Abstract
A more thorough understanding of the differences in DNA methylation (DNAm) profiles in populations may hold promise for identifying molecular mechanisms through which genetic and environmental factors jointly contribute to human diseases. Inflammation is a key molecular mechanism underlying several chronic diseases including cardiovascular disease, and it affects DNAm profile on both global and locus-specific levels. To understand the impact of inflammation on the DNAm of the human genome, we investigated DNAm profiles of peripheral blood leukocytes from 966 African American participants in the Genetic Epidemiology Network of Arteriopathy (GENOA) study. By testing the association of DNAm sites on CpG islands of over 14,000 genes with C-reactive protein (CRP), an inflammatory biomarker of cardiovascular disease, we identified 257 DNAm sites in 240 genes significantly associated with serum levels of CRP adjusted for age, sex, body mass index and smoking status, and corrected for multiple testing. Of the significantly associated DNAm sites, 80.5% were hypomethylated with higher CRP levels. The most significant Gene Ontology terms enriched in the genes associated with the CRP levels were immune system process, immune response, defense response, response to stimulus, and response to stress, which are all linked to the functions of leukocytes. While the CRP-associated DNAm may be cell-type specific, understanding the DNAm association with CRP in peripheral blood leukocytes of multi-ethnic populations can assist in unveiling the molecular mechanism of how the process of inflammation affects the risks of developing common disease through epigenetic modifications.
Introduction
Inflammation plays a key role in the development of atherosclerosis and cardiovascular diseases [1]. The molecular mechanism underlying atherosclerosis involves the interplay between the immune system and environmental risk factors such as diet, exercise, and smoking. Biomarkers of the immune system, including serum levels of several inflammatory markers, such as C-reactive protein (CRP), fibrinogen, and interleukin 6 are independently associated with cardiovascular diseases (CVD) after adjustment for other known risk factors [2].
Epigenetic modification, through DNA methylation (DNAm) and other molecular mechanisms, can regulate gene expression levels and is an important molecular mechanism underlying disease development. Reports have suggested that epigenetic alteration may lead to the development of immune disorders [3–5]. Further, epigenetic modifications in the immune cells can affect their functionality in inflammatory responses of the human body, which play a key role in developing chronic conditions such as CVD.
CRP is a biomarker of systemic inflammation and a risk factor for the development of inflammation-mediated diseases such as CVD, metabolic syndrome, type 2 diabetes and hypertension [6,7]. The production of CRP in the liver is triggered by cytokines (e.g. IL6 which is secreted by macrophages and T-cells) in response to inflammatory conditions. As a downstream biomarker of inflammatory conditions, CRP also integrates upstream signals from cytokine activation and environmental stimuli. Serum CRP has a long half-life with low circadian variation, and can be easily measured using standardized methods [8,9]. CRP level is heritable [10] and is associated with common genetic variants [6,11,12]. CRP-associated genetic loci have been identified through the genome-wide association study (GWAS) in large sample size in multiple ethnic groups [13,14]. CRP level is also associated with age, sex and environmental factors such as secondhand smoke exposure, air pollution and diet [15]. Epigenetic profile is associated with cigarette smoking [16–18], pesticides [19] and other environmental toxicants [20]. These non-genetic factors can modify the epigenetic profile of genes to alter gene expression levels, potentially leading to changes in disease phenotypes. We hypothesized that the serum CRP level is associated with the epigenetic profile, specifically DNAm, which may represent the joint effect of both genetic and environmental factors.
In this study, we investigated the epigenetic associations of CRP, an inflammatory biomarker of CVD, by measuring over 27,000 DNA methylation (DNAm) sites in peripheral blood leukocytes (PBLs) of 966 African Americans. We identified DNAm sites significantly associated with serum levels of CRP across the genome.
Materials and Methods
Sample
The final sample for analysis consisted of 966 African Americans from Jackson Mississippi in the Genetic Epidemiology Network of Arteriopathy (GENOA) study, a community-based study of hypertensive sibships that aims to identify genes influencing blood pressure [21,22]. GENOA data was collected in two phases. Phase I (1996-1999) and Phase II (2000-2004) data consist of demographic information, medical history, clinical characteristics, lifestyle factors, and blood samples for genotyping and biomarker assays. The blood samples and phenotypic data used in this study were collected during GENOA Phase II study. The GENOA study was approved by the Institutional Review Boards of the University of Michigan, Mayo Clinic and Emory University. Each participant gave written informed consent.
Phenotype Measurement
Age, sex and other phenotypic data were collected from the physical examination and laboratory assessment at the time of the Phase II study visit. Serum CRP levels were measured by a highly sensitive immunoturbidimetric assay on fasting serum samples that had been stored at -80°C and thawed at room temperature [23].
Genome-wide Methylation Assay
The genomic DNA was extracted from stored PBL samples of 1,008 GENOA Phase II African American (AA) participants from 498 sibships, bisulfite converted and then genotyped for methylation profiling of 27,578 CpG loci using the Illumina Infinium HumanMethylation27 BeadChip (Illumina, San Diego, CA) as previously described [24]. The intensity data of the methylated and unmethylated bead sites from Illumina iScan system were then loaded into the GenomeStudio Methylation Module for analysis. Forty nine samples were excluded from the following analysis due to poor quality of the intensity measurements of control probes. The cleaned data set included DNAm profiles of 972 AA individuals from 493 sibships. After merging with the phenotypic data, 966 AAs had complete phenotypes and DNAm measurements.
There are 56 control probes on each chip representing a) sample independent measures of staining, hybridization, target removal, and DNA extension and b) sample dependent measures of bisulfite conversion, G/T mismatch, non-polymorphic and negative controls. The sample independent controls allow for the evaluation of the quality of the chip processing steps, while the sample dependent controls allow for the evaluation of the performance across samples. We removed sites with control probe values greater than 4 standard deviations from their mean values. In addition, we developed a normalization scheme to reduce batch and chip effects by linearly regressing the methylated and non-methylated intensity signals onto the set of control probes that are orthogonal (i.e. independent) predictors of the control spot distributions. We also excluded sites that are located on the X and Y chromosomes. Sites that are deemed multimodal based on the Dip Test proposed by Hartigan [25] using a cut-off of p<0.001 on either the methylated or non-methylated signal intensities were set aside for more specialized methods of normalization that take into account their modality. Finally, we flagged the 2,984 sites identified by Chen et al. [26] as having non-specific binding probes (over 10% of the probes map to highly homologous genomic sequences at 40 or more of their base pairs), as well as the 875 sites that have probes overlapping with SNPs reported in dbSNP, which may influence the methylation levels reported by the microarray. We separated out these nonspecific and polymorphic sites after all analyses to aid in interpretation of results. Total number of 22,927 DNAm sites are tested for their associations with serum level of CRP in this study.
We implemented an internal replication design [27,28] to validate our findings of CRP-associated DNA methylation. We took advantage of the sibship-based design of GENOA study and created two datasets, each with 393 unrelated individuals, to test for replication of epigenetic associations with CRP in study groups with similar genetic and environmental backgrounds. We randomly sampled one sibling from each sibship with at least two sibs (n=294) without replacement to create the first dataset (referred to here as Dataset 1). From the remaining participants, we randomly sampled a second sibling from each sibship with at least two sibs to establish the second dataset (referred to here as Dataset 2). The same number of singletons (total n=198) was randomly assigned to each dataset. Therefore, the subjects within each dataset (n=393) were unrelated to each other. Within each of Dataset 1 and Dataset 2, we conducted the multiple linear regression analysis of lnCRP adjusted for the same set of covariates as in the analysis of the pooled 966 samples.
Table 1. Summary of demographic variables.
| Females (N=685, 70.9%) | Males (N=281, 29.1%) | |
|---|---|---|
| Mean±SD | Mean±SD | |
| Age (yr.) | 66.10±7.56 | 66.70±7.64 |
| BMI* (kg/m2) | 32.06±6.58 | 28.98±4.81 |
| SBP (mm Hg) | 140.88±21.62 | 138.05±20.75 |
| DBP* (mm Hg) | 77.52±10.83 | 80.39±11.06 |
| CRP* (mg/L), median (Q1, Q3) | 0.38 (0.19, 0.80) | 0.27 (0.12, 0.58) |
* statistically different between males and females (p-value < 0.05).
Table 2. Summary of the 30 most significant CRP-associated DNA methylation sites.
| DNAm | Gene | Chr. | Location* (bp) | Strand | Beta (SE) | P-value | P-value2** | ds1 P-value | ds2 P-value |
|---|---|---|---|---|---|---|---|---|---|
| cg07073964 | KLK10 | 19 | 649371 | - | -4.12 (0.58) | 5.85×10-12 | 4.43×10-12 | 3.51×10-3 | 1.81×10-6 |
| cg09358725 | LMO2 | 11 | 33870664 | - | -3.60 (0.52) | 1.69×10-11 | 2.21×10-11 | 1.83×10-4 | 1.80×10-5 |
| cg04121771 | TM4SF4 | 3 | 150674314 | + | -4.42 (0.68) | 2.05×10-10 | 6.88×10-11 | 2.25×10-4 | 1.46×10-4 |
| cg08458487 | SFTPD | 10 | 81699171 | - | -2.79 (0.43) | 2.26×10-10 | 3.07×10-10 | 4.09×10-4 | 3.48×10-5 |
| cg09305224 | FUT7 | 9 | 139047066 | - | -3.38 (0.52) | 2.48×10-10 | 2.76×10-10 | 6.95×10-4 | 3.82×10-5 |
| cg00645579 | IRF7 | 11 | 607140 | - | -3.80 (0.59) | 2.94×10-10 | 2.85×10-10 | 8.55×10-4 | 7.06×10-6 |
| cg05556717 | CCL26 | 7 | 75257240 | - | -3.32 (0.52) | 3.94×10-10 | 3.12×10-10 | 2.13×10-4 | 8.39×10-7 |
| cg17496921 | TSPAN16 | 19 | 11267993 | + | -2.94 (0.46) | 4.97×10-10 | 4.78×10-10 | 5.68×10-4 | 1.12×10-4 |
| cg03801286 | KCNE1 | 21 | 34806378 | - | -2.62 (0.41) | 5.61×10-10 | 5.74×10-10 | 2.52×10-4 | 2.34×10-4 |
| cg21969640 | GPR84 | 12 | 53043844 | - | -3.08 (0.49) | 6.03×10-10 | 6.28×10-10 | 2.69×10-4 | 4.88×10-5 |
| cg05501357 | HIPK3 | 11 | 33264845 | + | -3.35 (0.53) | 6.29×10-10 | 7.41×10-10 | 8.58×10-4 | 1.83×10-4 |
| cg03600318 | SFTPD | 10 | 81698971 | - | -3.58 (0.57) | 7.01×10-10 | 6.80×10-10 | 1.65×10-4 | 1.99×10-3 |
| cg18084554 | ARID3A | 19 | 880046 | + | -2.68 (0.43) | 7.91×10-10 | 1.03×10-9 | 1.19×10-4 | 7.68×10-5 |
| cg06625767 | F12 | 5 | 176769301 | - | -2.80 (0.45) | 1.04×10-9 | 1.07×10-9 | 3.82×10-3 | 3.38×10-5 |
| cg15248035 | CCIN | 9 | 36159949 | + | -2.62 (0.42) | 1.22×10-9 | 1.36×10-9 | 7.39×10-4 | 1.79×10-4 |
| cg05546038 | NOL3 | 16 | 65764534 | + | -3.96 (0.64) | 1.40×10-9 | 1.58×10-9 | 2.21×10-5 | 2.23×10-3 |
| cg09303642 | NFE2 | 12 | 52977085 | - | -2.70 (0.44) | 1.60×10-9 | 2.28×10-9 | 1.94×10-4 | 1.01×10-4 |
| cg03330678 | SEPT9 | 17 | 72827828 | + | -2.62 (0.43) | 1.69×10-9 | 2.27×10-9 | 1.62×10-3 | 3.57×10-5 |
| cg17753124 | IER2 | 19 | 13120872 | + | -3.34 (0.54) | 1.72×10-9 | 1.31×10-9 | 1.59×10-3 | 3.50×10-4 |
| cg22242539 | SERPINF1 | 17 | 1611970 | + | -3.25 (0.53) | 2.08×10-9 | 1.86×10-9 | 2.31×10-4 | 8.51×10-4 |
| cg17166812 | NDUFS2 | 1 | 159436198 | + | -3.89 (0.64) | 2.28×10-9 | 2.30×10-9 | 4.44×10-3 | 1.82×10-4 |
| cg22266967 | S100P | 4 | 6746599 | + | -2.84 (0.47) | 2.29×10-9 | 2.39×10-9 | 2.58×10-3 | 6.12×10-5 |
| cg12380764 | IL19 | 1 | 205037818 | + | -2.93 (0.48) | 2.35×10-9 | 2.93×10-9 | 2.38×10-4 | 4.40×10-4 |
| cg10275770 | ICAM2 | 17 | 59437937 | - | -3.64 (0.60) | 2.51×10-9 | 2.21×10-9 | 5.35×10-4 | 1.06×10-3 |
| cg21492378 | CEP1 | 9 | 122890100 | + | -3.68 (0.60) | 2.53×10-9 | 2.08×10-9 | 1.29×10-4 | 9.80×10-6 |
| cg22381196 | DHODH | 16 | 70598877 | + | -2.24 (0.37) | 2.99×10-9 | 3.53×10-9 | 3.67×10-4 | 7.47×10-4 |
| cg23140706 | NFE2 | 12 | 52975545 | - | -4.22 (0.70) | 2.99×10-9 | 3.90×10-9 | 6.65×10-4 | 1.32×10-3 |
| cg20283107 | FAM91A1 | 8 | 124858150 | + | -3.65 (0.60) | 3.12×10-9 | 3.57×10-9 | 2.48×10-4 | 4.31×10-6 |
| cg27606341 | FYB | 5 | 39255389 | - | -2.55 (0.42) | 3.21×10-9 | 3.31×10-9 | 1.67×10-4 | 8.62×10-4 |
| cg26861460 | PARVG | 22 | 42906788 | + | -2.90 (0.48) | 3.27×10-9 | 3.42×10-9 | 3.68×10-4 | 1.29×10-4 |
* Chromosomal location is based on NCBI build 36.1.
** P-value2 was from the sensitivity analysis adjusted for age, gender, BMI, current smoking and hypertension status.
An internal replication was conducted by randomly splitting 966 samples into two mutually exclusive subsets (see details in the Methods), ds1 and ds2, each with 393 unrelated individuals. The beta coefficients and p-values were calculated using linear regression models for each subset.
Statistical Methods
Serum CRP level was transformed with the natural logarithm (ln) because of its skewed distribution. We forced age and sex in the multivariable regression models and identified body mass index (BMI) as significantly associated with lnCRP. LnCRP values were adjusted for age, sex, BMI and cigarette smoking (current smoker) in a multivariable regression model to identify CRP-associated DNAm sites in the association analyses. The “current smoker” was defined as a binary variable indicating if an individual had smoked within the past year or not. To identify sex-specific epigenetic associations with serum CRP, we evaluated the sex-DNAm interaction term in the multivariable-adjusted model for each DNAm site significantly associated with CRP level.
Based on chromosomal location (NCBI 36.1), we identified CRP-associated genes on human autosomes. Bonferroni corrected p-value of 0.05 (nominal p-value of 2.18×10-6) was applied to adjust for multiple testing of 22,927 autosomal DNAm sites. We used gProfile [29] to estimate the enrichment of Gene Ontology (GO) terms and identify the over-represented GO terms linked to the CRP-associated genes.
Linear mixed models were implemented in a multiple regression framework to adjust for the relatedness of the studied individuals. The sibship relationship was modeled as a random effect using the sibship identifiers. All statistical analyses were performed with R statistical environment version 2.11.1 from R Project (http://www.r-project.org/). The authors had full access to the data and take responsibility for its integrity.
Results
Table 1 summarizes the key phenotypes of the GENOA AA participants. The final analysis dataset included 966 AA participants from 492 sibships. This AA sample had various sizes of sibships with up to 10 siblings per sibship, with average sibship size of 1.96 siblings. About one fifth of the participants were singletons without any relatives included in the sample. Within the sample, 80.1% of males and 83.5% of females were diagnosed as having hypertension at the time of visit. Females had significantly higher BMI than males in this study. Serum CRP levels for female participants (median of 0.38 mg/L) were significantly higher than that of male participants (median of 0.27 mg/L).
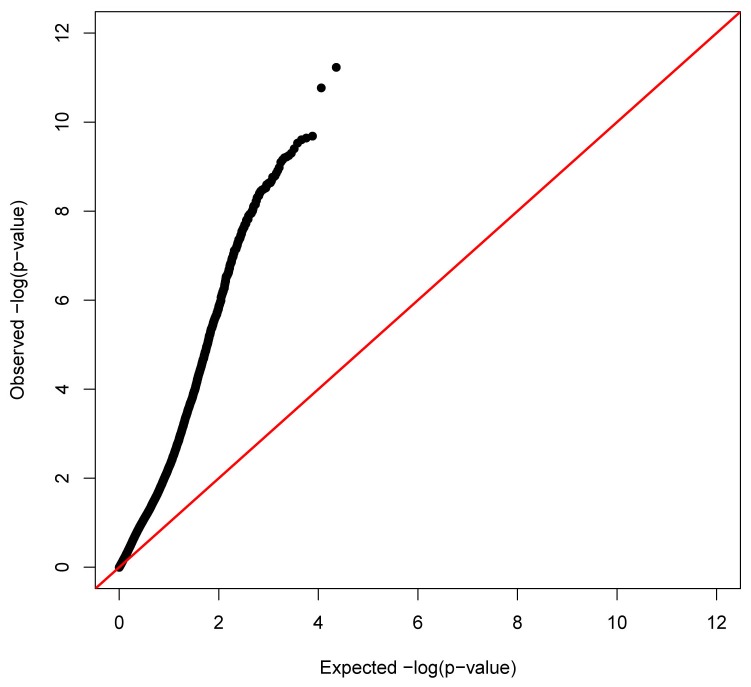
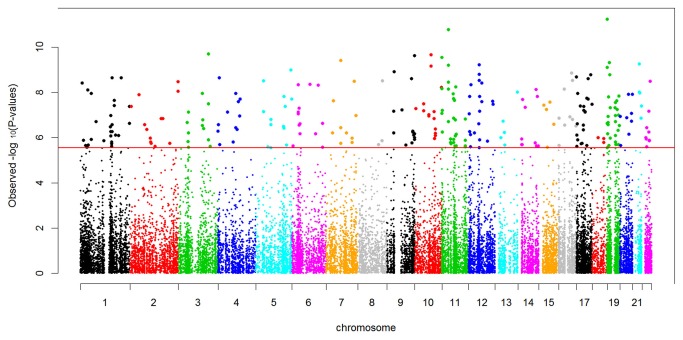
Using stringent Bonferroni correction for multiple testing in the analyses of linear mixed models to identify CRP-associated DNAm sites influencing natural log-transformed CRP (lnCRP), we identified 257 autosomal DNAm sites significantly associated with serum CRP levels (Table S1). The distribution of p-values was substantially inflated as shown in Figure 1 (Quantile-quantile plot). These DNAm sites mapped to 240 human genes across all 22 autosomes (Figure 2). Among 257 CRP-associated DNAm sites, 80.5% (207 out of 257 sites) exhibited an inverse correlation of greater methylation with lower level of CRP. Among the thirty most significant DNAm associations with CRP (Table 2), all DNAm sites exhibited a negative correlation of greater methylation with lower level of CRP. Using the two subsets each with 393 unrelated individuals, we confirmed that the top 30 associations (Table 2) have significant associations at the nominal p-value threshold correcting for 30 tests (1.67×10-3) in at least one subset (24 out of 30 have the significant p-values in both subset). By testing the sex-DNAm interaction term in a multiple regression model for each DNAm site, the most significant test had a p-value of 1.34×10-4. After correction of multiple testing, none of the interactions between sex and DNAm had statistically significant effects on serum CRP level.
Figure 1. Quantile-quantile plot of DNAm association with logCRP.
Figure 2. Manhattan plot of methylome-wide association with log CRP in 966 African Americans, adjusted for age, gender, BMI and cigarette smoking.
There were 240 unique genes annotated to the 257 CRP-associated DNAm sites. By searching the Gene Ontology (GO) database of homo sapiens through g: Profiler [29], we found five GO terms most over-represented among these genes with p-value less than 5×10-6 (Table 3). All five over-represented GO terms belonged to the Biological Process of “immune system process” or “response to stimulus”. There were 61 genes annotated to the GO category of “immune system process” and 126 genes annotated to “response to stimulus” (listed in Table S2).
Table 3. Over-represented GO terms for genes with CRP-associated DNAm sites.
| GO Term ID | GO term name | GO Domain* | Number of Genes | p-value |
|---|---|---|---|---|
| GO:0002376 | immune system process | BP | 61 | 1.09×10-12 |
| GO:0050896 | response to stimulus | BP | 126 | 1.84×10-9 |
| GO:0006950 | response to stress | BP | 68 | 6.28×10-7 |
| GO:0006952 | defense response | BP | 39 | 2.74×10-6 |
| GO:0006955 | immune response | BP | 46 | 1.16×10-10 |
* BP: Biological Process
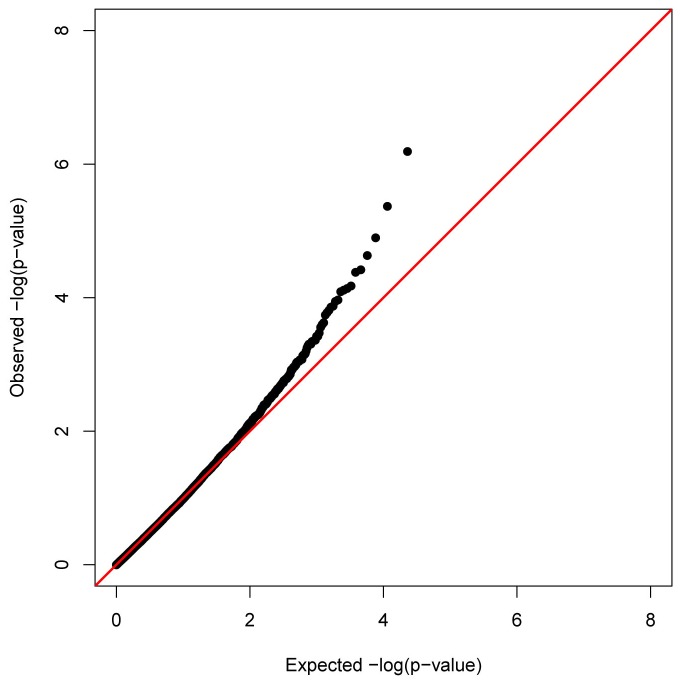
We applied a principal component analysis (PCA) to adjust for the inflation due to unmeasured confounders [30]. Using the top three principal components of the DNAm data, the inflation of the low p-values was well controlled with an inflation factor of 0.95 (Figure 3). Two DNAm sites, cg05316065 (MLZE) and cg27637521 (SOCS3), remained genome-wide significant with FDR q-values of 0.015 (nominal p-value of 6.44×10-7) and 0.049 (nominal p-value of 4.27×10-6) respectively. The most significant CRP-associated DNAm site for the analysis without PCA adjustment, cg07073964 (KLK10), was the fourth most significant DNAm site, with FDR q-value of 0.133 (nominal p-value of 2.33×10-5).
Figure 3. Quantile-quantile plot of DNAm association with lnCRP adjusted for top three principal components of the DNAm data.
Discussion
Using a large sample of AAs, we identified widespread CRP-associated DNAm sites across all 22 autosomes. These DNAm sites are mapped to a set of genes highly enriched for cellular defense mechanisms, immune responses and stress responses (Table 3), which are the key functions of leukocytes in the blood and are related to inflammatory responses.
The most significant CRP-associated DNAm (cg07073964) is located close to kallikrein-related peptidase 10 (KLK10) gene on chromosome 19. KLK10 encodes for a secreted protein, and is expression in many human tissues [31]. KLK10 may function as a tumor suppressor gene. The expression of KLK10 is down-regulated in several cancers (e.g. breast, prostate and non-small cell lung cancer, and hepatocellular carcinoma), and up-regulated in colorectal cancer, ovarian cancer, pancreatic ductal adenocarcinoma and uterine papillary serous carcinoma. The level of DNA methylation of KLK10 gene is reversely associated with the gene expression levels in human tissues [32,33]. In a recent study, expression of Klk10 was down-regulated in mouse carotid artery after a partial ligation which rapidly induces atherosclerosis [34]. In the same study, Lmo4 gene showed higher expression in mouse aortic arch after ligation, and in human coronary endothelium exposed to oscillatory shear [34]. The second most significant CRP-associated DNAm (cg09358725) is located on the promoter of LIM domain only 2 (LMO2) gene on chromosome 11. Another DNAm site (cg11822932) of LMO2 gene was also strongly associated with CRP levels (ranking 44th, Bonferroni corrected p-value of 1.65×10-4). The LMO2 protein is a highly conserved transcription factor and has a crucial role in early stage of hematopoietic development [35]. All cellular blood components including leukocytes are derived from hematopoietic stem cells. The modification of LMO2 expression level may have broad impact on the cellular functions of the blood cells.
There are several gene families related to the immune system that are enriched in the gene set of CRP-associated DNAm. Six immunorecepter (CD) genes, CD1D, CD7, CD22, CD27, CD59 and CD82, and five interleukin and receptor genes, IL1R2, IL2RΑ, IL19, IL21R, IL32 were identified by the epigenetic association analysis. The methylation sites in five G-protein coupled receptor (GPR) genes, GPR21, GPR65, GPR81, GPR84 and GPR171, were also found to be associated with CRP. The GPR gene family encodes receptors spanning the cellular membrane for signal transduction. GPRs play important roles in vision, smell, immune systems, and the autonomic nervous system, and are major drug targets of numerous human diseases [36].
To further understand the potential functional impact, we calculated the range of methylation difference of the most significant CRP-related CpG site, cg07073964 (KLK10). In the lowest quartile of serum level of CRP, the mean beta-value is 0.52, 6% higher than that in the highest quartile of CRP levels (mean beta-value of 0.49). Without the direct measurement of gene expression levels, we could not assess the impact of the methylation difference of cg07073964 on the transcription of KLK10. Due to the limited amount of change in DNA methylation, the functional consequence of the individual CRP-associated DNAm might not be substantial. However, the combination of a number of DNAm sites from multiple related pathways may have a much larger impact on the molecular function.
Despite the discovery that DNAm measured by LINE-1 repetitive elements, a proxy for global DNAm measurements, in PBLs of 593 elderly white males were not found to be significantly associated with CRP levels [37], DNAm has been associated with CRP levels at specific sites. Uddin et al. found a significant inverse correlation between methylation of the IL6 gene, involved in the inflammatory response, and serum levels of CRP in 33 individuals with a lifetime history of depression and 67 non-depressed controls [38]. Two DNAm sites located close to the IL6 gene, cg15703690 and cg01770232, were measured in our sample of AAs. Neither of these sites showed significant association with serum levels of CRP after correction of multiple testing. However, cg15703690 was inversely correlated with CRP levels with a nominal p-value of 0.0017 after adjustment for age, sex, BMI and smoking status.
Nearly 80% of the significant gene-specific DNAm sites exhibited an inverse correlation between hypomethylation and higher levels of CRP in our sample. Interestingly, we observed a similar trend between gene-specific DNAm and age in this AA sample: most age-related DNAm sites are hypomethylated in older age (unpublished). Age-related hypomethylation has also been previously reported in other ethnic groups [39]. A similar pattern of modifications of DNAm on CpG islands between chronic aging and inflammatory markers may indicate shared molecular mechanisms underlying chronic diseases through epigenetic changes.
The GENOA participants were enriched for hypertensives. Thus, this study’sAA sample is a high-risk cohort for cardiovascular diseases. The epigenetic associations with CRP identified in this study may not be generalizable to the non-diseased population. However, our epigenome-wide association study (EWAS) may greatly contribute toward the understanding of the disease etiology among AAs, who have the highest prevalence of hypertension among racial groups in the U.S. and are under-represented in genetic and epigenetic research. To address the potential impact of hypertension, we adjusted for hypertension status, in addition to age, gender, BMI and cigarette smoking in a sensitivity analysis. The test statistics of the DNAm sites between the primary model and the model adjusted for hypertension were consistent (R2=0.998), and the p-values of the top 30 sites were similarly significant between the two models (table 2). These results suggest that hypertension status did not affect the findings of the CRP-associated DNAm in the primary analysis.
Significant findings in epigenetic association studies should be validated in replicate samples as recommended for genetic association studies [40] in order to prevent false-positive results which can be caused by the variation in the biosample/experimental procedure [41] or analytical bias [42]. In this study of epigenetic associations with serum levels of CRP, we were not able to replicate the significant associations with CRP because we could not locate an independent sample with measurements of both CRP and methylome. Instead, we capitalized on the sibship structure of our data to create two replicate sets of unrelated individuals (one sib in unrelated subset 1, second sib in unrelated subset 2) to perform replication analysis of significant epigenetic associations. Among the top 30 associations, the test statistics were consistent between the two subsets and were mostly significant. Although this internal replication partially addressed some issues that may cause false-positive findings, future studies on independent populations are needed to fully validate the findings of epigenetic associations with CRP. We also observed substantial inflation of low p-values in the primary EWAS analysis. Such inflation in EWAS can be caused by unmeasured confounders (e.g. batch effect) or wide-spread associations on the methylome (e.g. age-related DNAm). Because of the overall inflation, we might not have fully controlled the type-1 error. By adjusting for the top three PCs of the DNAm data and multiple testing in a secondary analysis, we were able to fully control the inflation and identified two significant CRP-associated DNAm sites. However, without knowing the true underlying distribution of the epigenome-wide association, we could have over-corrected for the inflation and potentially increased the type-II error.
DNAm profiles are tissue and cell type-specific [24,43]. DNA methylation profiles have been commonly studied in PBLs due to the easy access to the biosample. The choice of PBLs is meaningful to study certain environmental exposures such as smoking, and chronic conditions involving the circulation and immune system. However, since PBLs comprise a mixture of multiple cell types, it is possible that the results reported here and elsewhere reflect inflammation-related DNAm changes that influence a single cell type component of PBLs. Since different DNAm profiles have been observed in distinct leukocyte subtypes using dozens of samples [44,45], the association between DNA methylation and CRP can be confounded by differences in the proportion of leukocyte subtypes between samples. Therefore we cannot rule out the impact of shift of cell populations on DNAm. Future studies of epigenetic associations in a single targeted cell population would be valuable for identifying cell-type specific associations between DNAm and CRP and other inflammatory biomarkers.
In conclusion, we identified over two hundred genes containing CRP-associated DNAm sites. The results highlight immune response and other cellular response genes involved in the regulation of chronic inflammation. Furthermore, the epigenetic variants associated with CRP levels do not directly overlap with the genetic variants influencing CRP levels, but they are involved in common pathways and gene families related to inflammation. Although we observed strong gene-specific epigenetic associations with CRP levels, for each identified gene, the underlying molecular mechanisms related to inflammation are largely unknown. These epigenetic modifications can be the triggers or consequences of inflammatory responses. Future replication studies are warranted to confirm the association between the DNA methylation sites and serum level of CRP.
Supporting Information
Summary of DNA methylation sites significantly associated with serum levels of CRP.
(PDF)
List of CRP-associated genes annotated to two GO terms “immune system process” and “response to stimulus.
(XLS)
Acknowledgments
We appreciate technical assistance from Jodie L. Van de Rostyne, Pamela I. Hammond and the Mayo Advanced Genomics Technology Center.
Funding Statement
This work was supported by National Institutes of Health grant HL100185 and HL100245. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Hansson GK (2005) Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med 352: 1685-1695. doi:10.1056/NEJMra043430. PubMed: 15843671. [DOI] [PubMed] [Google Scholar]
- 2. Luc G, Bard JM, Juhan-Vague I, Ferrieres J, Evans A et al. (2003) C-reactive protein, interleukin-6, and fibrinogen as predictors of coronary heart disease: the PRIME Study. Arterioscler Thromb Vasc Biol 23: 1255-1261. doi:10.1161/01.ATV.0000079512.66448.1D. PubMed: 12775578. [DOI] [PubMed] [Google Scholar]
- 3. Ballestar E (2011) Epigenetic alterations in autoimmune rheumatic diseases. Nat Rev Rheumatol 7: 263-271. doi:10.1038/nrrheum.2011.16. PubMed: 21343899. [DOI] [PubMed] [Google Scholar]
- 4. Bell CG, Teschendorff AE, Rakyan VK, Maxwell AP, Beck S et al. (2010) Genome-wide DNA methylation analysis for diabetic nephropathy in type 1 diabetes mellitus. BMC Med Genomics 3: 33. doi:10.1186/1755-8794-3-33. PubMed: 20687937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Burrell AM, Handel AE, Ramagopalan SV, Ebers GC, Morahan JM (2011) Epigenetic mechanisms in multiple sclerosis and the major histocompatibility complex (MHC). Discov Med 11: 187-196. PubMed: 21447278. [PubMed] [Google Scholar]
- 6. Hage FG, Szalai AJ (2007) C-reactive protein gene polymorphisms, C-reactive protein blood levels, and cardiovascular disease risk. J Am Coll Cardiol 50: 1115-1122. doi:10.1016/j.jacc.2007.06.012. PubMed: 17868801. [DOI] [PubMed] [Google Scholar]
- 7. Chae CU, Lee RT, Rifai N, Ridker PM (2001) Blood pressure and inflammation in apparently healthy men. Hypertension 38: 399-403. doi:10.1161/01.HYP.38.3.399. PubMed: 11566912. [DOI] [PubMed] [Google Scholar]
- 8. Libby P, Ridker PM (2004) Inflammation and atherosclerosis: role of C-reactive protein in risk assessment. Am J Med 116 Suppl 6A: 9S-16S. doi:10.1016/j.amjmed.2004.02.006. PubMed: 15050187. [DOI] [PubMed] [Google Scholar]
- 9. Ridker PM (2003) Clinical application of C-reactive protein for cardiovascular disease detection and prevention. Circulation 107: 363-369. doi:10.1161/01.CIR.0000053730.47739.3C. PubMed: 12551853. [DOI] [PubMed] [Google Scholar]
- 10. Pankow JS, Folsom AR, Cushman M, Borecki IB, Hopkins PN et al. (2001) Familial and genetic determinants of systemic markers of inflammation: the NHLBI family heart study. Atherosclerosis 154: 681-689. doi:10.1016/S0021-9150(00)00586-4. PubMed: 11257270. [DOI] [PubMed] [Google Scholar]
- 11. Ridker PM, Pare G, Parker A, Zee RY, Danik JS et al. (2008) Loci related to metabolic-syndrome pathways including LEPR,HNF1A, IL6R, and GCKR associate with plasma C-reactive protein: the Women’s Genome Health Study. Am J Hum Genet 82: 1185-1192. doi:10.1016/j.ajhg.2008.03.015. PubMed: 18439548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Elliott P, Chambers JC, Zhang W, Clarke R, Hopewell JC et al. (2009) Genetic Loci associated with C-reactive protein levels and risk of coronary heart disease. JAMA 302: 37-48. doi:10.1001/jama.2009.954. PubMed: 19567438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dehghan A, Dupuis J, Barbalic M, Bis JC, Eiriksdottir G et al. (2011) Meta-analysis of genome-wide association studies in >80 000 subjects identifies multiple loci for C-reactive protein levels. Circulation 123: 731-738. doi:10.1161/CIRCULATIONAHA.110.948570. PubMed: 21300955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Reiner AP, Beleza S, Franceschini N, Auer PL, Robinson JG et al. (2012) Genome-wide association and population genetic analysis of C-reactive protein in African American and Hispanic American women. Am J Hum Genet 91: 502-512. doi:10.1016/j.ajhg.2012.07.023. PubMed: 22939635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kushner I, Rzewnicki D, Samols D (2006) What does minor elevation of C-reactive protein signify? Am J Med 119: 166: e117-e128. PubMed: 16443421. [DOI] [PubMed] [Google Scholar]
- 16. Breitling LP, Yang R, Korn B, Burwinkel B, Brenner H (2011) Tobacco-smoking-related differential DNA methylation: 27K discovery and replication. Am J Hum Genet 88: 450-457. doi:10.1016/j.ajhg.2011.03.003. PubMed: 21457905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shenker NS, Polidoro S, van Veldhoven K, Sacerdote C, Ricceri F et al. (2012) Epigenome-wide association study in the European Prospective Investigation into Cancer and Nutrition (EPIC-Turin) identifies novel genetic loci associated with smoking. Hum: Mol Genet. [DOI] [PubMed] [Google Scholar]
- 18. Sun YV, Smith AK, Conneely KN, Chang Q, Li W et al. (2013) Epigenomic association analysis identifies smoking-related DNA methylation sites in African Americans. Hum: Genet. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Collotta M, Bertazzi PA, Bollati V (2013) Epigenetics and pesticides. Toxicology 307: 35-41. doi:10.1016/j.tox.2013.01.017. PubMed: 23380243. [DOI] [PubMed] [Google Scholar]
- 20. Pogribny IP, Rusyn I (2013) Environmental toxicants, epigenetics, and cancer. Adv Exp Med Biol 754: 215-232. doi:10.1007/978-1-4419-9967-2_11. PubMed: 22956504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. The FBPP Investigators, Boerwinkle E, Brown CA, Carrejo M, Ferrell R et al. (2002) A Multi-Center Genetic Study of Hypertension: The Family Blood Pressure Program (FBPP). Hypertension 39: 3-9. doi:10.1161/hy1201.100415. PubMed: 11799070. [DOI] [PubMed] [Google Scholar]
- 22. Daniels PR, Kardia SL, Hanis CL, Brown CA, Hutchinson R et al. (2004) Familial aggregation of hypertension treatment and control in the Genetic Epidemiology Network of Arteriopathy (GENOA) study. Am J Med 116: 676-681. doi:10.1016/j.amjmed.2003.12.032. PubMed: 15121494. [DOI] [PubMed] [Google Scholar]
- 23. Kullo IJ, Seward JB, Bailey KR, Bielak LF, Grossardt BR et al. (2005) C-reactive protein is related to arterial wave reflection and stiffness in asymptomatic subjects from the community. Am J Hypertens 18: 1123-1129. doi:10.1016/j.amjhyper.2005.03.730. PubMed: 16109328. [DOI] [PubMed] [Google Scholar]
- 24. Sun YV, Turner ST, Smith JA, Hammond PI, Lazarus A et al. (2010) Comparison of the DNA methylation profiles of human peripheral blood cells and transformed B-lymphocytes. Hum Genet 127: 651-658. doi:10.1007/s00439-010-0810-y. PubMed: 20238126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hartigan JA, Hartigan PM (1985) The dip test of unimodality. Ann Statist 13: 70-84. doi:10.1214/aos/1176346577. [Google Scholar]
- 26. Chen YA, Choufani S, Ferreira JC, Grafodatskaya D, Butcher DT et al. (2011) Sequence overlap between autosomal and sex-linked probes on the Illumina HumanMethylation27 microarray. Genomics 97: 214-222. doi:10.1016/j.ygeno.2010.12.004. PubMed: 21211562. [DOI] [PubMed] [Google Scholar]
- 27. Sun YV, Bielak LF, Peyser PA, Turner ST, Sheedy PF 2nd et al. (2008) Application of machine learning algorithms to predict coronary artery calcification with a sibship-based design. Genet Epidemiol 32: 350-360. doi:10.1002/gepi.20309. PubMed: 18271057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Smith JA, Turner ST, Sun YV, Fornage M, Kelly RJ et al. (2009) Complexity in the genetic architecture of leukoaraiosis in hypertensive sibships from the GENOA Study. BMC Med Genomics 2: 16. doi:10.1186/1755-8794-2-16. PubMed: 19351393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Reimand J, Kull M, Peterson H, Hansen J, Vilo J (2007) g: Profiler--a web-based toolset for functional profiling of gene lists from large-scale experiments. Nucleic Acids Res 35: W193-W200. doi:10.1093/nar/gkl929. PubMed: 17478515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bell JT, Pai AA, Pickrell JK, Gaffney DJ, Pique-Regi R et al. (2011) DNA methylation patterns associate with genetic and gene expression variation in HapMap cell lines. Genome Biol 12: R10. doi:10.1186/gb-2011-12-s1-i10. PubMed: 21251332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Luo LY, Grass L, Howarth DJ, Thibault P, Ong H et al. (2001) Immunofluorometric assay of human kallikrein 10 and its identification in biological fluids and tissues. Clin Chem 47: 237-246. PubMed: 11159772. [PubMed] [Google Scholar]
- 32. Lu CY, Hsieh SY, Lu YJ, Wu CS, Chen LC et al. (2009) Aberrant DNA methylation profile and frequent methylation of KLK10 and OXGR1 genes in hepatocellular carcinoma. Genes Chromosomes Cancer 48: 1057-1068. doi:10.1002/gcc.20708. PubMed: 19760608. [DOI] [PubMed] [Google Scholar]
- 33. Talieri M, Alexopoulou DK, Scorilas A, Kypraios D, Arnogiannaki N et al. (2011) Expression analysis and clinical evaluation of kallikrein-related peptidase 10 (KLK10) in colorectal cancer. Tumour Biol 32: 737-744. doi:10.1007/s13277-011-0175-4. PubMed: 21487810. [DOI] [PubMed] [Google Scholar]
- 34. Ni CW, Qiu H, Rezvan A, Kwon K, Nam D et al. (2010) Discovery of novel mechanosensitive genes in vivo using mouse carotid artery endothelium exposed to disturbed flow. Blood 116: e66-e73. doi:10.1182/blood-2010-04-278192. PubMed: 20551377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yamada Y, Warren AJ, Dobson C, Forster A, Pannell R et al. (1998) The T cell leukemia LIM protein Lmo2 is necessary for adult mouse hematopoiesis. Proc Natl Acad Sci U S A 95: 3890-3895. doi:10.1073/pnas.95.7.3890. PubMed: 9520463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lagerström MC, Rabe N, Haitina T, Kalnina I, Hellström AR et al. (2007) The evolutionary history and tissue mapping of GPR123: specific CNS expression pattern predominantly in thalamic nuclei and regions containing large pyramidal cells. J Neurochem 100: 1129-1142. doi:10.1111/j.1471-4159.2006.04281.x. PubMed: 17212699. [DOI] [PubMed] [Google Scholar]
- 37. Baccarelli A, Tarantini L, Wright RO, Bollati V, Litonjua AA et al. (2010) Repetitive element DNA methylation and circulating endothelial and inflammation markers in the VA normative aging study. Epigenetics 5: ([MedlinePgn:]) PubMed: 20305373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Uddin M, Koenen KC, Aiello AE, Wildman DE, de los Santos R et al. (2011) Epigenetic and inflammatory marker profiles associated with depression in a community-based epidemiologic sample. Psychol Med 41: 997-1007. doi:10.1017/S0033291710001674. PubMed: 20836906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Teschendorff AE, Menon U, Gentry-Maharaj A, Ramus SJ, Weisenberger DJ et al. (2010) Age-dependent DNA methylation of genes that are suppressed in stem cells is a hallmark of cancer. Genome Res 20: 440-446. doi:10.1101/gr.103606.109. PubMed: 20219944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Studies N-NWGoRiA, Chanock SJ, Manolio T, Boehnke M, Boerwinkle E et al. (2007) Replicating genotype-phenotype associations. Nature 447: 655-660. doi:10.1038/447655a. PubMed: 17554299. [DOI] [PubMed] [Google Scholar]
- 41. Fiegler H, Redon R, Andrews D, Scott C, Andrews R et al. (2006) Accurate and reliable high-throughput detection of copy number variation in the human genome. Genome Res 16: 1566-1574. doi:10.1101/gr.5630906. PubMed: 17122085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ioannidis JP (2005) Why most published research findings are false. PLOS Med 2: e124. doi:10.1371/journal.pmed.0020124. PubMed: 16060722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Pai AA, Bell JT, Marioni JC, Pritchard JK, Gilad Y (2011) A genome-wide study of DNA methylation patterns and gene expression levels in multiple human and chimpanzee tissues. PLOS Genet 7: e1001316 PubMed: 21383968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Houseman EA, Accomando WP, Koestler DC, Christensen BC, Marsit CJ et al. (2012) DNA methylation arrays as surrogate measures of cell mixture distribution. BMC Bioinformatics 13: 86. doi:10.1186/1471-2105-13-86. PubMed: 22568884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Koestler DC, Marsit CJ, Christensen BC, Accomando W, Langevin SM et al. (2012) Peripheral blood immune cell methylation profiles are associated with nonhematopoietic cancers. Cancer Epidemiol Biomarkers Prev 21: 1293-1302. doi:10.1158/1055-9965.EPI-12-0361. PubMed: 22714737. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Summary of DNA methylation sites significantly associated with serum levels of CRP.
(PDF)
List of CRP-associated genes annotated to two GO terms “immune system process” and “response to stimulus.
(XLS)