Abstract
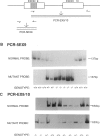
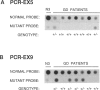
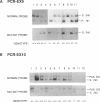
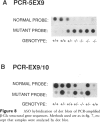
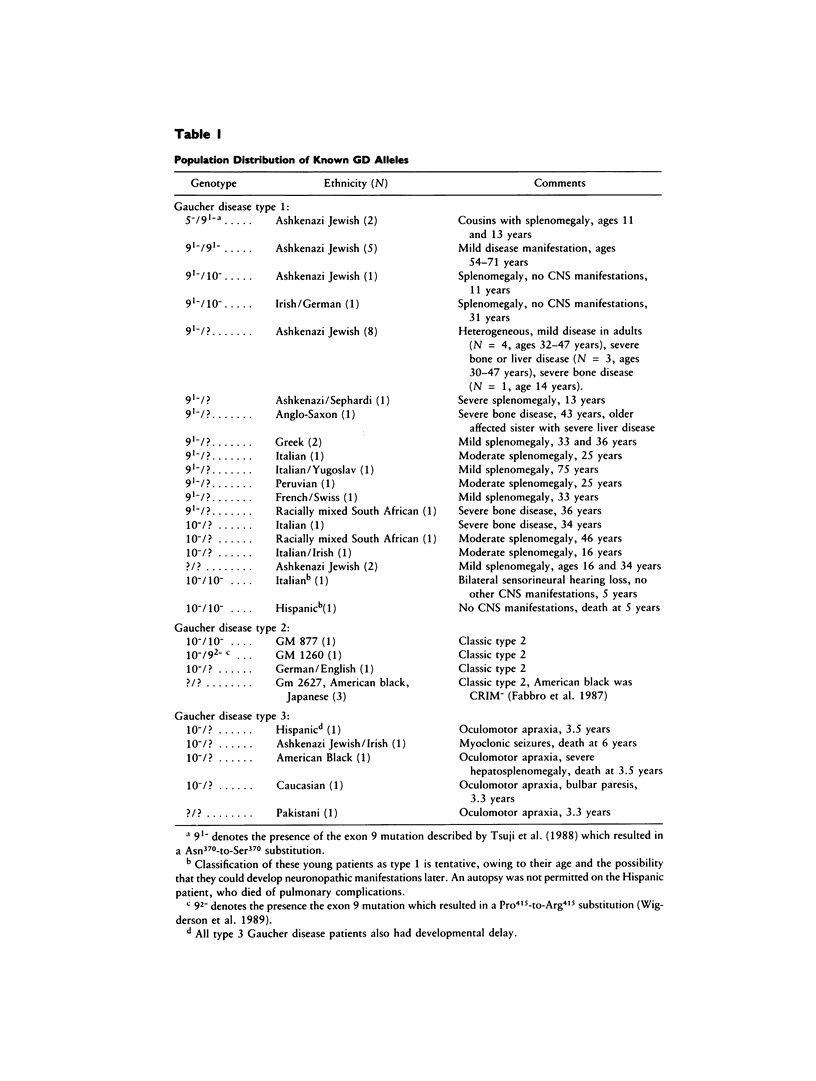
Gaucher disease (GD) is the most prevalent lysosomal storage disease. This autosomal recessive trait results from the defective activity of acid beta-glucosidase (beta-Glc). Four different exonic point mutations have been identified as causal alleles for GD. To facilitate screening for these alleles, assays were developed using allele-specific oligonucleotide hybridization to amplified genomic DNA sequences. Specifically, intron bases flanking exons 5, 9, and 10 were determined, and conditions for PCR amplification of these exons were obtained. Two different procedures were developed to distinguish signals obtained from the structural beta-Glc gene exons and those from the pseudogene. These procedures were used to determine the distribution of all known GD alleles in a population of 44 affected patients of varying phenotypes and ethnicity. The high frequency of one of the exon 9 mutations in Ashkenazi Jewish GD type 1 patients was confirmed, and, in addition, this mutation was present in ethnically diverse non-Jewish type 1 GD patients. Homozygotes (N = 5) for this allele were midly affected older individuals, and this mutant allele was not found in any patient with neuronopathic disease. The exon 10 mutation was confirmed as the predominant allele in types 2 and 3 GD. However, several type 1 GD patients, including one of Ashkenazi-Jewish heritage, also were heterozygous for this allele. The presence of this allele in type 1 patients did not correlate with the severity of clinical symptoms. The second exon 9 mutation and the exon 5 mutation were rare, since they occurred only heterozygously either in one type 2 GD patient or in two related Ashkenazi-Jewish GD patients, respectively. Although most GD patients (38 of 44) had at least one of the known mutant alleles, 57% were heterozygotes for only one of these mutations. Fourteen percent of patients were negative for all mutations. A total of 73% of GD patients had at least one unknown allele. The varying clinical phenotypes and ethnic origins of these incompletely characterized patients suggest that multiple other GD alleles exist.
Full text
PDF





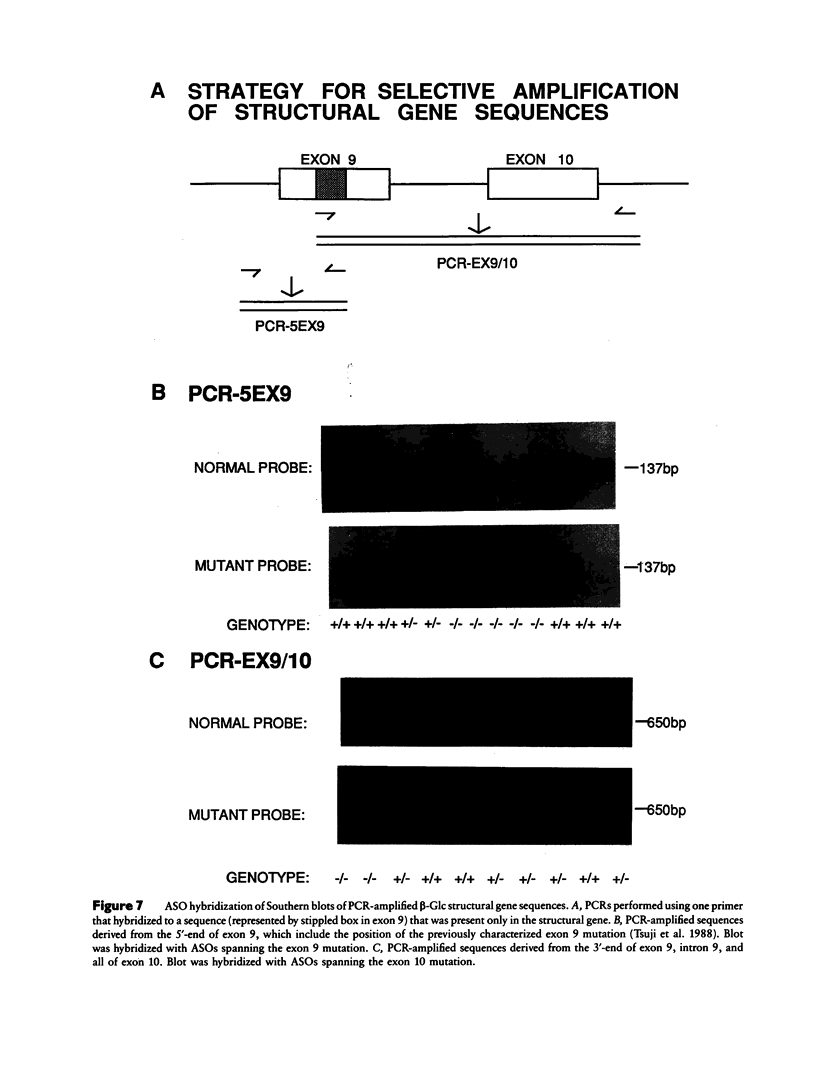



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen E. Y., Seeburg P. H. Supercoil sequencing: a fast and simple method for sequencing plasmid DNA. DNA. 1985 Apr;4(2):165–170. doi: 10.1089/dna.1985.4.165. [DOI] [PubMed] [Google Scholar]
- Fabbro D., Desnick R. J., Grabowski G. A. Gaucher disease: genetic heterogeneity within and among the subtypes detected by immunoblotting. Am J Hum Genet. 1987 Jan;40(1):15–31. [PMC free article] [PubMed] [Google Scholar]
- Ginns E. I., Brady R. O., Pirruccello S., Moore C., Sorrell S., Furbish F. S., Murray G. J., Tager J., Barranger J. A. Mutations of glucocerebrosidase: discrimination of neurologic and non-neurologic phenotypes of Gaucher disease. Proc Natl Acad Sci U S A. 1982 Sep;79(18):5607–5610. doi: 10.1073/pnas.79.18.5607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabowski G. A., Dinur T., Osiecki K. M., Kruse J. R., Legler G., Gatt S. Gaucher disease types 1, 2, and 3: differential mutations of the acid beta-glucosidase active site identified with conduritol B epoxide derivatives and sphingosine. Am J Hum Genet. 1985 May;37(3):499–510. [PMC free article] [PubMed] [Google Scholar]
- Grabowski G. A., Goldblatt J., Dinur T., Kruse J., Svennerholm L., Gatt S., Desnick R. J. Genetic heterogeneity in Gaucher disease: physicokinetic and immunologic studies of the residual enzyme in cultured fibroblasts from non-neuronopathic and neuronopathic patients. Am J Med Genet. 1985 Jul;21(3):529–549. doi: 10.1002/ajmg.1320210316. [DOI] [PubMed] [Google Scholar]
- Graves P. N., Grabowski G. A., Eisner R., Palese P., Smith F. I. Gaucher disease type 1: cloning and characterization of a cDNA encoding acid beta-glucosidase from an Ashkenazi Jewish patient. DNA. 1988 Oct;7(8):521–528. doi: 10.1089/dna.1.1988.7.521. [DOI] [PubMed] [Google Scholar]
- Graves P. N., Grabowski G. A., Ludman M. D., Palese P., Smith F. I. Human acid beta-glucosidase: Northern blot and S1 nuclease analysis of mRNA from HeLa cells and normal and Gaucher disease fibroblasts. Am J Hum Genet. 1986 Dec;39(6):763–774. [PMC free article] [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983 Jun 5;166(4):557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Horowitz M., Wilder S., Horowitz Z., Reiner O., Gelbart T., Beutler E. The human glucocerebrosidase gene and pseudogene: structure and evolution. Genomics. 1989 Jan;4(1):87–96. doi: 10.1016/0888-7543(89)90319-4. [DOI] [PubMed] [Google Scholar]
- Novotný J., Tonegawa S., Saito H., Kranz D. M., Eisen H. N. Secondary, tertiary, and quaternary structure of T-cell-specific immunoglobulin-like polypeptide chains. Proc Natl Acad Sci U S A. 1986 Feb;83(3):742–746. doi: 10.1073/pnas.83.3.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiner O., Wigderson M., Horowitz M. Structural analysis of the human glucocerebrosidase genes. DNA. 1988 Mar;7(2):107–116. doi: 10.1089/dna.1988.7.107. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Scharf S., Faloona F., Mullis K. B., Horn G. T., Erlich H. A., Arnheim N. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985 Dec 20;230(4732):1350–1354. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- Tsuji S., Choudary P. V., Martin B. M., Stubblefield B. K., Mayor J. A., Barranger J. A., Ginns E. I. A mutation in the human glucocerebrosidase gene in neuronopathic Gaucher's disease. N Engl J Med. 1987 Mar 5;316(10):570–575. doi: 10.1056/NEJM198703053161002. [DOI] [PubMed] [Google Scholar]
- Tsuji S., Martin B. M., Barranger J. A., Stubblefield B. K., LaMarca M. E., Ginns E. I. Genetic heterogeneity in type 1 Gaucher disease: multiple genotypes in Ashkenazic and non-Ashkenazic individuals. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2349–2352. doi: 10.1073/pnas.85.7.2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigderson M., Firon N., Horowitz Z., Wilder S., Frishberg Y., Reiner O., Horowitz M. Characterization of mutations in Gaucher patients by cDNA cloning. Am J Hum Genet. 1989 Mar;44(3):365–377. [PMC free article] [PubMed] [Google Scholar]