Abstract
Background
The Republic of Congo has had no cases of wild poliovirus type 1 (WPV1) since 2000. In October 2010, a neurologist noted an abnormal number of cases of acute flaccid paralysis (AFP) among adults, which were later confirmed to be caused by WPV1.
Methods
Those presenting with AFP underwent clinical history, physical examination, and clinical specimen collection to determine if they had polio. AFP cases were classified as laboratory-confirmed, clinical, or nonpolio AFP. Epidemiologic features of the outbreak were analyzed.
Results
From 19 September 2010 to 22 January 2011, 445 cases of WPV1 were reported in the Republic of Congo; 390 cases were from Pointe Noire. Overall, 331 cases were among adults; 378 cases were clinically confirmed, and 64 cases were laboratory confirmed. The case-fatality ratio (CFR) was 43%. Epidemiologic characteristics differed among polio cases reported in Pointe Noire and cases reported in the rest of the Republic of Congo, including age distribution and CFR. The outbreak stopped after multiple vaccination rounds with oral poliovirus vaccine, which targeted the entire population.
Conclusions
This outbreak underscores the need to maintain high vaccination coverage to prevent outbreaks, the need to maintain timely high-quality surveillance to rapidly identify and respond to any potential cases before an outbreak escalates, and the need to perform ongoing risk assessments of immunity gaps in polio-free countries.
The Global Polio Eradication Initiative began in 1988 with the goal of interrupting all wild poliovirus (WPV) transmission. Progress continues, but poliomyelitis remains endemic in Nigeria, Afghanistan, and Pakistan [1]. In addition, outbreaks due to importations have occurred in countries where indigenous WPV transmission had been interrupted, such as in Tajikistan, Ivory Coast, and China in 2010–2011 [2–4]. In some countries that had achieved the goal of interrupting WPV transmission, such as Angola, Chad, and the Democratic Republic of Congo (DRC), importations have resulted in ongoing, reestablished transmission, creating the threat of WPV spread to other polio-free countries.
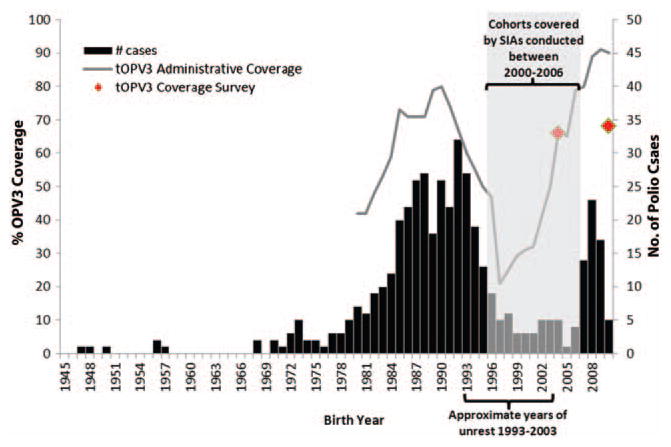
The Expanded Programme on Immunization began in the Republic of Congo (ROC) in 1981. However, civil unrest during 1993–2003, including a civil war in 1997–1999, disrupted routine immunization services for multiple birth cohorts. The recommended routine schedule includes 4 doses of trivalent oral polio vaccine (tOPV) administered at birth and at 6, 10, and 14 weeks of age. National reported routine immunization coverage for ≥3 doses of tOPV3 rose from 42% in 1980 to 90% in 2010; however, tOPV3 coverage dropped to 20% during the civil unrest in 1993–2003 (Figure 1) [5]. In 2010, administrative tOPV3 coverage of 90% was reported among 1-year-olds; however, a survey found tOPV3 coverage to be 68% [6]. Supplementary immunization activities (SIAs) targeting children aged <5 years were conducted starting in 2000, which reduced the number of susceptible children born from 1996 to 2006 (Figure 1). With these vaccination efforts, WPV transmission was interrupted; the last reported WPV type 1 (WPV1) case prior to 2010 was in 2000. However, ROC was at high risk for WPV importation and transmission because of low routine tOPV3 coverage among multiple birth cohorts not targeted by SIAs and active poliomyelitis outbreaks occurring in the bordering countries of DRC and Angola.
Figure 1.
Laboratory-confirmed and clinical poliomyelitis cases from the 2010–2011 outbreak and coverage with 3 doses of trivalent oral polio vaccine (tOPV3) by birth year, Republic of Congo. Data sources: tOPV3 administrative coverage is from the World Health Organization Joint Reporting Form [5]; coverage survey data is from the 2005 Demographic and Health Survey [20] and 2009 Expanded Program on Immunization Coverage Survey [6]. Abbreviations: OPV, oral polio vaccine; SIAs, supplementary immunization activities.
On 9 October 2010, a neurologist in Pointe Noire (PN), ROC, saw a 39-year-old man who reported 4 days of fever, headache, myalgias, dysphagia, dyspnea, and constipation, followed by acute, flaccid, rapidly progressing, asymmetric bilateral lower extremity paralysis. The patient’s vaccination history was unknown. The neurologist initiated treatment for suspected Guillain-Barre syndrome but the patient died the following day. During the following week, the neurologist noted an unusual number of adults presenting with acute flaccid paralysis (AFP) and alerted health officials. On 4 November 2010, WPV1 most closely genetically related to WPV1 circulating in Angola was isolated from a cerebrospinal fluid (CSF) specimen obtained from the patient [7], which prompted an extensive outbreak response by the ROC Ministry of Health, World Health Organization (WHO), the Centers for Disease Control and Prevention (CDC), United Nations Children’s Fund, and Médecin Sans Frontières. This report adds to preliminary accounts of the outbreak [7–9], providing a more complete description of the epidemiology and control efforts.
METHODS
Case Ascertainment
Surveillance for AFP to detect cases of paralytic poliomyelitis began in 1999 using the WHO-recommended poliomyelitis surveillance standard definition for an AFP case: sudden onset of flaccid paralysis in a child <15 years of age or suspected poliomyelitis in a person of any age [10]. Most persons with AFP during the outbreak were identified through a passive facility-based reporting system, but this was supplemented with active case finding by reviewing medical records at health facilities throughout ROC. All reported AFP cases were investigated by taking a clinical history from the patient (or their surrogate), conducting a physical examination, and collecting 2 stool samples for testing when possible. Alternative specimens, including serum, CSF, and rectal, throat, and nasopharyngeal swabs, were collected at the outset of the outbreak, before the etiology was established. Vaccination status was determined by vaccination card, where available, or by recall. For surviving persons from whom 2 adequate stool specimens (ie, collected within 14 days of onset and arriving in the laboratory in good condition) were not collected, clinical status was reevaluated at least 60 days after onset of paralysis.
Laboratory Testing
Specimens were tested at a WHO-accredited laboratory. WPV was isolated from stool specimens in RD and L20B cells in accordance with standard protocols [11] and characterized by real-time reverse-transcription polymerase chain reaction assays using enterovirus-specific and poliovirus group-, sero-type-, and Sabin strain-specific primer sets [12]. Testing of nonfecal specimens was conducted using a seminested amplification of viral RNA and sequencing of part of the VP1 region directly from clinical specimens as previously described [13]. WPV isolates were further characterized by sequencing of the complete VP1 coding region [14].
Case Classification
An AFP case was classified as laboratory-confirmed polio if poliovirus was isolated from any clinical specimen. Cases with inadequate stool specimens or alternative specimens from which WPV1 was not isolated were formally reviewed by the ROC National Polio Expert Committee (NPEC) for final classification based on clinical characteristics. An AFP case was classified by NPEC as clinically confirmed polio if it occurred in a person (1) with a strong likelihood of having poliomyelitis based on clinical presentation, (2) with residual paralysis at least 60 days after onset (when follow-up information was available), and (3) who lived in a province that had at least 1 laboratory-confirmed case. An AFP case was classified as clinically compatible polio if it occurred in a person who had a strong likelihood of having poliomyelitis and who lived in a province that did not have any laboratory-confirmed cases. For analysis, clinically confirmed or clinically compatible polio cases are grouped together and termed “clinical polio cases.” AFP cases classified as nonpoliomyelitis AFP were excluded from the outbreak analysis.
Data Management and Analysis
All data were stored in an EpiInfo version 3.5.1 database (CDC, Atlanta, Georgia) and analyzed using SAS software, version 9.2 (SAS Institute, Cary, North Carolina). Characteristics of the outbreak by case classification and location were evaluated as were the demographics, symptoms, and outcomes for cases. Age-group incidence rates were calculated. To assess associations between categorical variables, χ2 P values were calculated, except in cases where an expected cell count was <5, in which case 2-tailed Fisher exact P values were calculated. To assess differences in continuous variables, Wilcoxon rank-sum P values were calculated. The Cochran-Armitage test for trend was used to evaluate case-fatality ratios (CFRs) by age strata.
Ethics
As this was an outbreak, institutional review board approval was not needed to conduct this public health response.
RESULTS
Epidemiology of the Outbreak
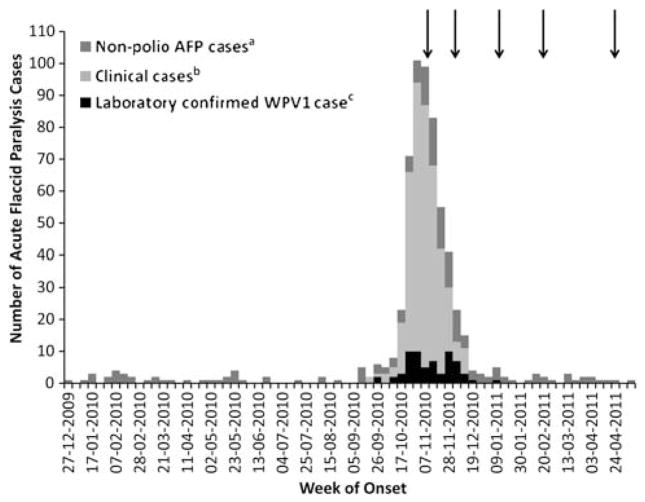
In ROC, 611 AFP cases were reported with onset from 1 January 2010 through 8 May 2011 (Figure 2). The first polio case had onset of paralysis on 19 September 2010 in PN. From 19 September 2010 through 22 January 2011, 445 polio cases were reported: 64 laboratory-confirmed, 378 clinically confirmed, and 3 clinically compatible (Figure 2). Genetically related cases were also reported from Gabon, DRC, and the bordering areas of Angola; this report focuses on cases in Congo.
Figure 2.
Classification of acute flaccid paralysis (AFP) cases by the National Poliomyelitis Expert Committee (NPEC) plotted by week of onset (n = 611). Arrows indicate start of an oral polio vaccine campaign. aNon-polio AFP case: patients with AFP with inadequate clinical specimens and who did not have symptoms consistent with poliomyelitis as determined by the NPEC. bClinical case: patients with AFP with inadequate clinical specimens with a strong likelihood of having poliomyelitis (ie, based on clinical presentation and residual paralysis at least 60 days after onset), as determined by the NPEC. This includes all clinically confirmed as well as clinically compatible cases. cLaboratory-confirmed WPV1 case: WPV1 isolated from clinical specimen.
The median age among all patients with laboratory-confirmed and clinical polio was 20 years (range, 0.6–63 years); the age-group incidence per 100 000 was 9 among 0–4-year-olds, 4 among 5–14-year-olds, 25 among 15–29-year-olds, and 4 among those aged ≥ 30 years. Among persons with polio, 265 (60%) were born from 1984 to 1995, and 54 (12%) were born from 2007 to 2009 (Figure 1). Two adequate stool specimens were collected for 54 (12%) cases. Vaccination status was missing for 348 (78%) of cases; among the 97 persons with vaccination history available, 54 (56%) were unvaccinated or undervaccinated (Table 1).
Table 1.
Characteristics of All Persons With Poliomyelitis, Persons Defined as Having Laboratory-Confirmed Poliomyelitis, and Persons Defined as Having Clinical Poliomyelitis, Republic of Congo (2010–2011)
| Characteristic | All Poliomyelitis (n = 445)
|
Laboratory-Confirmed (n = 64)
|
Clinical (n = 381)
|
χ2 P Value | |||
|---|---|---|---|---|---|---|---|
| No. Positive/Total | % | No. Positive/Total | % | No. Positive/Total | % | ||
| Male sex | 301/440 | 68 | 43/63 | 68 | 258/377 | 68 | .97 |
|
| |||||||
| Diagnosed in Pointe Noire | 390/445 | 88 | 39/64 | 61 | 351/381 | 92 | <.0001 |
|
| |||||||
| Clinical symptoms at time of presentation | |||||||
|
| |||||||
| Fever at onset | 322/341 | 94 | 48/52 | 92 | 274/289 | 95 | .47 |
|
| |||||||
| Progression of paralysis within 3 days | 314/331 | 95 | 50/52 | 96 | 264/279 | 95 | .65 |
|
| |||||||
| Asymmetrical paralysis | 116/342 | 34 | 19/51 | 37 | 97/291 | 33 | .59 |
|
| |||||||
| Limb paralysis | .002 | ||||||
|
| |||||||
| Monoplegia | 76/359 | 21 | 7/53 | 13 | 69/306 | 23 | |
|
| |||||||
| 2 limbs paralyzed | 212/359 | 59 | 26/53 | 49 | 186/306 | 61 | |
|
| |||||||
| Triplegia | 13/359 | 4 | 5/53 | 9 | 8/306 | 3 | |
|
| |||||||
| Quadriplegia | 58/359 | 16 | 15/53 | 28 | 43/306 | 14 | |
|
| |||||||
| Paralysis at >60 days after onset among survivors with follow-up | 91/99 | 92 | 21/22 | 95 | 70/77 | 91 | .68a |
|
| |||||||
| Death | 193/445 | 44 | 9/64 | 14 | 184/381 | 48 | <.0001 |
|
| |||||||
| Missing data on OPV vaccination history | 348/445 | 78 | 36/64 | 56 | 312/381 | 82 | <.0001 |
|
| |||||||
| OPV doses (by card or recall) | .03 | ||||||
|
| |||||||
| 0 | 29/97 | 30 | 13/28 | 46 | 16/69 | 23 | |
|
| |||||||
| 1–2 | 25/97 | 26 | 8/28 | 29 | 17/69 | 25 | |
|
| |||||||
| ≥3 | 43/97 | 44 | 7/28 | 25 | 36/69 | 52 | |
Abbreviation: OPV, oral polio vaccine.
Two-tailed Fisher exact P value.
Clinical signs and symptoms were similar among patients with laboratory-confirmed and clinical polio, except that a greater percentage of those with laboratory-confirmed polio had 3- or 4-limb paralysis (Table 1). Available data did not permit determination of whether bulbar paralysis was present.
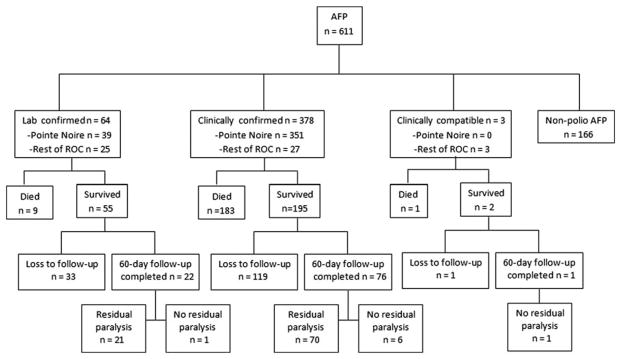
Of the 252 polio survivors, 60-day follow-up was completed for 99 (39%); 91 (92%) of these had residual paralysis (Figure 3). The overall CFR was 43% (193/445). The CFR increased with age: 29% (18/63) among 0–4-year-olds, 36% (16/45) among 5–14-year-olds, 47% (136/289) among 15–29-year-olds, and 52% (22/42) among persons aged ≥30 years (trend test = 0.0024).
Figure 3.
Clinical status of acute flaccid paralysis cases, including paralysis on 60-day follow-up, loss to follow-up, and death, Republic of Congo, 1 January 2010–8 May 2011. Abbreviations: AFP, acute flaccid paralysis; ROC, Republic of Congo.
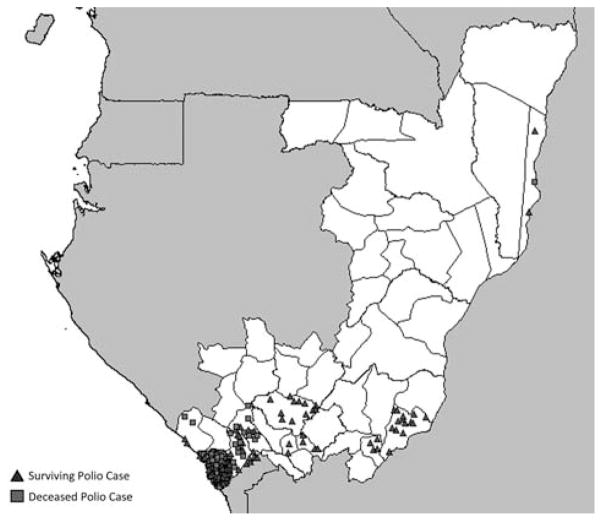
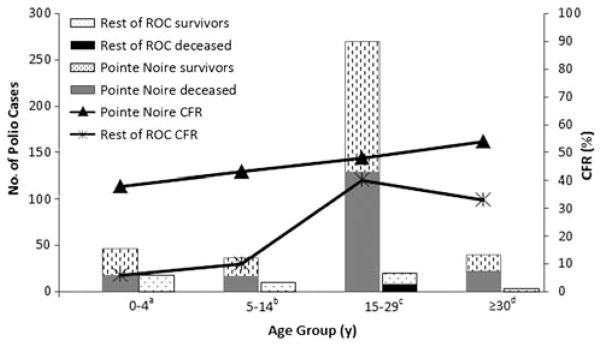
Overall, 390 (88%) cases were from PN (Figure 4) and differed epidemiologically from those in the rest of ROC (Table 2). In PN, the age-group incidence per 100 000 was 29 among 0–4-year-olds, 14 among 5–14-year-olds, 112 among 15–29-year-olds, and 17 among those aged ≥ 30 years. In the rest of ROC, this was 3 among 0–4-year-olds, 1 among 5–14-year-olds, 2 among 15–29-year-olds, and 0.3 among those aged ≥30 years. The CFR was 47% (182/390) among persons with polio reported in PN compared with 20% (11/55) among those in the rest of ROC (P = .0001) (Table 2); PN had a higher CFR in all age groups but was statistically significant only in the 0–4-year-old group (Figure 5). Persons with polio in PN and in the rest of ROC had similar clinical features including fever, asymmetrical paralysis, and 4-limb paralysis (data not shown).
Figure 4.
Laboratory-confirmed and clinical poliomyelitis cases by district of residence, Republic of Congo. Dots are placed randomly within a district and are not representative of the exact village of a given poliomyelitis case.
Table 2.
Comparison of Characteristics of Persons With Poliomyelitis in Pointe Noire Compared With Persons in the Rest of the Republic of Congo (2010–2011)
| Characteristic | Pointe Noire | Rest of Republic of Congo | χ2 P Value |
|---|---|---|---|
| No. of poliomyelitis cases | 390 | 55 | |
| No. of deaths | 182 | 11 | |
| Case-fatality ratio | 47% | 20% | .0002 |
| Median age, years (range) | 20 (0.6–63) | 9 (0.9–54) | <.0001a |
| Male sex | 69% | 65% | .61 |
| 2 stool samples collected within 14 days of onset arriving at the laboratory in good condition | 8% | 38% | <.0001 |
| Laboratory confirmed | 10% | 45% | <.0001 |
Wilcoxon rank-sum P value.
Figure 5.
Case-fatality ratio (CFR) among persons with poliomyelitis reported in Pointe Noire and in the rest of the Republic of Congo, stratified by age group, September 2010–January 2011. P values provided compare CFR by geographic location within each age group. aCFR χ2 P = .02. bCFR 2-tailed Fisher exact P = .07. cCFR χ2 P = .51. dCFR 2-tailed Fisher exact P = .60. Abbreviations: CFR, case-fatality ratio; ROC, Republic of Congo.
Outbreak Response
A national SIA was conducted 12–22 November 2010 using monovalent OPV against WPV1, followed by additional national SIAs in December, January, and February using bivalent OPV against WPV1 and WPV3 (bOPV). The first 3 national SIAs were synchronized with bordering regions of Angola and DRC. A subregional SIA targeting 7 districts in southern ROC, including Brazzaville and PN, was conducted in April using bOPV and was synchronized with Gabon, Angola, Namibia, and DRC. Because most cases were among adults, SIAs targeted the entire population of 4.5 million persons. To reach adults, normal SIA vaccination strategies needed to be adapted; in addition to vaccinating by visiting each house, vaccinators needed to visit businesses, farms, and restaurants.
DISCUSSION
This explosive poliomyelitis outbreak in ROC, centered in PN and originating from Angola, was unusual in its scope. In addition, the incidence of clinical polio was higher among young adults than among children, the CFR was much higher than reported in other WPV1 outbreaks, and the epidemiology of the outbreak varied based on residence. Also, in this outbreak, most cases were clinically confirmed rather than laboratory-confirmed, which is the global standard. Finally, in response to the right-shifted age distribution, the response effort had added challenges beyond those seen in outbreaks among younger age cohorts. A national campaign targeting the entire population had to be planned and executed quickly; the campaign’s effectiveness is apparent in the deceleration in cases soon after the SIAs started. The quick deceleration can be attributed to the fact that the entire population was targeted for vaccination, with added contribution from environmental secondary immunization through shed Sabin OPV strains.
Children usually comprise a majority of cases in poliomyelitis outbreaks; outbreaks among adults are unusual but have been described. In the 1996 WPV1 outbreak in Albania, 96 of 138 (70%) clinical and laboratory-confirmed cases were among persons aged ≥ 15 years [15]. The unusual age distribution in the Albanian outbreak was attributed to multiple factors. First, the adults were unlikely to be exposed to WPV1 and thus had little natural immunity; second, adults were recommended to receive only 2 doses of vaccine in infancy according to the country’s strategy. Finally, mismanagement of cold chain during the years that these adults were eligible for vaccination could have resulted in vaccination with nonimmunogenic vaccine. In a 2000 poliomyelitis outbreak in Namibia, >90% of 45 cases were among persons aged ≥15 years (N. Yusuf, personal written communication, February 2012); persons with poliomyelitis were suspected of being either unvaccinated or incompletely vaccinated due to civil conflict. Furthermore, these age cohorts had not been eligible for catch-up SIAs and were not exposed to circulating WPV.
In ROC, poor immunity is the likely reason that this outbreak affected predominantly 15–29-year-olds (birth years: 1981–1995). This cohort likely had little natural immunity due to low exposure to WPV1, as well as suboptimal vaccine-derived immunity due to disrupted routine services in the 1990s and ineligibility for the catch-up SIAs. The SIAs appear to have protected cohorts of children born from 1996 to 2006, accounting for the low incidence in 5–14-year-olds. The findings of a 2010 vaccine coverage survey conducted in one PN neighborhood are consistent with poor immunity in adults; 1-dose tOPV coverage was 87% among children aged <5 years, 74% among 5–14-year-olds, 50% among 15–29-year-olds, and 34% among adults aged ≥30 years [9]. Although there are limitations with these data in that they only cover one neighborhood in PN and much of the vaccination data are based on recall, they are consistent with an immunity gap in adults in PN.
Another unusual feature is that the mortality associated with this outbreak is much higher than the 5%–10% CFR typically seen in WPV1 outbreaks. Most polio outbreaks primarily affect children, and children are less likely to die as a result of WPV1 infection [16, 17]. However, even among children aged <15 years in ROC, the CFR was 32%. Furthermore, the CFR among those aged ≥15 years was 48%, and while a higher CFR is expected in adults, CFRs this high have not been observed in other recent outbreaks in low-income countries. In the 2010 WPV1 outbreak in Tajikistan, the CFR was 6% overall, with those aged ≥15 years having a CFR of 6% [3]. In a 1996 WPV1 outbreak in Albania, the CFR was 12% overall and 14% among adults [15]. An epidemiologic study of risk factors for death is presented separately [18].
The epidemiology differed between cases in PN and those from the rest of ROC. The outbreak spread broadly throughout the PN region, affecting older age groups. In the rest of ROC, fewer cases were reported, with most cases among children. One contributing factor could have been the higher population density in PN as well as the slum-like conditions in parts of the city, both of which can help to propagate the virus. However, this does not fully explain the burden in PN as Brazzaville is approximately 20 times as dense with similar slum-like conditions and only reported 13 cases of polio. Furthermore, children with polio in PN were more likely to die than those in the rest of ROC. Why the outbreak affected cases more severely in PN is unknown, although a variety of reasons were investigated. There were no significant genetic differences among the virus strains, including the Angolan parental virus, the viruses from different locations within ROC, and the related cases in neighboring countries; thus, viral differences were not driving the differential epidemiology. There was no evidence of a detection bias in PN with persons having less severe polio failing to seek care [19]. No yearly provincial immunization data were available to see if there was a larger immunity gap in PN than the rest of ROC.
A limitation of this investigation was that only 39% of survivors had follow-up; thus, the NPEC had to rely on presenting clinical data when classifying those without positive specimens. Additionally, a high number of cases had to be confirmed clinically due to the lack of specimens; adequate stool samples were collected from only 12% of persons with polio compared with the global benchmark of 80% [10]. Clinical confirmation is unreliable in areas where poliomyelitis is now rare; therefore, laboratory confirmation is the gold standard. Laboratory surveillance was challenging in PN, particularly at the peak of the outbreak when the 2 main hospitals in PN were caring for >200 paralyzed individuals, straining clinical and public health staff. However, because this was a confirmed WPV outbreak, the specificity of the definition increases. Because of the increased specificity and the similarities between the laboratory-confirmed and clinical cases, we are confident that the vast majority of clinical cases represent true WPV paralysis. A limitation of accepting the clinical case definition is that some cases might have been misclassified.
Another limitation of this investigation was surveillance. While country-wide active surveillance was conducted, surveillance was facility-based. However, once the outbreak was confirmed and vaccination campaigns commenced, media coverage stressed the need to visit health facilities, and medical care for polio was free, helping to decrease the impact of this surveillance bias. Additionally, surveillance only focused on paralytic polio, and it is estimated that for every case of paralytic polio there are 200 more cases that are nonparalytic and thus not reported. Finally, we could not obtain more details about the cases, especially among the deceased; we are unable to account for bulbar paralysis, living conditions, socioeconomic status, and vaccination history.
Several lessons from this outbreak are applicable to other countries. First, ROC had periods of civil conflict with low routine vaccination coverage which created age-specific immunity gaps. Although these gaps were mitigated to some extent through SIAs, older children were not vaccinated, leaving a vulnerable cohort. Health authorities in other polio-free countries, especially those with a history of armed conflict or other ruptures of immunization service delivery, should carefully evaluate polio immunity level by age cohorts and mitigate the risk for an outbreak both among young children and among older cohorts. Second, countries in close proximity to countries with active or recent polio circulation are at high risk for importation. ROC is adjacent to Angola and DRC, both of which had ongoing, reestablished transmission that should have led to the anticipation of an importation. Appropriate measures such as increased surveillance and vaccination of nonimmune populations should have been taken prophylactically. Third, a high index of suspicion for polio is required in the evaluation of all AFP cases, even among adults. Appropriate specimen collection is vital to rapidly identifying the first cases of poliomyelitis in previously polio-free countries. Delays in processing stool specimens from cases reported during September and October resulted in delayed identification of this outbreak until November, causing avoidable delays in response activities which prolonged and magnified this outbreak. Fourth, WPV transmission was stopped <6 months after onset of the first case with multiple coordinated SIAs targeting the entire population. Finally, routine immunization coverage needs to be strengthened dramatically in order to mitigate the risk of future importations.
Outbreaks such as the one in the ROC undermine confidence and drain scarce resources. At this critical juncture in polio eradication, preventing importations by increasing population immunity in vulnerable countries is imperative. Heightened vigilance for WPV importation is critical to ending an outbreak quickly.
Acknowledgments
The authors thank Professor George Moyen, Minister of Health, and all his staff for their leadership and mobilization of national resources for the rapid response to the epidemic. The authors are also grateful to Dr Yousouf Gamatie and Mrs Mariam Flach, the World Health Organization and United Nations Children’s Fund Representatives in the Republic of Congo, respectively, for supporting the technical coordination and mobilization of resources from the United Nations system; the Bill & Melinda Gates Foundation; Rotary Club International; and the European Union. We also thank Alexis Elira Dokekias, Pani Obengui, Cyriaque Ndjobomamadou, Geoffrey Koubemba, Ndamaba Bebene Bandzouzi, Aboubacar Ndiaye, Christopher Giles Wolff, Lydie Maoungou-Minguel, Samuel Okiror, Norbert Ngendekanyia, Brigitte Toure, Salvator Nibitinga, Jean Jacques Muyembe, and members of the National Polio Expert Committee for their assistance with this outbreak investigation and response.
Financial support. This work was supported by the Republic of Congo Ministry of Health; the World Health Organization; the US Centers for Disease Control and Prevention; United Nations Children’s Fund; Médecin Sans Frontières; the Bill & Melinda Gates Foundation; Rotary Club International; and the European Union.
Footnotes
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Centers for Disease Control and Prevention. Progress toward interruption of wild poliovirus transmission–worldwide, January 2010–March 2011. MMWR Morb Mortal Wkly Rep. 2011;60:582–6. [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. Progress toward interrupting wild poliovirus circulation in countries with reestablished transmission—Africa, 2009–2010. MMWR Morb Mortal Wkly Rep. 2011;60:306–11. [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. Outbreaks following wild poliovirus importations—Europe, Africa, and Asia, January 2009–September 2010. MMWR Morb Mortal Wkly Rep. 2010;59:1393–9. [PubMed] [Google Scholar]
- 4.Global Polio Eradication Initiative. [Accessed 1 November 2011];Wild poliovirus confirmed in China. 2011 Nov 1; Available at: http://www.polioeradication.org/tabid/408/iid/148/Default.aspx.
- 5.World Health Organization. [Accessed 8 July 2011];Immunization profile—Congo. 2011 Jun 1; Available at: http://apps.who.int/immunization_monitoring/en/globalsummary/countryprofileselect.cfm.
- 6.Republic of Congo Ministry of Health. Revue externe du programme elargi de vaccination au Congo. Brazzaville, Congo: 2010. [Google Scholar]
- 7.Grard G, Drexler JF, Lekana-Douki S, et al. Type 1 wild poliovirus and putative enterovirus 109 in an outbreak of acute flaccid paralysis in Congo October–November 2010. Euro Surveill. 2010;15 doi: 10.2807/ese.15.47.19723-en. pii=19723. [DOI] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention. Poliomyelitis outbreak—Republic of the Congo, September 2010–February 2011. MMWR Morb Mortal Wkly Rep. 2011;60:312–3. [PubMed] [Google Scholar]
- 9.Le Menach A, Llosa AE, Mouniaman-Nara I, et al. Poliomyelitis outbreak, Pointe-Noire, Republic of the Congo, September 2010–February 2011. Emerg Infect Dis. 2011;17:1506–9. doi: 10.3201/eid1708.110195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.World Health Organization. [Accessed 8 July 2011];WHO—recommended surveillance standard of poliomyelitis. Available at: http://www.who.int/immunization_monitoring/diseases/poliomyelitis_surveillance/en/index.html.
- 11.World Health Organization. Polio laboratory manual. 4. Geneva, Switzerland: World Health Organization; 2004. [Google Scholar]
- 12.Kilpatrick DR, Yang CF, Ching K, et al. Rapid group-, serotype-, and vaccine strain-specific identification of poliovirus isolates by real-time reverse transcription-PCR using degenerate primers and probes containing deoxyinosine residues. J Clin Microbiol. 2009;47:1939–41. doi: 10.1128/JCM.00702-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nix WA, Oberste MS, Pallansch MA. Sensitive, seminested PCR amplification of VP1 sequences for direct identification of all enterovirus serotypes from original clinical specimens. J Clin Microbiol. 2006;44:2698–704. doi: 10.1128/JCM.00542-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu HM, Zheng DP, Zhang LB, Oberste MS, Pallansch MA, Kew OM. Molecular evolution of a type 1 wild-vaccine poliovirus recombinant during widespread circulation in China. J Virol. 2000;74:11153–61. doi: 10.1128/jvi.74.23.11153-11161.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Prevots DR, Ciofi degli Atti ML, Sallabanda A, et al. Outbreak of paralytic poliomyelitis in Albania, 1996: high attack rate among adults and apparent interruption of transmission following nationwide mass vaccination. Clin Infect Dis. 1998;26:419–25. doi: 10.1086/516312. [DOI] [PubMed] [Google Scholar]
- 16.Weinstein L. Influence of age and sex on susceptibility and clinical manifestations in poliomyelitis. N Engl J Med. 1957;257:47–52. doi: 10.1056/NEJM195707112570201. [DOI] [PubMed] [Google Scholar]
- 17.Weinstein L, Shelokov A, Seltser R, Winchell GD. A comparison of the clinical features of poliomyelitis in adults and in children. N Engl J Med. 1952;246:297–302. doi: 10.1056/NEJM195202212460805. [DOI] [PubMed] [Google Scholar]
- 18.Gregory CJ, Ndiaye S, Patel M, et al. Investigation of elevated case-fatality rate in poliomyelitis outbreak in pointe noire, Republic of Congo - 2010 [published online ahead of print 21 August 2012] Clin Infect Dis. 2012;55:1299–306. doi: 10.1093/cid/cis715. [DOI] [PubMed] [Google Scholar]
- 19.Le Menach A, Llosa A. Epidémie de poliomyélite à Pointe-Noire; Congo. Octobre à Décembre 2010; Epicentre: European Center for Disease Control and Prevention; Health Protection Agency; 2010. [Google Scholar]
- 20.Centre National de la Statistique et des Études Économiques (CNSEE), ORC Macro. Enquête démographique et de Santé du Congo 2005. Calverton, Maryland: CNSEE et ORC Macro; 2006. [Google Scholar]