SUMMARY
Borrelia burgdorferi (the agent of Lyme disease) is unusual in that it contains free cholesterol and cholesterol glycolipids. It is also susceptible to complement-independent bactericidal antibodies, such as CB2, a monoclonal IgG1 against outer surface protein B (OspB). The bactericidal action of CB2 requires the presence of cholesterol glycolipids and cholesterol. Through ultrastructural, biochemical and biophysical approaches, we show that these cholesterol glycolipids exist as lipid raft-like microdomains in the outer membrane of cultured and mouse-derived B. burgdorferi, and in model membranes from B. burgdorferi lipids. The order and size of the microdomains of intact cells and model membranes are temperature sensitive and correlate with the bactericidal activity of CB2. Here we demonstrate the existence of cholesterol-containing lipid raft-like microdomains in a prokaryote.
Keywords: Cholesterol, lipid rafts, Borrelia, bactericidal antibody
INTRODUCTION
The Borrelia include the agents of Lyme disease (Benach et al., 1983; Burgdorfer et al., 1982) and relapsing fever (Johnson, 1977), and are extracellular spirochetes that are susceptible to the action of antibodies (Connolly and Benach, 2005; LaRocca and Benach, 2008). The antibody response is the primary means of host defense against the Borrelia and is linked to the pathogenesis of these infections. Antibodies were first used to confirm the spirochetal etiology of Lyme disease, and serology is critical for diagnosis of the disease.
Lytic complement is not required for clearance of Borrelia infections (Bockenstedt et al., 1993; Connolly and Benach, 2001; Newman and Johnson, 1981) and resistance to complement is mediated by the binding of complement regulator inhibitors (Bykowski et al., 2008). There are complement-independent antibodies to Borrelia that are bactericidal in vitro and in vivo. The monoclonal antibodies CB2 (IgG1) and H6831 (IgG2a) against the outer surface lipoprotein B (OspB) of B. burgdorferi and CB515 (IgM) and H4825 (IgG2a) against variable major proteins (Vmps) of relapsing fever Borrelia exhibit this unique bactericidal activity. (Coleman et al., 1992; Connolly et al., 2004; Sadziene et al., 1993). Monovalent Fab fragments and the variable regions alone had the same effect (Coleman et al., 1992; Sadziene et al., 1993, Larocca et al., 2009). Structural changes occur in OspB upon binding of CB2 or H6831 but could not explain the bactericidal mechanism (Becker et al., 2005; Katona et al., 2000).
Complement-deficient mice generated IgM antibodies coincident with the clearance of relapsing fever spirochetemia identical to clearance in wild type (WT) mice (Connolly and Benach, 2001; Connolly et al., 2004). B cell-deficient mice had a peak spirochetemia that was longer and higher than WT mice. These experiments demonstrated that complement-independent bactericidal antibodies function in vivo. CB2 creates pores that lead to osmotic lysis as part of its bactericidal mechanism (LaRocca et al., 2009). Ultrastructural studies showed that exposure to CB2 resulted in the creation of outer membrane projections and openings. Additionally, CB2 bound to E. coli expressing OspB was not bactericidal, indicating that factors specific to the Borrelia outer membrane are required for the bactericidal mechanism (LaRocca et al., 2009).
The outer membrane of Borrelia contains phosphatidylcholine and phosphatidylglycerol as well as numerous lipoproteins (Belisle et al., 1994; Brandt et al., 1990; Jones et al., 1995; Radolf et al., 1994; Radolf et al., 1995). Incorporation of cholesteryl glucoside into the membrane of B. hermsii (Livermore et al., 1978), prompted the demonstration that B. burgdorferi also contained antigenic glycolipids (Wheeler et al., 1993). Antibodies to these glycolipids crossreact with gangliosides and vice versa (Garcia Monco et al., 1993; Garcia-Monco et al., 1995).
B. burgdorferi has three glycolipids, two of which contain cholesterol. These glycolipids were identified as cholesteryl 6-O-acyl-β-D-galactopyranoside or cholesteryl 6-O-palmitoyl-β-D-galactopyranoside (ACGal/Bb-GL-I), cholesteryl-β-D-galacto-pyranoside (CGal), and mono-α-galactosyl-diacylglycerol (MGalD) and exist in other Borrelia species (Ben-Menachem et al., 2003; Schroder et al., 2003; Stubs et al., 2009). Free cholesterol and cholesterol esters also exist in the outer membrane of Borrelia (Stubs et al., 2009). The presence of cholesterol and cholesterol glycolipids in prokaryotes is unusual although there are some exceptions such as Helicobacter, Mycoplasma, Ehrlichia, Anaplasma, and Brachyspira (Haque et al., 1995; Hirai et al., 1995; Lin and Rikihisa, 2003; Smith, 1971; Trott et al., 2001),. In eukaryotic cell membranes, sterols help form microdomains called lipid rafts (Brown and London, 2000; London, 2002). Lipid rafts are ordered, rigid, cholesterol-rich areas of the membrane that also are rich in certain lipid-anchored proteins, notably, the glycosylphosphatidylinositol (GPI)-anchored proteins that have important roles in maintaining lateral heterogeneities in cell membranes and in biological sorting processes. Lipid rafts are important for receptor clustering and lateral sorting of proteins (Brown, 1998; Epand, 2008) as well as elasticity, endocytosis, exocytosis, and vesicle formation and budding (Chen and Rand, 1997; Huttner and Zimmerberg, 2001; Nichols, 2003; Salaun et al., 2004; Wang et al., 2000). The presence of cholesterol in the outer membrane of Borrelia suggests that lipid raft microdomains, similar to those in eukaryotes, may exist in these bacteria. If this is the case, lipid rafts may be important for lateral sorting and coalescence of certain Borrelia antigens and could influence membrane effects in response to CB2.
We show here that cholesterol and cholesterol glycolipids are critical for the bactericidal mechanism of the complement-independent MAb, CB2. We also show evidence for the existence of lipid raft microdomains in B. burgdorferi.
RESULTS
CB2 causes removal of antigens from the surface of B. burgdorferi in membrane vesicles
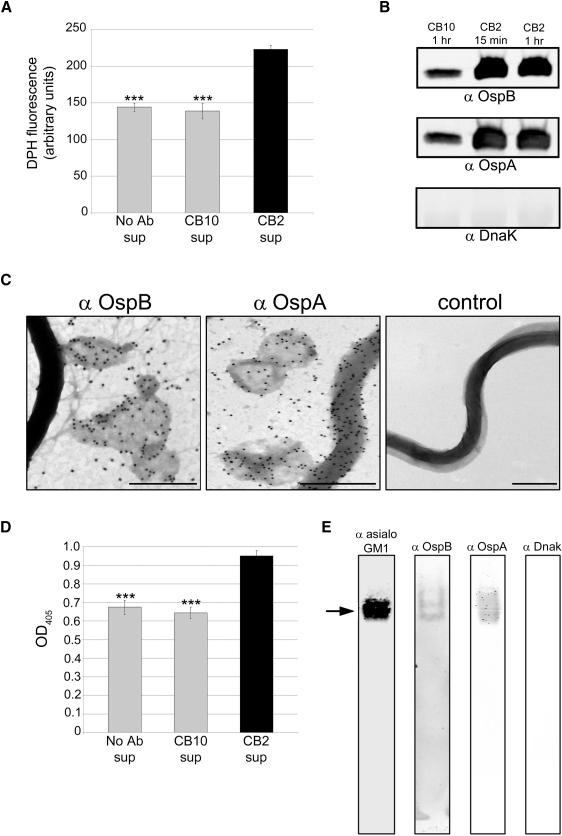
Previously, we showed that CB2 exerted its bactericidal effects by creating membrane projections and blebs on the surface of B. burgdorferi and proposed that vesicle removal formed the lytic pores (LaRocca et al., 2009). To determine whether vesicle removal is important in the bactericidal mechanism of CB2, we measured release of vesicles in Borrelia supernatants with the fluorescent, lipophilic probe 1,6-diphenyl-1,3,5-hexatriene (DPH) . DPH fluoresces in hydrophobic but not aqueous environments, and shows a linear response with membrane bilayer concentration (London and Feligenson, 1978). Spirochetes were treated with CB2, CB10, (a complement-dependent IgG1 against OspA used as a control), or a control without antibody in the presence of dextran T500 to prevent lysis (LaRocca et al., 2009). Greater release of vesicles from CB2-treated B. burgdorferi was observed relative to the controls that had the normal, constitutive level of membrane vesicle release (Figure 1A). Vesicle release was correlated with an increased release of OspB, and OspA, but not of cytosolic DnaK (Figure 1B). Negative-stain transmission electron microscopy (TEM) demonstrated removal of membrane vesicles that contained OspB (Figure 1C). The membrane vesicles also contained OspA, concordant with our earlier observations using confocal microscopy where we observed release of vesicles containing OspA as a later event in the bactericidal mechanism following release of vesicles containing OspB (Escudero et al., 1997). The late release of vesicles containing OspA may be due to the lack of membrane stability induced by the specific binding of CB2 to OspB.
Figure 1. CB2 removes antigens from the surface of B. burgdorferi in membrane vesicles.
A. CB2 treatment caused increased release of membrane vesicles from B. burgdorferi relative to treatment with CB10 (control IgG against OspA) or no antibody (Ab) as measured by DPH fluorescence in the cell-free supernatant (sup). B. Immunoblots showing that OspB and OspA are released into the supernatant upon CB2 treatment for 15 min or 1 h while the cytosolic DnaK is not. C. Negative-stain TEM images showing that CB2 causes removal of OspB and OspA (18 nm colloidal gold) from the spirochete surface in large and small membrane vesicles (dark gray around colloidal gold) after 15 min of exposure. Controls (secondary colloidal gold conjugate only) did not show labeling. Size bars = 500 nm. D. ELISA showing greater release of cholesterol glycolipids into the supernatant upon CB2 exposure relative to controls (see panel A). E. Native PAGE immunoblot of supernatants from spirochetes treated with CB2 for 30 min. Bands representing OspB, OspA, and cholesterol glycolipids (anti-asialo GM1) had the same mobility (arrow) indicating colocalization of these molecules in CB2-induced vesicles; cytosolic DnaK was not present in the supernatant. Results in A, B, D, and E are from triplicate experiments. ANOVA, *** p < 0.001.
The release of vesicles caused by CB2 suggested that cholesterol and cholesterol glycolipids could be constituents of these structures, and they were quantified in supernatants following CB2 treatment. Polyclonal rabbit anti-asialo GM1 cross-reacts with B. burgdorferi cholesterol glycolipids and was used to detect the cholesterol glycolipids in the vesicles induced by treatment with CB2 (Figure 1D). When these supernatants were resolved by electrophoresis on native gels and transferred to nitrocellulose, immunoblot reactivity to OspB, OspA and cholesterol glycolipids occurred at the same mobility, but not DnaK (Figure 1E) confirming the inclusion of the lipoproteins in the membrane vesicles, and also the results in Figure 1B and 1D.
Cholesterol is required for the bactericidal mechanism of CB2
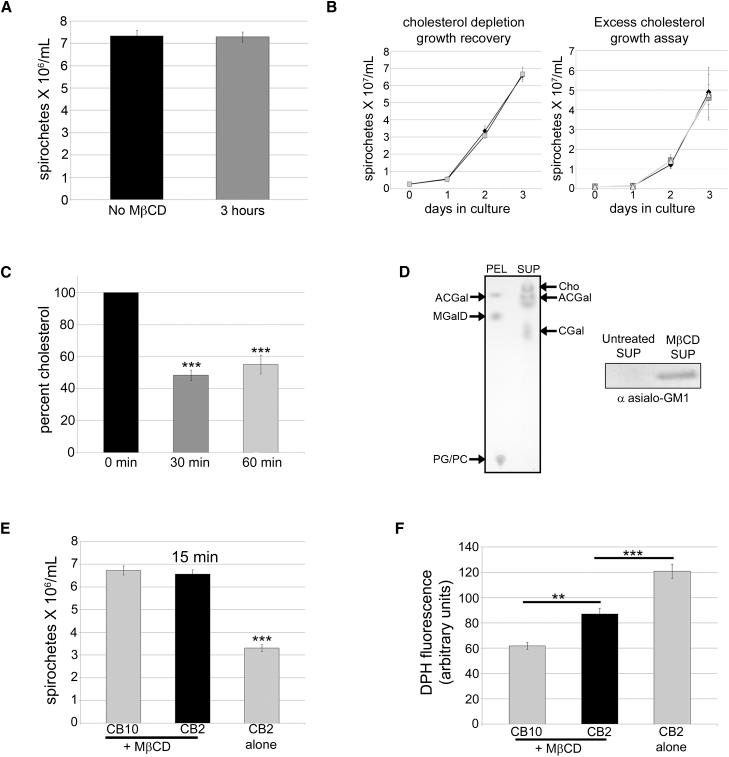
The possibility of a molecular interaction between OspB and cholesterol glycolipids led us to test whether the bactericidal mechanism of CB2 is dependent on cholesterol. Methyl-β-cyclodextrin (MβCD) has been used to deplete cholesterol in eukaryotic cells (Zidovetzki and Levitan, 2007) and bacteria (Lin and Rikihisa, 2003). Given the known toxicity of MβCD, we determined the experimental conditions for its use. There was no change in spirochete numbers after an MβCD exposure of 3 h (Figure 2A), and spirochetes exposed to 10 mM MβCD for 30 min grew normally once returned to normal medium (Figure 2B). Protein levels did not change in the pellet or supernatant following MβCD treatment for 1 h (Figure S1A). Furthermore, adding cholesterol was not toxic as exposure to 10 and 20 μg/ml of excess cholesterol did not affect spirochete growth (Figure 2B). These experiments show that there are no obvious pleiotropic effects of MβCD, or of excess cholesterol at the concentrations and incubation times used for the cholesterol-depletion experiments, and that the spirochetes remain viable (do not lyse). When B. burgdorferi were treated with 10 mM MβCD for 30 or 60 min, there was a 50% reduction in total cholesterol detected fluorometrically (Figure 2C). Lipid extracts from supernatants and pellets of MβCD-treated spirochetes were separated by thin-layer chromatography (TLC) and showed that MβCD removes free cholesterol, and the glycolipids ACGal/Bb-GL-I, and CGal from B. burgdorferi while not affecting MGalD or phospholipids (Figure 2D). Probing MβCD-treated supernatants with antibody to asialo GM1 in immunoblots confirmed that cholesterol glycolipids were removed from B. burgdorferi (Figure 2D). Lipid extracts were also analyzed by mass spectrometry, which showed that MβCD removed the two cholesterol glycolipids and confirmed the TLC findings (Table S1).
Figure 2. Cholesterol depletion affects the bactericidal mechanism of CB2 against B. burgdorferi. See also Table S1 and Figures S1 – S2.
A. 10 mM of MβCD is not toxic to B. burgdorferi after a 3 hr exposure. B. Left graph: B. burgdorferi grow normally following cholesterol depletion with MβCD for 30 min (black diamonds) compared to untreated spirochetes (gray squares). Right graph: there was no difference in the growth of B. burgdorferi with 10 (dark gray squares), or 20 μg/ml (light gray squares) of excess cholesterol; controls (black diamonds). C. 10 mM MβCD depletes cholesterol in B. burgdorferi by 50% after 30 or 60 min of treatment. D. Chloroform-methanol (85:15) TLC showing that MβCD removes cholesterol (Cho) and cholesterol glycolipids (ACGal, CGal) into the supernatant (SUP) while the MβCD-treated B. burgdorferi pellet (PEL) which contains ACGal, MGalD (cholesterol-free glycolipid), phosphatidyl choline (PC), and phosphatidyl glycerol (PG). Right: immunoblot of supernatants (sup) from control or MβCD-treated spirochetes probed with anti asialo GM1 confirming that MβCD removes the cholesterol glycolipids. E. Spirochetes were exposed to MβCD for cholesterol depletion and then treated with CB2 or the control IgG CB10 for 15 min F. DPH fluorescence vesicle assay of spirochete supernatants shows that depletion of cholesterol with MβCD reduces the number of vesicles released from B. burgdorferi upon CB2 exposure compared to spirochetes that have not been depleted of cholesterol. Spirochete counts were done by direct darkfield microscopy enumeration. Results for A, B, C, E, and F are from triplicate experiments. ANOVA, *** p < 0.001, ** p < 0.01.
To determine the role of cholesterol and cholesterol glycolipids in the bactericidal mechanism of CB2, spirochetes were pre-treated with MβCD for 30 min and exposed to the antibody. In contrast to the 50% decrease in organisms without MβCD treatment, after a 15 min incubation with CB2, there was no reduction in the number of organisms (Figure 2E). Spirochetes depleted of cholesterol exhibited decreased vesicle release after CB2 treatment compared to those treated with CB2 alone and CB10 (Figure 2F). The difference in vesicle release resulting from treatment with CB2 or CB10 may be due to the greater affinity of CB2 for OspB. The affinity of CB2 for recombinant OspB is three orders of magnitude greater (Kd = 1.1 nM) than the affinity of CB10 for recombinant OspA (Kd = 1.6 μM). Thus, membrane cholesterol greatly enhances the bactericidal action of CB2.
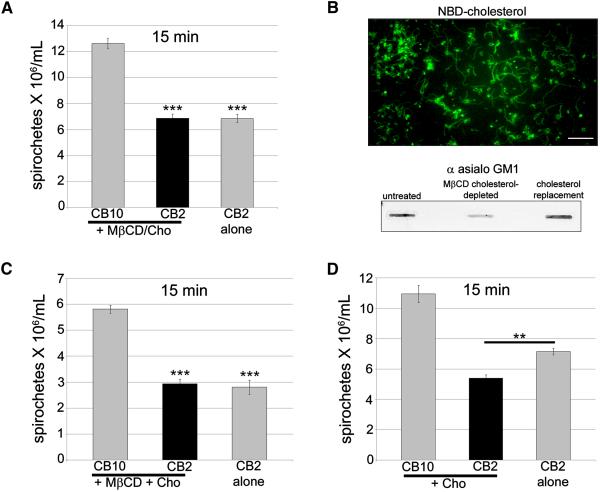
To show that the decreased bactericidal effect of CB2 was specific for cholesterol depletion, MβCD was mixed with 20 μg/ml of cholesterol prior to treatment of spirochetes followed by CB2 treatment. Spirochete numbers decreased to levels similar to treatment with CB2 alone (Figure 3A). We next assessed the bactericidal effect of CB2 when cholesterol was replaced following depletion. Cholesterol replacement was confirmed by incorporation of the fluorescent NBD-cholesterol into B. burgdorferi, following MβCD treatment (Figure 3B). Replacing cholesterol in spirochetes resulted in its incorporation into the cholesterol glycolipid fraction as detected with anti asialo GM1 in slot blots (Figure 3B). Cholesterol replacement also restored the bactericidal effect of CB2 (Figure 3C). Furthermore, addition of 20 μg/ml exogenous cholesterol during CB2 treatment enhanced the bactericidal effect of the antibody (Figure 3D). To determine if cholesterol replacement or deletion have an effect on CB2 binding to OspB, we performed surface labeling of OspB by indirect immunofluorescence of B. burgdorferi, and western blots quantitated by densitometry. Using both experimental approaches, we concluded that the binding of CB2 to OspB after cholesterol replacement or deletion does not change, indicating that the accessibility of OspB to the antibody remains the same (Figures S2A-C and S2B). These experiments show that membrane cholesterol is required for the bactericidal mechanism of CB2.
Figure 3. Cholesterol is required for the bactericidal mechanism of the complement-independent antibody, CB2.
A. Inhibition of 10 mM MβCD with 20 μg/ml of soluble cholesterol (+ MβCD/Cho) restored the bactericidal effect of CB2. B. Fluorescent spirochetes exposed to 10 mM MβCD and treated with cholesterol-NBD show that cholesterol has been re-incorporated into their membrane. Below: slot blot shows that cholesterol replacement results in its incorporation in the cholesterol glycolipids as detected by anti asialo GM1. C. CB2 regains its bactericidal effect against spirochetes that have had their cholesterol replaced following depletion (MβCD + Cho). D. Addition of excess cholesterol (20 μg/ml, + Cho) during CB2 treatment enhances the bactericidal effect of the antibody against spirochetes. Results for A, C, and D are from triplicate experiments. ANOVA, *** p < 0.001, ** p < 0.01.
The cholesterol glycolipids of B. burgdorferi are constituents of the membrane vesicles created by CB2
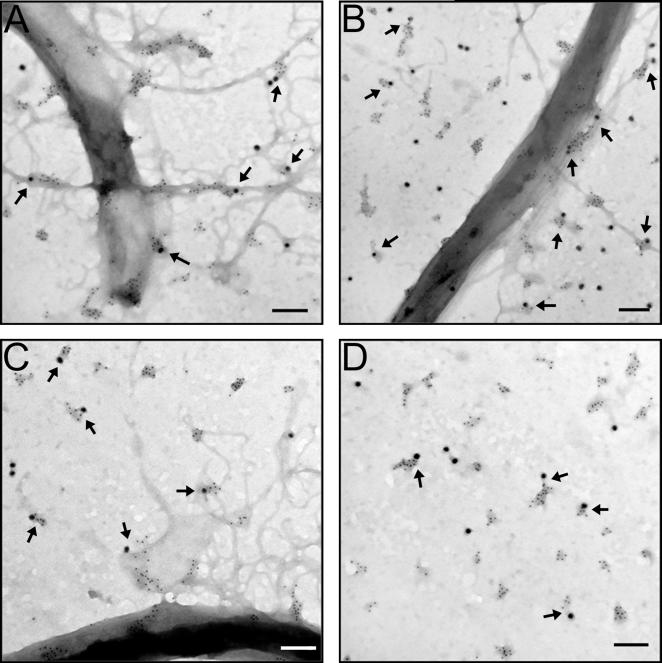
The localization of the cholesterol glycolipids in relation to OspB was characterized using transmission electron microscopy (TEM) following treatment of spirochetes with CB2. An anti-rabbit IgG conjugated to colloidal gold of 6 nm was used to detect anti-asialo GM1 bound to cholesterol glycolipids and an anti-mouse IgG conjugated to colloidal gold of 18 nm was used to detect CB2 bound to OspB (Figure 4). There was an abundance of cholesterol glycolipids in outer membrane projections created by CB2 (Figure 4A – C) and in released membrane vesicles containing OspB and bound CB2 (Figure 4A – D), demonstrating the presence of these lipids in the released structures. The cholesterol glycolipids formed distinct clusters or microdomains surrounding OspB in membrane vesicles and projections.
Figure 4. The cholesterol glycolipids of B. burgdorferi are constituents in the projections and vesicles induced by CB2. See also Figure S3. A – D.
Negative-stain TEM images of CB2-treated B. burgdorferi that were labeled with immunogold for CB2 bound to OspB (18 nm colloidal gold) and cholesterol glycolipids (6 nm colloidal gold). Cholesterol glycolipids are associated with projections (A – C) and vesicles (B – D) induced by CB2. Panel D specifically focuses on released membrane vesicles. Projections and released vesicles contain cholesterol glycolipids clustered around OspB (arrows) suggesting the existence of cholesterol-rich microdomains. Controls included secondary gold conjugates alone, and normal mouse and rabbit serum controls. Size bars = 100 nm unless otherwise indicated.
The cholesterol glycolipids of B. burgdorferi bind OspB
The clustering of cholesterol glycolipids around OspB in the outer membrane and in released vesicles (Figure 4) shows that these molecules associate. This suggests that OspB exists in microdomains in the outer membrane of B. burgdorferi. It is possible that OspB interacts with cholesterol glycolipid directly or via the interaction of its covalently-linked palmitates with the tightly packed hydrophobic core of the ordered domains, as is the case for other lipid-anchored proteins that interact with lipid rafts. Crystal structures of both OspA and OspB share a putative binding cleft for which there is no proposed ligand (Becker et al., 2005; Li et al., 1997). At the bottom of this cleft are three charged amino acids (arg-162, glu-184, and arg-214) that may be functionally important due to their location and sequence conservation. It has been postulated that this cleft could bind small molecule ligands (Becker et al., 2005; Li et al., 1997), so we tested whether the cholesterol glycolipids bind to this cleft through the galactose. Blind docking analyses with the program VINA were performed using the crystal structure of OspB and ACGal, Bb-GL-I, and CGal. The top scored binding sites in OspB were those involving the cleft lined with the previously mentioned triad of arg-162, glu-184, and arg-214. The binding energy calculated in silico by VINA of the top ranking solution of each docked complex at 25°C was −6.5 kcal/mol (Ki ~ 17 μM) for ACGal, −7.3 kcal/mol (Ki ~ 4 μM) for CGal, and −5.4 kcal/mol for Bb-GL-I (Ki ~ 109 μM). However, the conformations adopted by these molecules, the weak hydrogen bond and steric interactions with OspB, and the extended solvent exposure of these hydrophobic molecules, point to a weak interaction of these cholesterol glycolipids with the OspB cleft, consistent with the relatively low binding energies (Figure S3). This suggests that the cleft in OspB has low affinity for the cholesterol glycolipids of B. burgdorferi, although it could also be enhanced if OspB is held in the outer membrane by other interactions. Docking analyses and modeling show that the glycolipid binding site faces the membrane and that it would be relatively near to its surface (~30-40 Å). Furthermore, the N-terminus of OspB is close to the cleft, and in all the binding models predicted by the docking analyses, the cleft faces the cellular membrane (Figure S3, arrow) which strengthens the possibility of an interaction.
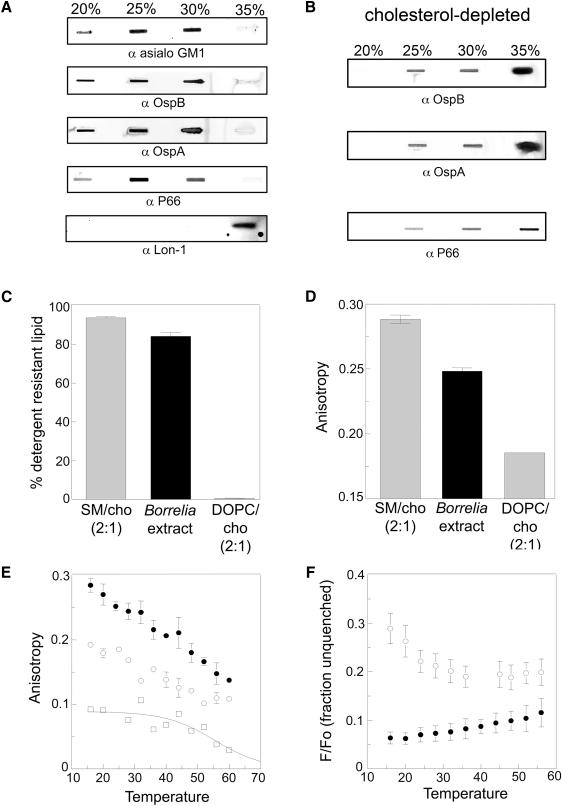
Detergent resistant membranes containing OspB and cholesterol glycolipids can be isolated from B. burgdorferi
The clustering of cholesterol into distinct microdomains (Figure 4) is a characteristic of lipid rafts. To determine whether the cholesterol glycolipid-rich regions of B. burgdorferi exhibit other characteristics of lipid rafts, we performed solubilization experiments on whole B. burgdorferi with Triton X-100 (TX-100) at 4°C, followed by separation of the lysate on a discontinuous OptiPrep density gradient. Lipid rafts resist solubilization by TX-100 at 4°C forming detergent-resistant membranes (DRM; (Brown and Rose, 1992). DRM float in the gradient and are found predominantly at the 25% density with some at 20% and 30%. Each density fraction was analyzed by slot blot for cholesterol glycolipids as well as specific proteins that may associate with the DRM. Cholesterol glycolipids were concentrated in the 25% and 30% fractions with some in the 20% fraction, demonstrating association of these glycolipids with DRM (Figure 5A). The outer membrane proteins OspB, OspA, and P66 partitioned with cholesterol glycolipids in the DRM in the 20%, 25% and 30% fractions, suggesting that these proteins normally reside in lipid rafts (Figure 5A). The cytoplasmic Lon-1 protease (Coleman et al., 2009) was included as a control and was only found in the 35% fraction, typical for a soluble protein (Figure 5A). We also determined the lipid composition of the DRM and non-DRM (solubilized) fractions. We found that TX-100 treatment solubilized PC, PG, and MGalD but not ACGal/Bb-GL-I, CGal, or free cholesterol when lipids were analyzed by TLC (Figure S4). This result shows that the DRM are composed of a distinct subset of B. burgdorferi lipids. The observation that DRM can be isolated from B. burgdorferi and that they are rich in cholesterol lipids is suggestive of the existence of lipid rafts. Indeed, following cholesterol depletion of spirochetes that disrupts lipid rafts, and thus the DRM, the same lipoproteins solubilize and are found in the 35% fraction (Figure 5B).
Figure 5. Lipid rafts exist in B. burgdorferi. See also Figure S4.
A. Whole spirochetes were subjected to TX-100 treatment at 4°C and Optiprep density gradient separation. Gradient fractions were slot blotted and probed with anti-asialo GM1, anti-OspB, anti-OspA, anti-P66, or anti-Lon-1 protease. B. Spirochetes were treated as in A but were exposed to 10 mM MβCD prior to solubilization in TX-100. Cholesterol depletion leads to solubilization of lipoproteins (35% fraction) C. B. burgdorferi lipids in the form of MLV model membranes display resistance to solubilization by TX-100. Lipid ratios are mol:mol. SM/cho (2:1) Lo = sphingomyelin/cholesterol vesicles; DOPC/cho (2:1) Ld = dioleoylphosphatidylcholine /cholesterol vesicles. D. Fluorescence anisotropy of DPH demonstrates a high degree of order among lipids in B. burgdorferi MLV. E. DPH fluorescence anisotropy in Borrelia MLV as a function of temperature. MLV contained B. burgdorferi lipid extract (filled circles) or 2:1 DOPC/cholesterol (open circles). Open squares show the difference between the value for Borrelia lipids and that for the DOPC/cho fit to a sigmoidal curve that has a limiting value of zero at high temperature. F. FRET as a function of temperature for Borrelia lipids in model membranes demonstrates the co-existence of Lo (raft) and Ld domains (high F/Fo values). MLV contained Borrelia lipid extract (open circles) or DOPC/cholesterol 2:1 (filled circles). F/Fo is the ratio of fluorescence in samples containing donor (NBD-DPPE) and acceptor (rhodamine-DOPE), to that in samples containing donor. A and B are representative experiments and C – F are from triplicate experiments. * p < 0.05, *** p < 0.001.
Multilamellar vesicles (MLV) of B. burgdorferi lipids spontaneously form ordered lipid domains
To confirm that the insolubility of B. burgdorferi lipids in TX-100 was linked to their ability to form ordered domains (lipid rafts), we studied the properties of MLV composed of extracted B. burgdorferi lipids and compared them to those of vesicles forming a raft (liquid ordered, Lo) state or a non-raft (liquid disordered, Ld) state. Figure 5C shows that DRMs can be obtained by treating B. burgdorferi MLV with TX-100 at room temperature (RT). The degree of insolubility in TX-100 is slightly less than that of sphingomyelin/cholesterol vesicles (SM/cho 2:1), which form the Lo state, and much more than for dioleoylphosphatidylcholine /cholesterol vesicles (DOPC/cho 2:1), which form the Ld state. DPH fluorescence anisotropy measurements (for membrane order) indicated that at RT the degree of order in the B. burgdorferi MLV was intermediate between Lo state SM/cho and Ld state DOPC/cho (Figure 5D). This shows that the tendency of B. burgdorferi lipids to form an ordered state bilayer is not a detergent artifact. Measurement of temperature dependence of fluorescence anisotropy (Figure 5E) shows that the high degree of order in Borrelia lipids relative to that in non-raft (DOPC/cho) vesicles persists to high temperatures.
Although detergent-resistance and anisotropy experiments demonstrate a degree of lipid order, they do not distinguish between a homogeneous bilayer that has a uniformly high degree of order and one that contains co-existing Lo (raft) and Ld domains. To distinguish this, a Förster Resonance Energy Transfer (FRET) assay was used. This evaluates the degree of segregation of NBD-1,2-dipalmitoyl-sn-glycero-3-phosphoethanolamine-N-7-nitro-2-1,3-benzoxadiazol-4-yl (NBD-DPPE), which has a moderate affinity for rafts, and 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine-N-lissamine rhodamine B sulfonyl (rhodamine-DOPE), which mainly localizes within disordered domains (Ayuyan and Cohen, 2008). When lipid raft and non-raft domains co-exist, these probes segregate from one another, and FRET is weak. The temperature dependence of FRET in the Borrelia MLV was compared to that of Ld state (non-raft) DOPC/cho vesicles. The Borrelia MLV show weak FRET (high F/Fo values) at low temperature in contrast to DOPC/cho (Figure 5F). At high temperature, the FRET values in the Borrelia MLV approach those in DOPC/cholesterol, showing that the segregation of the probes in the Borrelia lipid MLV is lost at high temperature (Figure 5F). This is typical of the melting of ordered domains at high temperature, such that ordered domain formation, and segregation of molecules into different domains is lost (Bakht et al., 2007; Xu et al., 2001). Combined, these results demonstrate that the B. burgdorferi lipids have the ability to form ordered domains (lipid rafts) that segregate from Ld domains over a wide range of temperatures.
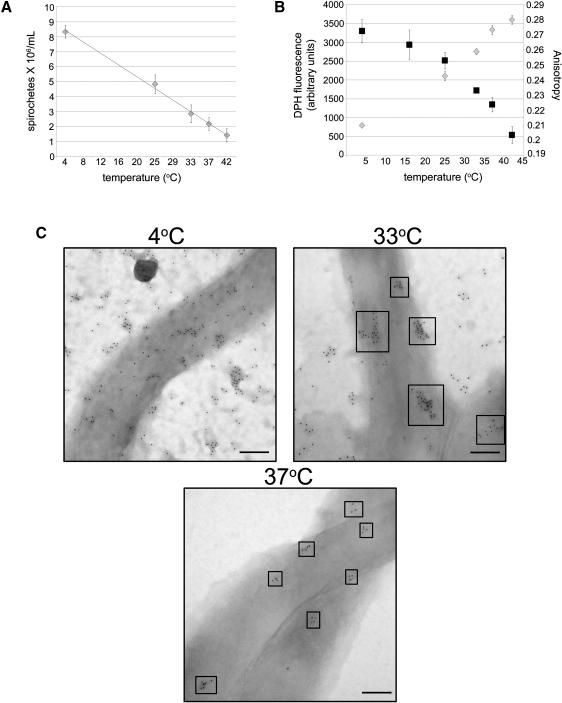
Temperature affects both the bactericidal action of CB2 and cholesterol glycolipid organization
To determine if the bactericidal effects of CB2 were temperature dependent, spirochetes were held at different temperatures between 4°C and 42°C and then treated with CB2. The bactericidal activity of CB2 increased with increasing temperature (Figure 6A). Additionally, the rate of CB2-induced lysis increased as temperature increased (Figure S5). A decrease in the degree of ordered microdomain formation as temperature increased was also detected by DPH fluorescence. The intensity of DPH fluorescence bound to B. burgdorferi increased with increasing temperature (Figure 6B, left y axis), suggesting that DPH inserts into the membrane of B. burgdorferi more readily at higher temperatures. In addition, the anisotropy of DPH fluorescence decreased as temperature increased due to greater membrane disorder (Figure 6B right y axis). Anisotropy values of whole B. burgdorferi and their temperature dependence were concordant with those obtained in model membranes of B. burgdorferi lipids (Figure 5E). This is consistent with a decrease in order and a decrease in the raft formation or size at high temperatures (Fujita et al., 2007),
Figure 6. Modulation of outer membrane order (fluidity) with temperature affects the bactericidal activity of CB2 and the organization of the cholesterol glycolipids. See also Figures S5 and S6.
A. Killing assay showing that the bactericidal activity of CB2 is directly proportional to temperature. B. Permeability of the B. burgdorferi membrane is directly proportional to temperature as measured by DPH incorporation (gray diamonds). Lipid order, as measured by anisotropy (black squares), is inversely proportional to temperature. Results are from triplicate experiments. C. B. burgdorferi were labeled for cholesterol glycolipids (6 nm colloidal gold) at 4, 33, and 37°C and analyzed to observe the native organization of microdomains and the effect of temperature on organization and size. At 4°C the microdomains are greatly enlarged and cholesterol glycolipids appear more dispersed. At 33°C the microdomains exist as distinct clusters of ~ 100 nm; at 37°C the microdomains form smaller (~40 nm) clusters. Size bars = 100 nm.
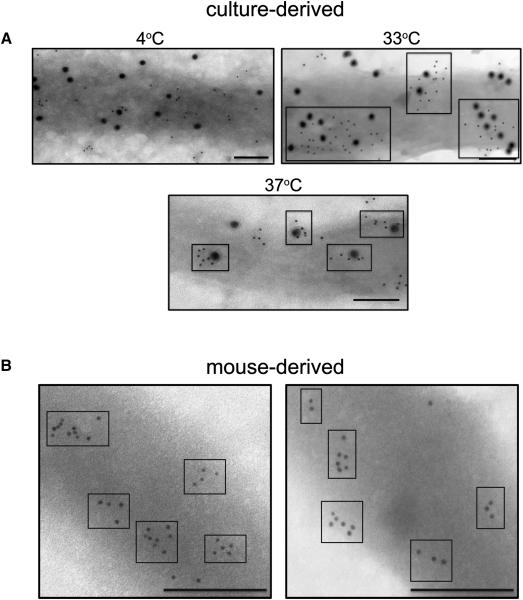
Indeed, temperature had a significant effect on the organization of lipid microdomains in the outer membrane of B. burgdorferi (Figure 6C). Cholesterol glycolipids organized into distinct clusters at 33°C (~100 nm,) and at 37°C (~40 nm,). At 4°C larger, more dispersed clusters were noted. Temperature might alter other membrane properties, however, it did not affect the levels of several membrane proteins or the level of CB2 binding to B. burgdorferi (Figure S6). The visualization of distinct cholesterol microdomains (clusters) in the outer membrane of intact B. burgdorferi cells is highly indicative of lipid rafts. That the cholesterol microdomains can be seen even without antibody treatment indicates that this is the native organization of the cholesterol glycolipids in the outer membrane of B. burgdorferi and not the result of the CB2-induced damage seen in Figure 4. Furthermore, OspB colocalizes with the cholesterol glycolipids rafts at different temperatures (Figure 7A) suggesting that this association is maintained as the size of rafts changes with temperatures. Lipid rafts are also present in spirochetes isolated from mouse organs, without cultivation, demonstrating that these microdomains occur in vivo (Figure 7B) That the cholesterol glycolipid microdomains are present in vivo suggests that this is the normal organization of the cholesterol glycolipids when spirochetes are in the mammalian host. The in vivo organization of the cholesterol glycolipids is similar to the organization of cultured organisms at 370C with tight clusters.
Figure 7. Cholesterol glycolipid microdomains associate with OspB at different temperatures and exist in B. burgdorferi harvested directly from mice.
A. B. burgdorferi held at 4, 33, or 37°C were fixed and labeled with anti-asialo GM1 (6 nm colloidal gold) and CB2 (18 nm colloidal gold) followed by secondary colloidal gold conjugates. OspB in the glycolipid microdomains organize differently depending on the temperature (boxes). B. B. burgdorferi harvested directly (without cultivation) from the bladders of C3H/HeN mice 20 days post inoculation were labeled for cholesterol glycolipids (6 nm colloidal gold) to demonstrate the presence of clustering in vivo (boxes). Size bars = 100 nm.
DISCUSSION
Three main conclusions are derived from these studies. First, we show that cholesterol glycolipids of B. burgdorferi are required for the bactericidal mechanism of CB2, the complement-independent IgG against OspB. Second, a cross-reaction with the cholesterol glycolipids that could have implications for the pathogenesis of Lyme disease has been documented. Third, we have also provided evidence for the existence of lipid rafts in Borrelia from cultures at various temperatures and directly isolated from experimentally infected mice. This has implications for other prokaryotes.
Cholesterol and cholesterol glycolipids could have profound effects on the dynamics and organization of the outer membrane of B. burgdorferi which may allow these molecules to contribute to the bactericidal mechanism induced by CB2. We demonstrated the requirement of cholesterol and cholesterol glycolipids for the bactericidal action of CB2. Depletion of cholesterol and cholesterol glycolipids decreased the bactericidal activity of CB2 whereas replacement of cholesterol restored it. Additional evidence for a role of cholesterol in CB2 action comes from the formation and composition of the vesicles and surface projections that increase upon CB2 exposure. Ultrastructural and biochemical approaches showed that released vesicles include the antigen OspB, with bound CB2, and cholesterol glycolipids in the form of microdomains. Lipid raft organization impacts CB2 function. Ultrastructural evidence suggests that the clustering of the cholesterol glycolipids into distinct microdomains is maintained in the membrane vesicles and projections observed after CB2 treatment.
The lipid binding properties of OspB would explain its association with these lipid rafts. The structural docking analyses showed that the cleft of OspB can bind the cholesterol glycolipids in a weak interaction. This could be enhanced by the interaction of OspB (and also very likely OspA) with the cholesterol glycolipids through its covalent palmitoylation at the amino terminal cysteine of the lipoprotein. Binding to cholesterol, a raft component, could induce raft association. This could be enhanced by palmitoylation which is also believed to impart an affinity for lipid rafts in eukaryotes (Epand, 2008). The hemagglutinin and neuraminidase of influenza do not associate with rafts if their palmitic acids are removed (Shvartsman et al., 2003; Zhang et at., 2000)
The model for the bactericidal action based on the evidence presented here requires that the binding of CB2 to OspB induces an unstable boundary between microdomains and the surrounding bilayer resulting in formation of projections, membrane fission leading to shedding of vesicles rich in cholesterol glycolipids and OspB, and formation of pores that result in the lysis of the spirochete (LaRocca et al., 2009). This raises the question as to how might the interaction of OspB with cholesterol glycolipids influence membrane fission induced by CB2. One possibility is that upon CB2 binding, OspB undergoes a conformational change so that it expands the outer leaflet of the membrane bilayer. This could result either from a penetration of OspB into the outer leaflet, a net movement of lipid into the outer leaflet from the inner leaflet, or a decrease in the packing of the outer leaflet lipids so that they take up more space per molecule. Whatever the mechanism for the expansion of the outer leaflet, it could be amplified by the high concentration of OspB within domains rich in cholesterol glycolipids. Once the outer leaflet expands, the bilayer would spontaneously bend to maintain contact with the inner leaflet thus forming projections. If this bending is sufficiently severe, the boundary between the domains rich in OspB and the remainder of the membrane would be unstable, leading to vesiculation from the surface of B. burgdorferi. When OspB is more dispersed within the membrane, such as when when cholesterol glycolipid is absent, CB2 binding may not cause the same changes in OspB conformation or membrane structure, thereby reducing membrane fission (vesiculation). An alternate model is that membrane disruption could arise from a change in the OspB-bound lipid upon CB2 binding. For example, the binding of CB2 could change OspB structure in such a manner that the distance between palmitates and bound cholesterol glycolipid increases, resulting in outer leaflet expansion, which would have the consequences noted above.
The ultrastructural evidence for the organization of cholesterol glycolipids into clustered microdomains suggests that these are analogous to the lipid rafts that form in cholesterol-rich eukaroytic cells. The microdomains were ~ 100 nm in diameter at the optimal in vitro growth temperature for B. burgdorferi (33°C), consistent with the small size of eukaryotic lipid rafts (Pike, 2009). This evidence was strengthened by biochemical and biophysical experiments that documented the existence of a prokaryotic version of lipid raft microdomains. First, we found that DRM could be isolated from B. burgdorferi and were rich in cholesterol glycolipids. OspB, OspA, and P66 were shown to reside in the DRM, suggesting that the outer membrane proteins normally localize in these microdomains. In addition, the physical behavior of B. burgdorferi lipids in MLV showed that they formed lipid raft-like ordered domains as determined by TX-100 detergent resistance, anisotropy, and FRET. Combined, these data indicate that microdomains form in the outer membrane of B. burgdorferi, and can be considered close analogs of eukaryotic lipid rafts. Therefore, the discovery that Borrelia has lipid rafts could be a key to the understanding of lipoprotein function, to the role of the cholesterol glycolipids in both physiological and pathological conditions, and to the pathogenesis of Lyme disease and relapsing fever.
The lack of lipid rafts in the membranes of prokaryotes may be due to the absence of cholesterol in all but a few bacteria, namely Helicobacter, Mycoplasma, Ehrlichia, Anaplasma, Brachyspira, and, of course, Borrelia. We now show that lipid rafts exist in prokaryotes. These findings represent a substantial shift in the thinking about lipid rafts, expanding their biological relevance to prokaryotes.
Lastly, the evolution of the eukaryotic cell membrane with its well-defined, segregated cholesterol-sphingolipid domains, is not completely understood. It is possible that prokaryotes, such as Borrelia, that have ordered lipid domains represent transitional forms that in the distant past led to the evolution of the eukaryotic cell membrane. Thus, there is an evolutionary significance to the ordered cholesterol-glycolipid domains in the outer membrane of Borrelia.
Changes in the organization of lipid rafts in B. burgdorferi in response to temperature may also be of functional importance. Ultrastructural evidence showed that the microdomains decrease in size as temperature increases. In fact, spirochetes harvested directly from mice have lipid rafts that are organized in the same manner and size as those from spirochetes in culture at 37°C. Therefore, the in vivo and culture (at 37°C) lipid raft organization is concordant. In addition, biophysical data showing that membrane order and fluidity decreases as temperature increases (both in cells and MLV) are in concordance with the ultrastructural observations. Furthermore, these changes were correlated to differences in CB2 bactericidal activity. Lower temperatures decreased CB2 bactericidal activity, while the opposite was true for higher temperatures. This is additional evidence of a link between CB2 action and lipid raft organization.
The temperature dependence of lipid raft organization in B. burgdorferi may have significant implications in the transmission cycle of the spirochetes. Due to their arthropod-to-mammal life cycle, B. burgdorferi are exposed to a wide range of temperatures from ambient temperature in the tick vector to 37°C during mammalian infection. Thus, lipid raft organization in Borrelia may change in response to environmental cues, and may play a significant role in their transmission, such as allowing the spirochetes to sense their location, within the vector or the mammalian host. The complex of outer membrane lipoproteins within lipid rafts could carry out a sensor function, and importantly, it could have a signaling role in the differential expression of these lipoproteins as B. burgdorferi moves from the tick to the mammal. A similar response to environmental cues may even occur for other tick-borne pathogens that contain cholesterol such as, E. chaffeensis and A. phagocytophilum if these bacteria were also shown to have lipid rafts.
The cross-reactivity of the antibody to asialo GM1 with lipid antigens of Borrelia has been known for some time and antibodies generated against the lipid antigens of B. burgdorferi cross-react with ganglioside GM1 [Gal-β-1-3-GalNAc-β-1-4-Gal-β-1-4-Glc-β-1-1-ceramide]. We now know that this cross-reactivity is at least partially due to the shared β1-3 linkage of the galactose (Willison and Kennedy, 1993). That these immunogenic glycolipids share an epitope with a prominent host molecule raises the possibility of an antibody-mediated effect in the pathogenesis of Lyme disease. This idea is not a new one, however, sufficient evidence for the contribution of autoreactivity to the pathologic manifestations of Lyme disease has been lacking. The cross-reactivity among the cholesterol glycolipids with GM1 may prompt a reappraisal of antibody and cell-mediated (Kinjo et al., 2006) damage as part of the pathogenesis of the spirochetoses.
In summary, using an antibody as a biological tool, we have documented the existence of membrane microdomains in the membrane of the prokaryote, B. burgdorferi. These microdomains contain the cholesterol glycolipids of Borrelia, and contribute to the bactericidal mechanism of CB2. Their lipid composition and physical properties indicates that they are similar to the lipid rafts of eukaryotic membranes. Several antigens of B. burgdorferi were shown to reside in lipid rafts, one of which, OspB, is the target of the complement-independent bactericidal IgG, CB2.
EXPERIMENTAL PROCEDURES
Bacteria, bacterial cultures, mice, and monoclonal antibodies
For all experiments B. burgdorferi strain B31 was used. Cultures were grown in microaerophillic conditions in Barbour-Stoenner-Kelly (BSK)–H medium (Sigma) at 33°C. CB2 and CB10 hybridoma supernatants were produced and purified as previously described (Coleman et al., 1992). C3H/HeN mice were inoculated intradermally with 2 × 104 spirochetes; after 20 days, the bladders were removed and dispersed in Hank’s Balanced Salt Solution (HBSS, GIBCO). The suspension was centrifuged at 400 × g and the supernatant examined for spirochetes.
Fluorometric assay for quantification and depletion of cholesterol
B. burgdorferi were treated with 10 mM MβCD (Sigma) in HBSS at 33°C for 30 or 60 min and centrifuged. The resulting pellets were analyzed for total cholesterol using the fluorometric Amplex Red Cholesterol Assay Kit (Invitrogen) and compared against pellets from untreated spirochetes.
HIGHLIGHTS.
Cholesterol lipids are critical for the bactericidal mechanism of a lytic antibody
Lipoproteins interact with cholesterol lipids in the Borrelia membrane
Lipid rafts exist in cultured and mouse-derived B. burgdorferi
These findings represent a shift in the relevance of lipid rafts to prokaryotes
Supplementary Material
ACKNOWLEDGEMENT
This work was supported by grants from the National Institutes of Health R37-AI-02744, ROI-AR-040445, and the Northeast Biodefense Center U54-AI-057158 (Lipkin) to JLB, and by grant R01-GM-048596 to EL. TJL was supported by T32-AI-007539 from the NIAID. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIAID or the NIH.
Footnotes
SUPPLEMENTAL INFORMATION Additional experimental procedures (CB2 bactericidal assays; detection methods for lipids; detergent-resistance of whole B. burgdorferi and model membrane vesicles; MβCD toxicity and protein level assays; blind docking analyses; DPH vesicle assays; FRET and fluorescence anisotropy measurements, and transmission electron microscopy) as well as Table S1 and Figures S1 – S6 are detailed in supplemental information.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Ayuyan AG, Cohen FS. Raft composition at physiological temperature and pH in the absence of detergents. Biophys J. 2008;94:2654–2666. doi: 10.1529/biophysj.107.118596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakht O, Pathak P, London E. Effect of the structure of lipids favoring disordered domain formation on the stability of cholesterol-containing ordered domains (lipid rafts): identification of multiple raft-stabilization mechanisms. Biophys J. 2007;93:4307–4318. doi: 10.1529/biophysj.107.114967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker M, Bunikis J, Lade BD, Dunn JJ, Barbour AG, Lawson CL. Structural investigation of Borrelia burgdorferi OspB, a bactericidal Fab target. J Biol Chem. 2005;280:17363–17370. doi: 10.1074/jbc.M412842200. [DOI] [PubMed] [Google Scholar]
- Belisle JT, Brandt ME, Radolf JD, Norgard MV. Fatty acids of Treponema pallidum and Borrelia burgdorferi lipoproteins. J Bacteriol. 1994;176:2151–2157. doi: 10.1128/jb.176.8.2151-2157.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Menachem G, Kubler-Kielb J, Coxon B, Yergey A, Schneerson R. A newly discovered cholesteryl galactoside from Borrelia burgdorferi. Proc Natl Acad Sci U S A. 2003;100:7913–7918. doi: 10.1073/pnas.1232451100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benach JL, Bosler EM, Hanrahan JP, Coleman JL, Habicht GS, Bast TF, Cameron DJ, Ziegler JL, Barbour AG, Burgdorfer W, et al. Spirochetes isolated from the blood of two patients with Lyme disease. N Engl J Med. 1983;308:740–742. doi: 10.1056/NEJM198303313081302. [DOI] [PubMed] [Google Scholar]
- Bockenstedt LK, Barthold S, Deponte K, Marcantonio N, Kantor FS. Borrelia burgdorferi infection and immunity in mice deficient in the fifth component of complement. Infect Immun. 1993;61:2104–2107. doi: 10.1128/iai.61.5.2104-2107.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt ME, Riley BS, Radolf JD, Norgard MV. Immunogenic integral membrane proteins of Borrelia burgdorferi are lipoproteins. Infect Immun. 1990;58:983–991. doi: 10.1128/iai.58.4.983-991.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown DA, London E. Structure and function of sphingolipid- and cholesterol-rich membrane rafts. J Biol Chem. 2000;275:17221–17224. doi: 10.1074/jbc.R000005200. [DOI] [PubMed] [Google Scholar]
- Brown DA, Rose JK. Sorting of GPI-anchored proteins to glycolipid-enriched membrane subdomains during transport to the apical cell surface. Cell. 1992;68:533–544. doi: 10.1016/0092-8674(92)90189-j. [DOI] [PubMed] [Google Scholar]
- Brown RE. Sphingolipid organization in biomembranes: what physical studies of model membranes reveal. J Cell Sci. 1998;111(Pt 1):1–9. doi: 10.1242/jcs.111.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgdorfer W, Barbour AG, Hayes SF, Benach JL, Grunwaldt E, Davis JP. Lyme disease-a tick-borne spirochetosis? Science. 1982;216:1317–1319. doi: 10.1126/science.7043737. [DOI] [PubMed] [Google Scholar]
- Bykowski T, Woodman ME, Cooley AE, Brissette CA, Wallich R, Brade V, Kraiczy P, Stevenson B. Borrelia burgdorferi complement regulator-acquiring surface proteins (BbCRASPs): Expression patterns during the mammal-tick infection cycle. Int J Med Microbiol. 2008;298(Suppl 1):249–256. doi: 10.1016/j.ijmm.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Rand RP. The influence of cholesterol on phospholipid membrane curvature and bending elasticity. Biophys J. 1997;73:267–276. doi: 10.1016/S0006-3495(97)78067-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman JL, Katona LI, Kuhlow C, Toledo A, Okan NA, Tokarz R, Benach JL. Evidence that two ATP-dependent (Lon) proteases in Borrelia burgdorferi serve different functions. PLoS Pathog. 2009;5:e1000676. doi: 10.1371/journal.ppat.1000676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman JL, Rogers RC, Benach JL. Selection of an escape variant of Borrelia burgdorferi by use of bactericidal monoclonal antibodies to OspB. Infect Immun. 1992;60:3098–3104. doi: 10.1128/iai.60.8.3098-3104.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly SE, Benach JL. Cutting edge: the spirochetemia of murine relapsing fever is cleared by complement-independent bactericidal antibodies. J Immunol. 2001;167:3029–3032. doi: 10.4049/jimmunol.167.6.3029. [DOI] [PubMed] [Google Scholar]
- Connolly SE, Benach JL. The versatile roles of antibodies in Borrelia infections. Nat Rev Microbiol. 2005;3:411–420. doi: 10.1038/nrmicro1149. [DOI] [PubMed] [Google Scholar]
- Connolly SE, Thanassi DG, Benach JL. Generation of a complement-independent bactericidal IgM against a relapsing fever Borrelia. J Immunol. 2004;172:1191–1197. doi: 10.4049/jimmunol.172.2.1191. [DOI] [PubMed] [Google Scholar]
- Epand RM. Proteins and cholesterol-rich domains. Biochim Biophys Acta. 2008;1778:1576–1582. doi: 10.1016/j.bbamem.2008.03.016. [DOI] [PubMed] [Google Scholar]
- Escudero R, Halluska ML, Backenson PB, Coleman JL, Benach JL. Characterization of the physiological requirements for the bactericidal effects of a monoclonal antibody to OspB of Borrelia burgdorferi by confocal microscopy. Infect Immun. 1997;65:1908–1915. doi: 10.1128/iai.65.5.1908-1915.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita A, Cheng J, Hirakawa M, Furukawa K, Kusunoki S, Fujimoto T. Gangliosides GM1 and GM3 in the living cell membrane form clusters susceptible to cholesterol depletion and chilling. Mol Biol Cell. 2007;18:2112–2122. doi: 10.1091/mbc.E07-01-0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia Monco JC, Wheeler CM, Benach JL, Furie RA, Lukehart SA, Stanek G, Steere AC. Reactivity of neuroborreliosis patients (Lyme disease) to cardiolipin and gangliosides. J Neurol Sci. 1993;117:206–214. doi: 10.1016/0022-510x(93)90175-x. [DOI] [PubMed] [Google Scholar]
- Garcia-Monco JC, Seidman RJ, Benach JL. Experimental immunization with Borrelia burgdorferi induces development of antibodies to gangliosides. Infect Immun. 1995;63:4130–4137. doi: 10.1128/iai.63.10.4130-4137.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haque M, Hirai Y, Yokota K, Oguma K. Steryl glycosides: a characteristic feature of the Helicobacter spp.? J Bacteriol. 1995;177:5334–5337. doi: 10.1128/jb.177.18.5334-5337.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai Y, Haque M, Yoshida T, Yokota K, Yasuda T, Oguma K. Unique cholesteryl glucosides in Helicobacter pylori: composition and structural analysis. J Bacteriol. 1995;177:5327–5333. doi: 10.1128/jb.177.18.5327-5333.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson RC. The spirochetes. Annu Rev Microbiol. 1977;31:89–106. doi: 10.1146/annurev.mi.31.100177.000513. [DOI] [PubMed] [Google Scholar]
- Jones JD, Bourell KW, Norgard MV, Radolf JD. Membrane topology of Borrelia burgdorferi and Treponema pallidum lipoproteins. Infect Immun. 1995;63:2424–2434. doi: 10.1128/iai.63.7.2424-2434.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katona LI, Ayalew S, Coleman JL, Benach JL. A bactericidal monoclonal antibody elicits a change in its antigen, OspB of Borrelia burgdorferi, that can be detected by limited proteolysis. J Immunol. 2000;164:1425–1431. doi: 10.4049/jimmunol.164.3.1425. [DOI] [PubMed] [Google Scholar]
- Kinjo Y, Tupin E, Wu D, Fujio M, Garcia-Navarro R, Benhnia MR, Zajonc DM, Ben-Menachem G, Ainge GD, Painter GF, et al. Natural killer T cells recognize diacylglycerol antigens from pathogenic bacteria. Nat Immunol. 2006;7:978–986. doi: 10.1038/ni1380. [DOI] [PubMed] [Google Scholar]
- LaRocca TJ, Benach JL. The important and diverse roles of antibodies in the host response to Borrelia infections. Curr Top Microbiol Immunol. 2008;319:63–103. doi: 10.1007/978-3-540-73900-5_4. [DOI] [PubMed] [Google Scholar]
- LaRocca TJ, Holthausen DJ, Hsieh C, Renken C, Mannella CA, Benach JL. The bactericidal effect of a complement-independent antibody is osmolytic and specific to Borrelia. Proc Natl Acad Sci U S A. 2009;106:10752–10757. doi: 10.1073/pnas.0901858106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Dunn JJ, Luft BJ, Lawson CL. Crystal structure of Lyme disease antigen outer surface protein A complexed with an Fab. Proc Natl Acad Sci U S A. 1997;94:3584–3589. doi: 10.1073/pnas.94.8.3584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin M, Rikihisa Y. Ehrlichia chaffeensis and Anaplasma phagocytophilum lack genes for lipid A biosynthesis and incorporate cholesterol for their survival. Infect Immun. 2003;71:5324–5331. doi: 10.1128/IAI.71.9.5324-5331.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livermore BP, Bey RF, Johnson RC. Lipid metabolism of Borrelia hermsi. Infect Immun. 1978;20:215–220. doi: 10.1128/iai.20.1.215-220.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- London E. Insights into lipid raft structure and formation from experiments in model membranes. Curr Opin Struct Biol. 2002;12:480–486. doi: 10.1016/s0959-440x(02)00351-2. [DOI] [PubMed] [Google Scholar]
- London E, Feligenson GW. A convenient and sensitive fluorescence assay for phospholipid vesicles using diphenylhexatriene. Anal Biochem. 1978;88:203–211. doi: 10.1016/0003-2697(78)90412-8. [DOI] [PubMed] [Google Scholar]
- Newman K, Jr., Johnson RC. In vivo evidence that an intact lytic complement pathway is not essential for successful removal of circulating Borrelia turicatae from mouse blood. Infect Immun. 1981;31:465–469. doi: 10.1128/iai.31.1.465-469.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols B. Caveosomes and endocytosis of lipid rafts. J Cell Sci. 2003;116:4707–4714. doi: 10.1242/jcs.00840. [DOI] [PubMed] [Google Scholar]
- Pike LJ. The challenge of lipid rafts. J Lipid Res. 2009;50(Suppl):S323–328. doi: 10.1194/jlr.R800040-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radolf JD, Bourell KW, Akins DR, Brusca JS, Norgard MV. Analysis of Borrelia burgdorferi membrane architecture by freeze-fracture electron microscopy. J Bacteriol. 1994;176:21–31. doi: 10.1128/jb.176.1.21-31.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radolf JD, Goldberg MS, Bourell K, Baker SI, Jones JD, Norgard MV. Characterization of outer membranes isolated from Borrelia burgdorferi, the Lyme disease spirochete. Infect Immun. 1995;63:2154–2163. doi: 10.1128/iai.63.6.2154-2163.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadziene A, Thompson PA, Barbour AG. In vitro inhibition of Borrelia burgdorferi growth by antibodies. J Infect Dis. 1993;167:165–172. doi: 10.1093/infdis/167.1.165. [DOI] [PubMed] [Google Scholar]
- Salaun C, James DJ, Chamberlain LH. Lipid rafts and the regulation of exocytosis. Traffic. 2004;5:255–264. doi: 10.1111/j.1600-0854.2004.0162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroder NW, Schombel U, Heine H, Gobel UB, Zahringer U, Schumann RR. Acylated cholesteryl galactoside as a novel immunogenic motif in Borrelia burgdorferi sensu stricto. J Biol Chem. 2003;278:33645–33653. doi: 10.1074/jbc.M305799200. [DOI] [PubMed] [Google Scholar]
- Shvartsman DE, Kotler M, Tall RD, Roth MG, Henis YI. Differently anchored influenza hemagglutinin mutants display distinct interaction dynamics with mutual rafts. J Cell Biol. 2003;163:879–888. doi: 10.1083/jcb.200308142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith PF. Biosynthesis of cholesteryl glucoside by Mycoplasma gallinarum. J Bacteriol. 1971;108:986–991. doi: 10.1128/jb.108.3.986-991.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stubs G, Fingerle V, Wilske B, Gobel UB, Zahringer U, Schumann RR, Schroder NW. Acylated cholesteryl galactosides are specific antigens of borrelia causing lyme disease and frequently induce antibodies in late stages of disease. J Biol Chem. 2009;284:13326–13334. doi: 10.1074/jbc.M809575200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trott DJ, Alt DP, Zuerner RL, Wannemuehler MJ, Stanton TB. The search for Brachyspira outer membrane proteins that interact with the host. Anim Health Res Rev. 2001;2:19–30. [PubMed] [Google Scholar]
- Wang Y, Thiele C, Huttner WB. Cholesterol is required for the formation of regulated and constitutive secretory vesicles from the trans-Golgi network. Traffic. 2000;1:952–962. doi: 10.1034/j.1600-0854.2000.011205.x. [DOI] [PubMed] [Google Scholar]
- Wheeler CM, Garcia Monco JC, Benach JL, Golightly MG, Habicht GS, Steere AC. Nonprotein antigens of Borrelia burgdorferi. J Infect Dis. 1993;167:665–674. doi: 10.1093/infdis/167.3.665. [DOI] [PubMed] [Google Scholar]
- Xu X, Bittman R, Duportail G, Heissler D, Vilcheze C, London E. Effect of the structure of natural sterols and sphingolipids on the formation of ordered sphingolipid/sterol domains (rafts). Comparison of cholesterol to plant, fungal, and disease-associated sterols and comparison of sphingomyelin, cerebrosides, and ceramide. J Biol Chem. 2001;276:33540–33546. doi: 10.1074/jbc.M104776200. [DOI] [PubMed] [Google Scholar]
- Zhang J, Pekosz A, Lamb RA. Influenza virus assembly and lipid raft microdomains: a role for the cytoplasmic tails of the spike glycoproteins. J Virol. 2000;74:4634–4644. doi: 10.1128/jvi.74.10.4634-4644.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zidovetzki R, Levitan I. Use of cyclodextrins to manipulate plasma membrane cholesterol content: evidence, misconceptions and control strategies. Biochim Biophys Acta. 2007;1768:1311–1324. doi: 10.1016/j.bbamem.2007.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.