only when feeling lonely? Results from the Amsterdam Study of the Elderly (AMSTEL) Psychol Med. 2012;42(4):843–853. doi: 10.1017/S0033291711001772. [DOI] [PubMed] [Google Scholar]
- 38.Luo Y, Waite LJ. Loneliness and mortality among older adults in China. J Gerontol B Psychol Sci Soc Sci. 2014;69(4):633–645. doi: 10.1093/geronb/gbu007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cole SW, Korin YD, Fahey JL, Zack JA. Norepinephrine accelerates HIV replication via protein kinase A-dependent effects on cytokine production. J Immunol. 1998;161(2):610–616. [PubMed] [Google Scholar]
- 40.Collado-Hidalgo A, Sung C, Cole S. Adrenergic inhibition of innate anti-viral response: PKA blockade of Type I interferon gene transcription mediates catecholamine support for HIV-1 replication. Brain Behav Immun. 2006;20(6):552–563. doi: 10.1016/j.bbi.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 41.Dimitrov S, et al. Differential TNF production by monocyte subsets under physical stress: Blunted mobilization of proinflammatory monocytes in prehypertensive individuals. Brain Behav Immun. 2013;27(1):101–108. doi: 10.1016/j.bbi.2012.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cacioppo JT, et al. Loneliness within a nomological net: An evolutionary perspective. J Res Pers. 2006;40:1054–1085. [Google Scholar]
- 43.McCulloch CE, Searle SR, Neuhaus JM. Generalized, Linear, and Mixed Models. Wiley; Hoboken, NJ: 2008. [Google Scholar]
- 44.Fredrickson BL, et al. A functional genomic perspective on human well-being. Proc Natl Acad Sci USA. 2013;110(33):13684–13689. doi: 10.1073/pnas.1305419110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fredrickson BL, et al. Psychological well-being and the human conserved transcriptional response to adversity. PLoS One. 2015;10(3):e0121839. doi: 10.1371/journal.pone.0121839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tung J, et al. Social environment is associated with gene regulatory variation in the rhesus macaque immune system. Proc Natl Acad Sci USA. 2012;109(17):6490–6495. doi: 10.1073/pnas.1202734109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Su AI, et al. A gene atlas of the mouse and human protein-encoding transcriptomes. Proc Natl Acad Sci USA. 2004;101(16):6062–6067. doi: 10.1073/pnas.0400782101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Abbas AR, et al. Immune response in silico (IRIS): Immune-specific genes identified from a compendium of microarray expression data. Genes Immun. 2005;6(4):319–331. doi: 10.1038/sj.gene.6364173. [DOI] [PubMed] [Google Scholar]
- 49.Ingersoll MA, et al. Comparison of gene expression profiles between human and mouse monocyte subsets. Blood. 2010;115(3):e10–e19. doi: 10.1182/blood-2009-07-235028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cole SW, Yan W, Galic Z, Arevalo J, Zack JA. Expression-based monitoring of transcription factor activity: The TELiS database. Bioinformatics. 2005;21(6):803–810. doi: 10.1093/bioinformatics/bti038. [DOI] [PubMed] [Google Scholar]
- 51.Sloan EK, et al. Social stress enhances sympathetic innervation of primate lymph nodes: Mechanisms and implications for viral pathogenesis. J Neurosci. 2007;27(33):8857–8865. doi: 10.1523/JNEUROSCI.1247-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Leutenegger CM, et al. Real-time TaqMan PCR as a specific and more sensitive alternative to the branched-chain DNA assay for quantitation of simian immunodeficiency virus RNA. AIDS Res Hum Retroviruses. 2001;17(3):243–251. doi: 10.1089/088922201750063160. [DOI] [PubMed] [Google Scholar]
- 53.Watson A, et al. Plasma viremia in macaques infected with simian immunodeficiency virus: Plasma viral load early in infection predicts survival. J Virol. 1997;71(1):284–290. doi: 10.1128/jvi.71.1.284-290.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lü X, et al. Targeted lymph-node immunization with whole inactivated simian immunodeficiency virus (SIV) or envelope and core subunit antigen vaccines does not reliably protect rhesus macaques from vaginal challenge with SIVmac251. AIDS. 1998;12(1):1–10. doi: 10.1097/00002030-199801000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fleiss JL. Statistical Methods for Rates and Proportions. Wiley; New York: 1981. [Google Scholar]
- 56.Efron B, Tibshirani RJ. An Introduction to the Bootstrap. Chapman & Hall; New York: 1993. [Google Scholar]