Abstract
Lineage plasticity, an ability to transition from one committed developmental pathway to another, has been proposed as a source of intratumoral heterogeneity and tumor adaptation to adverse environments including exposure to targeted anti-cancer treatments. Tumor conversion to a different histological subtype has been associated with loss of dependency on the original oncogenic driver, leading to therapeutic resistance. A particularly notable pathway of lineage plasticity in cancer, histologic transformation from adenocarcinoma to an aggressive neuroendocrine derivative, was initially described in lung cancers harboring an EGFR mutation, and has been subsequently found in multiple other adenocarcinomas, including prostate cancer under strong anti-androgenic selection. Squamous transformation is recently identified, and less well characterized, pathway of adenocarcinoma escape from suppressive anticancer therapy. Expansion of the practice of tumor re-biopsy upon disease progression has increased recognition of these mechanisms of resistance and has supported exploration of the underlying biology. In this review, we provide an overview of the impact of lineage plasticity on cancer progression and therapy resistance, with a focus on neuroendocrine transformation in lung and prostate tumors. We discuss our current understanding of the molecular drivers of this phenomenon, emerging management strategies, and open questions in the field.
Cancer cell plasticity can be operationally defined as the ability of a cell to substantially modify its identity and take on a new phenotype that more closely resembles a distinct developmental lineage. Such plasticity is increasingly recognized as playing a key role in drug resistance and metastasis, two major causes of cancer mortality. Lineage plasticity allows adaptation and survival of tumor cells in harsh environments notably including hypoxia and the selective pressure of potent targeted anti-cancer treatments1,2. Lineage plasticity may be both dependent on and a driver of intratumoral heterogeneity: increased diversity has been associated with therapeutic resistance and metastasis implying retention of pluripotent progenitors3, and the persistence of such progenitors can repopulate resistant or metastatic tumors with a diverse complement of cell phenotypes4. As such, lineage plasticity as a mechanism of tumor escape from a targeted dependency does not necessarily imply a complete or irreversible switch to another well-defined canonical lineage but may also include adoption of novel or hybrid lineages. As we discuss below, recent data from single cell profiling and other emerging technologies suggest that a pre-existing repertoire of cancer cell sub-populations exhibiting different epigenetic and transcriptomic characteristics, potentially coupled with adaptive shifts in gene expression programs under the selective pressure of therapy, may drive evolution of tumor assignment from one histologic category to another.
Tumor plasticity in preclinical models
Plasticity and metastasis
Loss of epithelial phenotype and induction of mesenchymal characteristics, a process known as epithelial-to-mesenchymal transition (EMT), has been associated with increased capacity for migration and invasion of tumor cells. EMT is typically characterized by downregulation of the cell adhesion molecule, E-cadherin, which may facilitate cancer cells escape from the primary tumor, entry into the bloodstream, and widespread dissemination. At metastatic sites, tumor cells have been described as undergoing a reverse process, mesenchymal-to-epithelial transition (MET), to generate metastases5.
EMT and MET are prime examples of tumor cell plasticity. Rios et al. recently established a large-scale single-cell resolution 3D imaging protocol allowing visualization of the clonal architecture of entire tumors6. In this study, imaging of multicolor-lineage tracing mouse models of breast cancer induced by the loss of Trp53 and Pten in either basal or luminal progenitor cells revealed the existence of multiple founder clones in every tumor. Analyses of each clone separately revealed cells exhibiting an EMT phenotype in almost every clone analyzed, characterized by the expression of typical mesenchymal genes, such as Zeb1, Cnn1 or Timp2 6. The observation that nearly all clones had the capacity for both mesenchymal and epithelial phenotypic differentiation supports a model of inherent plasticity for most tumor cells over an alternative model in which potential plasticity is limited to a rare subpopulation.
Several molecular drivers have been defined as triggering EMT. Hao et al. showed that TGFβ type II receptor (Tgfbr2) ablation in a PTEN-deficient mouse model of prostate cancer led to more proliferative and invasive tumors exhibiting an EMT signature with enrichment of E2F targets and stemness-related factors such as SOX2, KLF4, NANOG, and SALL4. Mutation of Pten and Tgfbr2 in luminal cells promoted emergence of a subset of dedifferentiated, invasive cells with an intermediate basal-luminal phenotype in the primary tumors; this population was substantially enriched in early metastases, supporting a role of Tgfbr2 as a suppressor of lineage plasticity in this setting7. These data illustrate the close relationship between plasticity, stemness and metastasis. In the context of the cancer stem cell theory – proposing that a subpopulation of tumor cells uniquely harbor indefinite progenitor capacity, including both self-renewal and differentiation into other tumor components – the reprogramming of epithelial cells to acquire metastatic potential would favor adoption of a progenitor state with a ¨deprogrammed¨ highly plastic phenotype over that of non-stem-like tumor cells exhibiting a higher level of differentiation 8–10. The EMT process may be able to induce a stem-like state, as the EMT transcriptional program shows partial overlap with that of stemness 11,12.
Adding a layer of complexity to the EMT process, plasticity may give rise to different EMT programs. Using a lineage-labeled murine model of Kras-mutated, P53-deficient pancreatic cancer, Aiello et al. described two different modes of EMT, characterized by downregulation of E-cadherin gene expression or internalization of E-cadherin protein from the membrane, with each displaying different migration patterns. Both EMT programs could reverse to an epithelial phenotype in vitro and in vivo, in agreement with the described epithelial phenotype of metastasis13.
There is accumulating insight into the epigenetic regulators that control EMT plasticity, with metastasis-specific methylation signatures identified in multiple malignancies, including breast and prostate tumors14–18. In some cases, during the initial stages of EMT, tumor cells exhibit a metastable intermediate phenotype, attributed to the coexistence of transcription-repressive (Histone H3 trimethylation on lysine 27 residue, H3K27me3) and -permissive (Histone H3 trimethylation on lysine 4 residue, H3K4me3) marks in histones associated with EMT genes such as CDH1 (encoding E-cadherin)19. This primed intermediate (bivalent) state may facilitate rapid gene expression pattern modification and interconversion between epithelial and mesenchymal phenotypes20–22, thereby facilitating adaptation of cells to environments favoring EMT, such as hypoxia or treatment-induced stress, as mesenchymal cells exhibit higher resistance to senescence and apoptosis23,24. Counterbalancing epigenetic modifiers including the histone demethylase LSD125,26 and the Polycomb repressive complexes 1 and 2 (PRC1/2)27–29 have been implicated as critical factors maintaining and controlling fate decisions from this bivalent state. Once mesenchymal-like tumor cells have reached a new, distant niche and established micrometastases, it is thought that a mesenchymal-to-epithelial transition takes place, involving reversal to an epithelial state. Recently, a role for protein kinase A (PKA) has been proposed in this setting. PKA is activated by cAMP, leading to the phosphorylation and activation of the histone demethylase PHF2 that promotes re-expression of epithelial genes30.
Stromal microenvironmental interactions at receptive sites for establishment of micrometastasis may also influence tumor plasticity leading to MET. Wingrove et al. compared the transcriptomes generated by brain metastases after arterial injection of cell lines of different cancer types (including breast, lung, and melanoma), to cells in 2D culture, or in subcutaneous and orthotopic xenografts. The brain metastases exhibited upregulation of neuronal-like pathways and CNS-enriched genes (DCLK1, HEY1, AKAP5, EFNB3, among others)31,32, effects that were reversible when these cells were separated from the brain stroma and cultured in vitro33,34.
These results highlight the robust plasticity between epithelial and mesenchymal states in tumor cells, dependent on an integration of both intrinsic and environmental cues. Maintenance of a flexible and bidirectional cellular potential for interconversion between epithelial and mesenchymal states appears to be essential for tumor capacity to colonize distant niches.
Plasticity and cell fate
Some of the same factors that drive deterministic cell fate decisions in development and organogenesis have been rediscovered in tumor biology as drivers of intratumoral heterogeneity and cancer lineage plasticity. Tata et al. showed in mouse models that loss of the lung lineage-specifying transcription factor Nkx2–1 in the alveolar epithelium leads to conversion to gastric-like cells, suggesting the existence of plasticity that would lead to the acquisition of cell fates characteristic of adjacent organs in the upper aerodigestive tract35. This transdifferentiation was not detected in epithelial cells lining the larger airways, suggesting that plasticity in this setting may be limited by the histologic/molecular context. In these models, concurrent oncogenic Kras activation increased apparent potential for plasticity, allowing conversion to gastric-like cells in both alveolar and airway epithelial cells, leading to mucinous adenocarcinoma formation. Loss of Nkx2–1 combined with overexpression of the transcription factor SOX2 was sufficient to generate squamous tumors with features of esophageal differentiation. SOX2 exhibits a dramatically altered genomic binding profile in the absence of Nkx2–1, which allows for the activation of a squamous differentiation program. This squamous differentiation occurred in vivo and in vitro, suggesting independence of this process from stroma35. These findings show that cancer cells can readily acquire cell fates associated with developmentally related organs, highlighting that tumor plasticity may mirror the developmental history of organs.
In the context of KRAS-driven lung adenocarcinoma, Lkb1 loss has been shown to induce an epigenomic reprogramming that drives tumor cells to a plastic state that enables squamous transformation36. Squamous transformed tumors from these Kras-mutant, Lkb1-deficient models, as well as human adenosquamous tumors, show loss of the PRC2-related H3K27me3 repressive chromatin mark, which promotes a squamous transcriptional program including upregulation of Ngfr, Sox2, ΔNp63, and Krt5/637,38. EZH2, the enzymatic subunit of the PRC2 complex, is actually upregulated in squamous tumors as compared to adenocarcinomas, presumably reflecting feedback as the levels of the EZH2-related H3K27me3 mark are reduced. The overall reduction in PRC2 activity has been attributed to downregulation of the PRC2 regulatory subunit EED, which is required for EZH2 canonical function39, and explains the lack of efficacy of pharmacologically or genetically targeting EZH2 to affect squamous conversion in these models. Interestingly, in these models only club cell and bronchioalveolar stem cell (BACS) progenitors appeared competent to generate adenosquamous tumors -- alveolar type II (AT2) cells did not, again supporting that particular plasticity modes may be limited by cell type.
Lineage tracing experiments have been key to identify relationships between progenitor and differentiated cells, and reveal differential plasticity in distinct tiers of differentiation. In the prostate setting, it has been shown that basal cells uniquely exhibit plasticity under inflammation conditions, supporting multi-lineage differentiation40, and similar results have been observed in the lung, where dedifferentiation of secretory cells and transdifferentiation into basal cells can occur following injury 41.
Plasticity and therapy resistance
Plasticity-driven intratumoral heterogeneity has been described to play a major role in therapy resistance acquisition in several settings, including prostate and lung tumors. Prostate adenocarcinomas are initially highly responsive to anti-androgen therapy, as are EGFR-mutant lung adenocarcinomas to EGFR inhibitors, but these therapies are suppressive rather than curative, and eventual emergence of acquired resistance occurs in nearly all patients42,43. Zou et al.44 provided evidence of plasticity in a TP53-/PTEN-deficient mouse model of prostate cancer in which tumors were less durably responsive to the anti-androgenic abiraterone than their counterparts from a PTEN deficient mouse model. TP53-/PTEN-deficient tumors display a variety of histologic subtypes, including squamous, sarcomatoid, small-cell neuroendocrine-like, and other non-adenocarcinoma phenotypes, which were not found in the single PTEN knock out model44. Interestingly, although the anti-androgenic abiraterone appeared to expand this histologic diversity, a full spectrum of histologic subtypes could be observed in untreated tumors, suggesting a shared role of oncogenic drivers and the selective pressure of anti-androgenic treatment in potentiating heterogeneity and thus, resistance. Multiple mechanisms of resistance to anti-androgens have been described in patients, some but not all of which include loss of androgen receptor (AR) expression. Mechanisms in therapy-resistant prostate cancers exhibiting AR loss include: (1) neuroendocrine-transformed prostate tumors (discussed in a subsequent section); (2) tumors with altered tyrosine kinase signaling (FGFR, MAPK) showing stemness characteristics, and sensitivity to the inhibition of these kinases45; and (3) tumors with upregulation of LSD1/KMD1A (a histone demethylase that regulates gene expression in stem cells) in which a demethylase activity-independent function of this enzyme leads to activation of an aggressive phenotype gene network46. In anti-androgen resistant tumors with retained AR expression, a subset of cases with an intermediate adenocarcinoma-neuroendocrine phenotype, displaying transcriptomic hallmarks of neuroendocrine tumors but with retained high AR expression have also been described. Here, resistance may be driven by epigenetic regulators such as BET (Bromodomain and Extra-Terminal Domain) family members, EZH2 or LSD1, inhibition of which restores sensitivity to anti-androgen therapy46,47.
Beyond prostate and lung, there are interesting examples of plasticity-derived therapy resistance in other malignancies. Basal cell carcinomas treated with a Hedgehog inhibitor have been shown to undergo epigenomic reprogramming to a stem-like phenotype mediated by Wnt signaling as a mechanism of acquired therapeutic resistance48,49. Similarly, there is emerging evidence of plasticity as a driver of treatment resistance in melanoma, with tumor cells adapting to MAPK inhibition by differentiation to a neural-crest-like state 50–52, and developing resistance to immunotherapy by induction of an EMT/stem-like phenotype with no expression of the melanocyte differentiation antigen53.
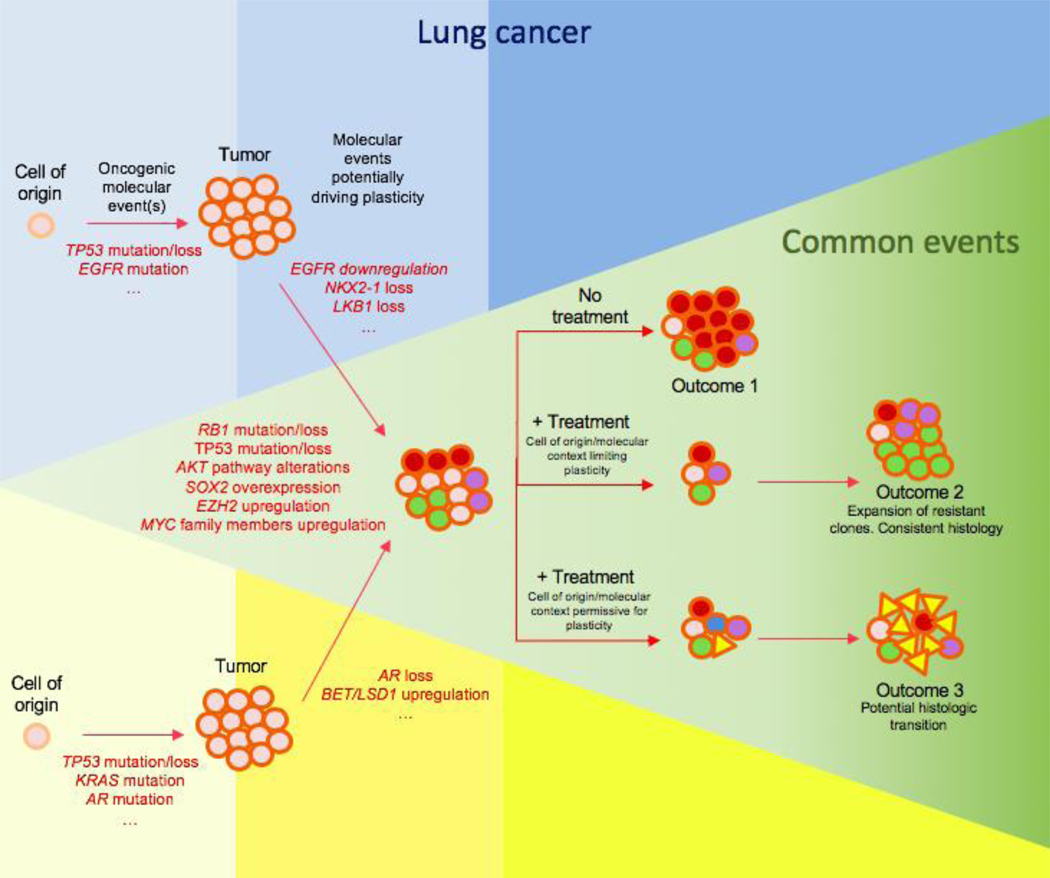
Taken together, these data support the idea that certain oncogenic mutations and suppressive treatments promote a plastic state in tumor cells, and that plasticity enables tumor cell diversification into, and potentially transdifferentiation among, multiple histological subtypes. These subtypes may differ in intrinsic oncogenic driver dependence: under the selective pressure of therapy, this heterogeneity promotes therapeutic escape and tumor progression (Figure 1).
Figure 1.
Schematic of how molecular context, lineage plasticity, and treatment-exerted selective pressure can lead to different outcomes, exhibiting exclusive molecular and cellular events for lung (blue) and prostate (yellow) tumors, and commonalities of these processes between both settings (green). In these tumors, a variety of molecular events can promote lineage plasticity, thus triggering intratumoral heterogeneity. A plasticity-permissive molecular environment, together with a selective pressure (i.e., treatment) may lead to intratumoral clones exhibiting an alternative histology to that initially diagnosed, which may become the predominant cell type in the progressed tumor. Colors in the circular figures (cells) represent different intratumoral subclones while shapes represent distinct histologies.
Molecular biology of NE transformation
The histological transformation from adenocarcinomas to high-grade neuroendocrine tumors has been the most thoroughly characterized lineage shift to date. Complementary molecular data has emerged from studies of EGFR-mutant lung adenocarcinomas with acquired resistance to anti-EGFR therapy and prostate adenocarcinomas with acquired resistance to anti-androgen therapy54–56. The derivative therapy-resistant neuroendocrine tumors consistently retain molecular features of the adenocarcinoma of origin including tumor-specific somatic mutations, supporting the derivation of the aggressive neuroendocrine tumor through lineage plasticity rather than emergence of a second primary cancer. These neuroendocrine-transformed tumors share many features of de novo high grade neuroendocrine tumors, including a high prevalence of RB1 and TP53 alterations, expression of neuroendocrine markers, and transient response to platinum-based chemotherapy regimens55,56. Acquired resistance in neuroendocrine derivatives of adenocarcinomas is typically associated with the downregulation the initial oncogenic driver protein, EGFR in lung and AR in prostate56,57. The rapid progression of these neuroendocrine derivative tumors despite silencing of the previous oncogenic driver implies a fundamental shift to an alternative mitogenic signaling mechanism. In this section, we will discuss the current understanding of this transition, and the potential therapeutic implications.
TP53 and RB1
Concomitant inactivation of RB1 and TP53, present in most neuroendocrine-transformed tumors (Figure 2A), is thought to be an early event in transformation, and at least in lung cancer appears to be consistently detectable in the pre-transformation adenocarcinoma (Figure 2B)54,55. Trp53 loss induced self-renewal activity in mammary luminal progenitors in a genetically engineered mouse model, consistent with the well-described role of TP53 as a repressor of genes involved in stemness, such as Nanog, Sox2, Oct4, and c-Myc58–60. Interestingly, Met upregulation, signaling of which has been associated with stemness and basal differentiation61, was detected in these cells62. However, the luminal phenotype of these cells was intact, suggesting that even if Trp53 loss could pave the way to lineage plasticity and basal differentiation through the induction of stemness and Met overexpression, the loss of function of this gene is not sufficient for full neuroendocrine transformation. On the other hand, in Pten loss-driven mouse models of prostate adenocarcinoma (PADC), Rb1 loss resulted in emergence of a population of tumor cells with low expression of the epithelial marker Krt8, high expression of the neuroendocrine marker Synaptophysin, and suppressed AR expression. Interestingly, a subset of the relapsed tumors after castration in this setting exhibited Trp53 mutations; these mutations were not detected in tumors from noncastrated mice, suggesting cooperation between RB1 and TP53 in treatment resistance. TP53 loss was associated with lower AR expression in the castration-resistant tumors, which again showed heterogeneity in terms of intratumoral synaptophysin expression. Gene expression analysis of these murine tumors revealed altered expression of E2F target genes and neuroendocrine lineage genes, together with increased expression of stemness- and epigenetic reprogramming-related genes such as SOX2 and EZH2; these results were validated in human prostate tumors63. These data, together with the high prevalence of RB1 and TP53 alterations in neuroendocrine-transformed tumors, emphasize the key role of TP53 and RB1 loss in neuroendocrine transition. However, additional molecular events or selective pressures (exerted by therapy, microenvironment, hypoxia, or other means) seem to be required in the lung setting, as TP53/RB1 abrogation was not enough to induce a neuroendocrine phenotype or anti-EGFR therapy resistance in lung cancer cell lines57, and most tumors harboring concurrent TP53/RB1 alterations do not undergo neuroendocrine transformation64. Pointing to pathways of interest, additional factors (e.g. MYC/BCL2 overexpression and AKT overactivation) were notably required to induce neuroendocrine prostate and lung differentiation and dysregulated growth from normal epithelial cells65.
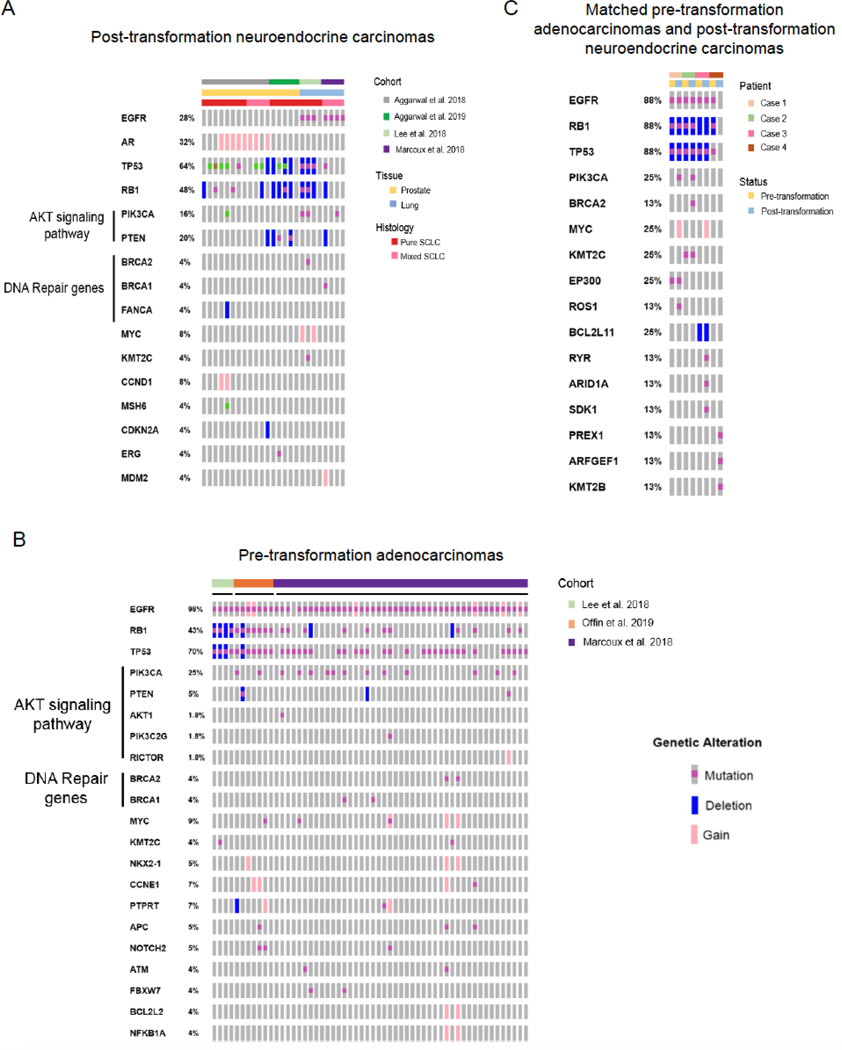
Figure 2.
Prevalent mutations in pre- and post- neuroendocrine transformed lung and prostate adenocarcinoma. (A) Oncoprint showing the most prevalent mutations in post-transformation neuroendocrine lung and prostate tumors from multiple studies55,56,97,130, including pure and mixed histology tumors. (B) Oncoprint showing the most prevalent mutations in lung adenocarcinoma samples that subsequently underwent neuroendocrine-transformation in several studies54,55,97. (C) Oncoprint showing the mutations detected in matched pre- and post-neuroendocrine transformation cases from 55. Grey boxes represent unaltered or untested alterations.
MYC family members
The MYC family of proteins has been extensively implicated in cellular reprogramming, functioning as master transcriptional regulators, modulating the activity of epigenetic control elements and in some instances promoting a plasticity-permissive stem-like state66,56. Expression of the N-MYC oncoprotein is higher in neuroendocrine than in non-neuroendocrine castration resistant prostate cancer67. Overexpression of this MYC family member in prostate epithelial cells in Pten-deficient murine models attenuates AR signaling, induces anti-androgen therapy resistance, and increases the incidence of neuroendocrine tumors67–69. Recent investigations using a Pten-deficient, Mycn-overexpressing mouse model have demonstrated an interplay in vitro and in vivo between N-Myc and EZH2, AR, and various AR co-factors including FOXA1 and HOXB1367,70. This interplay was characterized by (1) an increase of N-myc deregulated genes in the absence of AR signaling, including genes associated with neural development (e.g. SOX11, SOX21, NTRK1, NKX2–1), stemness (HOXA2/A3/A9/A10, WNT5A and SOX2), and neuroendocrine markers (CHGA) and (2) N-myc-mediated inhibition of androgen response gene sets in response to castration71.
Another member of the MYC family, c-MYC, has also been implicated in neuroendocrine transformation. MYC is frequently amplified in neuroendocrine transformed tumors in lung and prostate (Figure 2A), as well as in pre-transformation adenocarcinoma, implicating this key transcriptional regulator in the early steps of transformation (Figure 2B). In cooperation with Pim1 kinase, c-MYC has been reported to drive invasive prostate tumors with neuroendocrine differentiation in prostate xenografts72. Notably however, context clearly influences the result of c-MYC overexpression: c-MYC gene amplification is a common feature in human prostate adenocarcinoma and its overexpression has been used as a driver of murine models of this disease73,74. Going beyond lung and prostate, in a murine model of Kras-mutant pancreatic cancer, MYC overexpression drove a neuroendocrine phenotype in a subpopulation of tumor cells with increased resistance to gemcitabine. Gemcitabine treatment of pancreatic cancer cell lines and PDXs resulted in an increase in the fraction of tumor cell demonstrating hallmarks of neuroendocrine phenotype including expression of multiple neuroendocrine markers, a phenomenon that was abrogated by MYC knockdown75.
AKT/mTOR signaling pathway
Alterations in AKT signaling are associated with therapeutic resistance and may be involved in neuroendocrine transformation in both lung and prostate tumors (Figures 2A–B). Pten loss in murine models of prostate adenocarcinoma induces a shift from luminal to basal features within intraepithelial neoplasias76, consistent with a role in driving lineage plasticity77. In addition, together with other factors (TP53 abrogation, RB1 downregulation, and overexpression of MYC and BCL2), a constitutively activated variant of AKT (myristylated AKT) was essential for induction of a neuroendocrine phenotype in primary basal prostate epithelial cells and in primary normal human bronchial epithelial cells65. Mutations and copy number alterations in multiple members of the AKT signaling pathway, including PTEN, PIK3CA, RICTOR, and AKT1, are frequently found in transformed neuroendocrine tumors in both prostate65,69 and lung55,57, are potential promoters of neuroendocrine transformation in lung54, and have been similarly observed in pre-transformed adenocarcinoma (Figure 2B). Everolimus, an inhibitor of AKT/mTOR signaling, prolonged PFS in neuroendocrine pancreatic tumor patients, suggesting that AKT signaling may sustain neuroendocrine tumors of this type78.
SOX family members
The influence of the SOX transcription factor family on reprogramming and stemness has been deeply explored. Differential expression of SOX family members contributes to epigenomic remodeling and induction of differential transcriptional programs including those promoting dedifferentiation and plasticity permissiveness79, supporting a central role of SOX proteins in histological transformation. The transcription factor SOX2 appears to play a role in neuroendocrine lung tumors and as a driver of lineage plasticity leading to antiandrogen therapy resistance and neuroendocrine transformation in prostate tumors80–82. TP53 and RB1 knockdown led to enzalutamide (antiandrogen) resistance and to the upregulation of basal markers, neuroendocrine markers and lineage-defining/stemness-related transcription factors, as well as to the downregulation of luminal cell markers. The rapid global alteration in expression of these genes, and reversal to their original state after re-expression of TP53 and RB1, suggested that these effects were due to a population-wide shift in lineage, rather than to the selection of a rare subpopulation of enzalutamide-resistant cells under the pressure of the drug82. Furthermore, knockdown of SOX2 expression in the context of TP53/RB1 suppression, restored enzalutamide resistance and reduced basal and neuroendocrine marker expression82. In accordance with these results, SOX2 and another member of the SOX family, SOX4, were bioinformatically inferred as master regulator transcription factors defining the gene expression signature of treatment-induced neuroendocrine prostate tumors56.
Neuroendocrine tumors arising in the Tp53/Pten-deficient mouse model of prostate cancer under selection of anti-androgen therapy display transcriptional features similar to human neuroendocrine prostate cancer and are similarly resistant to anti-androgen treatment. These neuroendocrine derivates were notable for undetectable expression of AR and overexpression of neuroendocrine markers such as Synaptophysin, Chromogranin A, FOXA2, and neuron-specific enolase (NSE). These tumors also displayed expression of the luminal marker CK8 but not of the basal marker CK5, suggesting that neuroendocrine cells in this model were derived from luminal cells. This hypothesis was further confirmed in lineage tracing experiments, thus providing evidence of a lineage plasticity role in the derivation of these neuroendocrine-transformed tumors. The pan-neuronal differentiation factor SOX1183,84 was one of the most upregulated genes in these tumors. Downregulation of SOX11 led to decreased expression of NSE and synaptophysin, thus providing evidence of the role of this SOX family member in inducing, or at least maintaining, the acquired neuroendocrine phenotype.
Other molecular drivers
Multiple other factors have been implicated in promoting lineage plasticity in cancer. The cell cycle kinase Aurora kinase A (AURKA) cooperates with N-Myc in prostate neuroendocrine differentiation68. AURKA amplification is prevalent in anti-androgen resistant neuroendocrine prostate tumors and has been proposed as an early biomarker of neuroendocrine transformation in this setting 68,85. Recent reports also suggest that AURKA inhibitors may have efficacy against neuroendocrine tumors86,87.
The transcription factor FOXA1 may also be involved in lineage plasticity in prostate cancer. Castration leads to a rapid downregulation of tumor FOXA1. Exogenous silencing of FOXA1 in prostate cancer cell lines led to inhibition of neuroendocrine differentiation, supporting a potential role for this factor in neuroendocrine transformation88. Likewise, certain FOXA1 mutations exhibiting a gain of function phenotype promote a pro-luminal differentiation program, similar to wild type FOXA1 overexpression. These mutations, occurring in the R219 amino acid residue, were associated with a neuroendocrine phenotype, and to increased prevalence of invasive, intraductal basal disease, defined by the loss of AR expression in vivo in PTEN-deficient organoid xenografts89.
In addition, the ETS family transcription factor ERG appears to have a role in suppressing neuroendocrine transformation90,91. In PTEN-/TP53-deficient mouse models, ERG overexpression promoted the maintenance of AR and luminal epithelial marker expression. These effects were mediated by the suppression of cell-cycle related genes by ERG, leading to RB hyperphosphorylation and downregulation of E2F1-mediated EMT regulators, which restricted the plasticity of these tumors, resulting in maintained antiandrogen sensitivity. In contrast, tumors with no ERG expression were more prone to neuroendocrine transformation and exhibited a reliance on the RB1/E2F1 network with resultant sensitivity to the CDK4/6 inhibitor palbociclib92.
Other genes have been found to be altered in association with transformation. Recently, Offin et al. reported 7 cases of SCLC transformation in EGFR-mutant patients, where mutations in the pre-transformed adenocarcinoma tumors were analyzed and compared to never transformed EGFR-mutated adenocarcinomas with the same genomic signature (i.e. RB1 and TP53 mutations/loss). Several alterations were enriched in the cases that transformed, including NOTCH2, ELF3, and CCNE1, all of which regulate pathways involved in neuroendocrine tumor biology (Figure 2B). Whether these alterations in fact influence lineage plasticity has not been experimentally tested.
In addition to single genetic alterations, large scale genomic alterations and mutational signatures have been associated with neuroendocrine transformation. Enrichment in the activation-induced cytidine deaminase (AID)/apolipoprotein B mRNA editing enzyme, catalytic polypeptide-like (APOBEC) hypermutation signature and whole genome doubling have been observed in cohorts of SCLC-transformed tumors54,55,93. These molecular events, occurring after RB1 and TP53 mutation54, are enriched in the adenocarcinoma tumors that later underwent transformation, suggesting that RB1/TP53 loss may promote APOBEC mutagenesis and genome doubling, or that these mechanisms may promote a plasticity-permissive state where histological transformation is more likely to occur.
Lineage transition: Clinical approaches
Neuroendocrine transition management
While there has been a growing body of molecular and mechanistic insights into tumor lineage plasticity, the translational clinical implications of these data are still unclear. There have been no large-scale clinical studies to inform the optimal clinical management of histological transformation in the context of acquired resistance to targeted therapies in lung or prostate cancer patients. The increasing practice of re-biopsy of tumors after recurrence42,94, together with the optimization of the isolation and identification of neuroendocrine prostate cancer (NEPC) tumor cells from peripheral blood95, has improved our capacity to identify such cases, setting the stage for consideration of clinical trials in these patient populations.
Histologic transformation may occur in up to 5% of EGFR mutant lung adenocarcinomas64, and at least 20% of prostate adenocarcinomas on targeted therapies56. Median time to neuroendocrine transformation in EGFR-mutant lung adenocarcinomas is approximately 19 months after initiation of anti-EGFR therapy96, and, as noted, occurs primarily in a subset of tumors with concomitant detectable loss-of-function mutations in both RB1 and TP5354. Confirming the shared evolutionary history with the original adenocarcinoma55,94, transformed SCLCs harbor the original activating EGFR-mutation, but EGFR protein expression is downregulated following transdifferentiation57. Identifying the 3-gene mutational signature (EGFR/RB1/TP53) at diagnosis provides an opportunity for early intervention trials in these patients at risk.
The clinical outcomes after transformation mimic those of primary SCLC, exhibiting rapid progression on TKI treatment and transient response to SCLC-directed chemotherapies, with a median progression-free and overall survival of 3.4 and 10.9 months, respectively97, comparable to outcomes of classical extensive-stage SCLC (5.5 and 9.6 months, respectively)98. The most comprehensive analysis to date of treatment response in transformed cases was a retrospective multi-institutional report on 67 patients with EGFR-mutant SCLC and other high-grade neuroendocrine carcinomas97, including 9 patients with SCLC at initial diagnosis with EGFR-activating mutations. Transformed SCLC was highly responsive to small-cell directed systemic chemotherapy, including the chemotherapy doublet platinum-etoposide (54% response rate), and similar responses were observed in 8 out of 10 patients that had received platinum therapy for prior adenocarcinoma, comparable to the 60–70% response rate for induction regimens in de novo extensive stage SCLC99. Taxane chemotherapy was administered in 21 patients, with a median of 2 prior treatments following SCLC transformation and had a reported response rate of 50%. Although the numbers are small, chemotherapeutic treatment with paclitaxel and nab-paclitaxel had particularly high response rates of 71% (each with 5 responses out of 7 treated patients)5. By contrast, there were no responses among the 17 patients who received immunotherapy with either PD-1 or PD-L1 inhibitors, or even combination ipilimumab-nivolumab, seemingly underperforming the low response rates of immune checkpoint inhibitors in pre-treated SCLC or EGFR-mutant adenocarcinoma100. In 52% of patients, EGFR TKI therapy was continued beyond SCLC transformation, typically in combination with cytotoxic chemotherapy, presumably to target potential residual NSCLC clones, but the efficacy of this intervention remains unclear.
Due to the rapid relapse after chemotherapy, novel therapeutic options are being explored, but so far there are limited data on alternative treatments for transformed disease, with no randomized or prospective trials completed. Given the observation that SCLC histologic transformation of EGFR-mutant lung adenocarcinomas occurs in the context of concomitant RB1 and TP53 mutations, an ongoing clinical trial (NCT03567642) is exploring an interventional strategy of initial tumor suppression with the EGFR inhibitor osimertinib, followed by 4 cycles of platinum/etoposide and continued osimertinib, in patients with triple-mutant EGFR/RB1/TP53 adenocarcinomas, with the cytotoxic therapy intended to eliminate or maximally suppress the presumed precursor clone of transformed SCLC. Other novel approaches suggested by preclinical data have not yet reached clinical testing (Box 1). In terms of metastatic tropisms, brain metastases are frequent after transformation, occurring in 64% of these patients, similar to observations in classical SCLC97.
BOX1. Potential novel therapies for neuroendocrine-transitioned tumors.
The treatments most commonly employed for neuroendocrine-transformed tumors are essentially those used for primary small cell lung cancer, i.e. etoposide with a platinum agent122–124. However, targeted therapies that may have utility for transformed tumors are being defined. Niederst et al. showed that the antiapoptotic gene BCL2, a factor involved in potentiating neuroendocrine phenotypes and which is frequently overexpressed in neuroendocrine tumors65,125, may be a target in neuroendocrine-transformed tumors, as cell lines derived from SCLC-transformed tumors showed high sensitivity and a robust apoptotic response to the BCL2 inhibitor, navitoclax57. Puca et al. suggested another potential therapeutic target for neuroendocrine-prostate tumors, Delta-like protein 3 (DLL3). This receptor is expressed in over 75% of castration-resistant neuroendocrine prostate tumors and associated with worse overall survival in this setting. DLL3 was first noted as a therapeutic target in SCLC126,127.
Several studies have demonstrated effects of epigenetic modulators on plasticity. Pharmacologic or genetic inhibition of EZH2 increased AR expression, decreased synaptophysin expression, and restored sensitivity to the AR agonist enzalutamide in an anti-androgen resistant neuroendocrine Pten-/RB1-deficient mouse model63. LSD1 inhibitors (T-3775440 and SP-2509) have shown efficacy in transformed NEPC in vivo models, as well as in SCLC PDXs46,128. Bromodomain inhibitors such as JQ1, a small molecule inhibitor targeting the amino-terminal domain of BRD4, have shown promising efficacy in vivo in CRPC models 129.
In the prostate setting, neuroendocrine prostate cancer (NEPC) is rare at initial diagnosis, occurring in less than 2% of cases68. It is more commonly found upon recurrence in the setting of castrate-resistant prostate cancer (CRPC), as a primary mechanism of resistance to androgen deprivation therapy (ADT)57,101. Autopsy series demonstrate that 10–20% of patients dying from CRPC harbor small cell morphology, suggesting that NEPC may be substantially underdiagnosed102,103, probably due to failure to rebiopsy as well as to intratumoral heterogeneity and sampling variability. These tumors are typically associated with detectable serum biomarkers including neuroendocrine markers of chromogranin and synaptophysin, often accompanied by a drop in prostate specific antigen (PSA) levels56,104,105, and exhibit loss-of-function alterations in TP53, RB1, and PTEN56.
Optimal treatment options of NEPC remain to be defined. Pure small cell tumors have variable sensitivity to the platinum-based chemotherapy regimens that are used for SCLC, with reported response rates of 10–50%106–108. For non-pure small cell variant neuroendocrine tumors, several therapies have been explored, including taxanes (cabazitaxel, docetaxel) and platinum chemotherapy106. Given the generally poor outcomes in overall survival regardless of initial chemosensitivity, ongoing clinical trials are investigating novel options in this setting, such as olaparib (PARP inhibitor) maintenance following cabazitaxel-carboplatin in patients with aggressive-variant prostate cancer (NCT03263650), or the addition of the immune checkpoint inhibitor pembrolizumab to combination chemotherapy in patients with locally advanced or metastatic small cell/neuroendocrine cancers in either the bladder or prostate (NCT03582475).
Squamous transition management
In addition to neuroendocrine transformation, an alternative lineage plasticity mechanism involving squamous differentiation has been recently described in lung adenocarcinoma tumors, in the context of acquired resistance to EGFR TKI therapy109. A systematic review of 11 case reports identified squamous transformation associated with resistance to first-generation EGFR TKI as initial therapy, with median interval to treatment failure of 9.5 months (5 months – 2 years). While none of these cases definitively exclude an occult squamous component of an adenosquamous carcinoma being selected for under treatment pressure from EGFR TKI therapy, 3 patients had adenocarcinoma diagnosed from surgical resections as opposed to needle biopsies. The other 8 patients had a median duration of response to EGFR TKI therapy of 9 months, substantially exceeding the reported 3.1 month median for cases of non-adenocarcinoma NSCLC with activating EGFR mutation109, suggesting that initial response was maintained in pure adenocarcinoma until squamous transformation occurred. Although limited in number, available cases of apparent squamous transformation retained the characteristic EGFR mutation of the adenocarcinoma, supporting the hypothesis of lineage transformation. In addition, there are 2 case reports of squamous transformation conferring resistance to ALK inhibitors in the setting of EML4-ALK fusion, suggesting that squamous transdifferentiation may not be exclusive of EGFR mutant tumors treated with EGFR TKIs110,111.
Regarding the clinical management of these cases, no specific treatment of squamous transformation has been so far established. In de novo squamous lung carcinoma, pemetrexed-based chemotherapy is inferior to gemcitabine and docetaxel112. In one case report of a patient with progression on first-line EGFR TKI due to concomitant squamous transformation and secondary EGFR T790M mutation, osimertinib, but not combined pemetrexed-carboplatin, generated a response113. Prospective studies are needed to refine recommendations for treatment in patients with squamous transformation.
Conclusions and open questions
Increasing evidence links lineage plasticity, therapy resistance, and metastasis through mechanisms including EMT and histological transformation. Neuroendocrine transformation is increasingly recognized as an important mechanism of acquired therapeutic resistance in both lung and prostate adenocarcinomas. The data presented here support concomitant inactivation of TP53 and RB1 as a shared requirement of this transition across sites of origin. Loss of both tumor suppressor genes may facilitate transition to a plastic, stem-like state in which lineage switching is possible, but TP53/RB1 loss is not sufficient for the transformation to occur. Additional factors or genomic alterations, including aberrant overexpression of MYC/SOX family members, AKT pathway activation, and others, have been suggested as contributing to the shift to a neuroendocrine phenotype. EGFR (lung) or AR (prostate) signaling may oppose transformation; each of these mitogenic drivers is suppressed in the transformed cases. EGFR or AR signaling may activate a transcriptional program promoting an epithelial phenotype, pushing the tumor cells to a defined lineage and restraining plasticity. In this scenario, EGFR inhibitors and anti-androgens may reduce this lineage constraint, establishing permissivity and providing a selective pressure for EGFR-/AR-independent neuroendocrine transformation.
Neuroendocrine transformation may be a broader mechanism of acquired resistance to targeted therapies directed against key mitogenic drivers in cancer. A neuroendocrine gene expression signature associated with poor prognosis has been identified with application across all epithelial malignancies, suggesting that neuroendocrine transformation occurs in a wide variety of cancer types114. Further comprehensive molecular characterization of this histological transition in lung, prostate and other malignancies will determine if universal mechanisms may be governing this plasticity phenomenon across different tumor types. Among other contexts, a similar neuroendocrine transformation has been observed in ALK-translocated lung adenocarcinomas treated with potent and specific ALK inhibitors115,116. Neuroendocrine transformation may also occur independent of treatment, as in the cases of treatment-naïve SCLC with canonical oncogenic EGFR mutations 54,117. These cases are generally in non- or light-smokers, in contrast to typical SCLC but consistent with the population of EGFR-mutant lung adenocarcinoma patients. Neuroendocrine transformation may not be restricted to adenocarcinoma histologies, as there are reports of squamous tumors of the head and neck or lung that exhibit neuroendocrine differentiation, including combined squamous and SCLC tumors118–120.
Recently, a novel molecular classification of SCLC tumors has been proposed, based on relative expression of 4 transcriptional regulators: ASCL1, NEUROD1, POU2F3 and YAP1121. Little is known about the molecular subtyping of neuroendocrine-transformed lung tumors, or whether these tumors consistently align with one of these four defined subtypes. Characterization of these molecular features in neuroendocrine-transformed tumors will provide further insight into the molecular biology of these tumors, with potential implications for therapeutic response and patient outcome.
Importantly, most of the currently available data on lineage plasticity as a mechanism of acquired drug resistance relies on bulk sequencing, which is only able to estimate the average clonal genotype of a tumor and is likely to miss clonal populations with low representation in the tumors. Application of the current, emerging, and future single-cell sequencing technologies to the histological transformation question will provide further insight into the molecular biology of this phenomenon, potentially identifying intratumoral cell populations with molecular characteristics corresponding to novel or intermediate histologic subtypes. Detailed single cell profiling may help to unravel how relevant subpopulations within these tumors interact and under pressure of therapy undergo histological transformation.
Key Points.
Lineage plasticity can promote both metastasis and therapy resistance.
Histologic transformation may occur in up to 5% of EGFR mutant lung adenocarcinomas and at least 20% of prostate adenocarcinomas on targeted therapy.
RB1 and TP53 deficiency are implicated in but not sufficient for neuroendocrine transformation.
AKT pathway activation and aberrant activity of the MYC and SOX families of transcriptional regulators have been implicated as effectors of histological transformation.
Acknowledgements
This work was supported by grants from the US National Institutes of Health, including U24CA213274 and R01CA197936 (CMR).
REFERENCES
- 1.Sipos F, Constantinovits M & Muzes G Intratumoral functional heterogeneity and chemotherapy. World J Gastroenterol 20, 2429–2432, doi: 10.3748/wjg.v20.i10.2429 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zellmer VR & Zhang S Evolving concepts of tumor heterogeneity. Cell Biosci 4, 69, doi: 10.1186/2045-3701-4-69 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Calbo J et al. A functional role for tumor cell heterogeneity in a mouse model of small cell lung cancer. Cancer Cell 19, 244–256, doi: 10.1016/j.ccr.2010.12.021 (2011). [DOI] [PubMed] [Google Scholar]
- 4.Lim JS et al. Intratumoural heterogeneity generated by Notch signalling promotes small-cell lung cancer. Nature 545, 360–364, doi: 10.1038/nature22323 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dongre A & Weinberg RA New insights into the mechanisms of epithelial-mesenchymal transition and implications for cancer. Nat Rev Mol Cell Biol 20, 69–84, doi: 10.1038/s41580-018-0080-4 (2019). [DOI] [PubMed] [Google Scholar]
- 6.Rios AC et al. Intraclonal Plasticity in Mammary Tumors Revealed through Large-Scale Single-Cell Resolution 3D Imaging. Cancer Cell 35, 618–632 e616, doi: 10.1016/j.ccell.2019.02.010 (2019). [DOI] [PubMed] [Google Scholar]
- 7.Hao Y et al. TGFbeta signaling limits lineage plasticity in prostate cancer. PLoS Genet 14, e1007409, doi: 10.1371/journal.pgen.1007409 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guo W et al. Slug and Sox9 cooperatively determine the mammary stem cell state. Cell 148, 1015–1028, doi: 10.1016/j.cell.2012.02.008 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mani SA et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell 133, 704–715, doi: 10.1016/j.cell.2008.03.027 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grosse-Wilde A et al. Stemness of the hybrid Epithelial/Mesenchymal State in Breast Cancer and Its Association with Poor Survival. PLoS One 10, e0126522, doi: 10.1371/journal.pone.0126522 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McCoy EL et al. Six1 expands the mouse mammary epithelial stem/progenitor cell pool and induces mammary tumors that undergo epithelial-mesenchymal transition. J Clin Invest 119, 2663–2677, doi: 10.1172/JCI37691 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yu M et al. A developmentally regulated inducer of EMT, LBX1, contributes to breast cancer progression. Genes Dev 23, 1737–1742, doi: 10.1101/gad.1809309 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aiello NM et al. EMT Subtype Influences Epithelial Plasticity and Mode of Cell Migration. Dev Cell 45, 681–695 e684, doi: 10.1016/j.devcel.2018.05.027 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reyngold M et al. Remodeling of the methylation landscape in breast cancer metastasis. PloS one 9, e103896, doi: 10.1371/journal.pone.0103896 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Munoz DP et al. Activation-induced cytidine deaminase (AID) is necessary for the epithelial-mesenchymal transition in mammary epithelial cells. Proceedings of the National Academy of Sciences of the United States of America 110, E2977–2986, doi: 10.1073/pnas.1301021110 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lopez-Lago MA et al. Genomic deregulation during metastasis of renal cell carcinoma implements a myofibroblast-like program of gene expression. Cancer research 70, 9682–9692, doi: 10.1158/0008-5472.CAN-10-2279 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carmona FJ et al. Epigenetic disruption of cadherin-11 in human cancer metastasis. The Journal of pathology 228, 230–240, doi: 10.1002/path.4011 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ezponda T et al. The histone methyltransferase MMSET/WHSC1 activates TWIST1 to promote an epithelial-mesenchymal transition and invasive properties of prostate cancer. Oncogene 32, 2882–2890, doi: 10.1038/onc.2012.297 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cedar H & Bergman Y Linking DNA methylation and histone modification: patterns and paradigms. Nature reviews. Genetics 10, 295–304, doi: 10.1038/nrg2540 (2009). [DOI] [PubMed] [Google Scholar]
- 20.Bernstein BE et al. A bivalent chromatin structure marks key developmental genes in embryonic stem cells. Cell 125, 315–326, doi: 10.1016/j.cell.2006.02.041 (2006). [DOI] [PubMed] [Google Scholar]
- 21.Maruyama R et al. Epigenetic regulation of cell type-specific expression patterns in the human mammary epithelium. PLoS Genet 7, e1001369, doi: 10.1371/journal.pgen.1001369 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chaffer CL et al. Poised chromatin at the ZEB1 promoter enables breast cancer cell plasticity and enhances tumorigenicity. Cell 154, 61–74, doi: 10.1016/j.cell.2013.06.005 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lamouille S, Xu J & Derynck R Molecular mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell Biol 15, 178–196, doi: 10.1038/nrm3758 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen S et al. Conversion of epithelial-to-mesenchymal transition to mesenchymal-to-epithelial transition is mediated by oxygen concentration in pancreatic cancer cells. Oncol Lett 15, 7144–7152, doi: 10.3892/ol.2018.8219 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 25.Lim S et al. Lysine-specific demethylase 1 (LSD1) is highly expressed in ER-negative breast cancers and a biomarker predicting aggressive biology. Carcinogenesis 31, 512–520, doi: 10.1093/carcin/bgp324 (2010). [DOI] [PubMed] [Google Scholar]
- 26.Harris WJ et al. The histone demethylase KDM1A sustains the oncogenic potential of MLL-AF9 leukemia stem cells. Cancer Cell 21, 473–487, doi: 10.1016/j.ccr.2012.03.014 (2012). [DOI] [PubMed] [Google Scholar]
- 27.Dong C et al. Interaction with Suv39H1 is critical for Snail-mediated E-cadherin repression in breast cancer. Oncogene 32, 1351–1362, doi: 10.1038/onc.2012.169 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Herranz N et al. Polycomb complex 2 is required for E-cadherin repression by the Snail1 transcription factor. Molecular and cellular biology 28, 4772–4781, doi: 10.1128/MCB.00323-08 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dong C et al. G9a interacts with Snail and is critical for Snail-mediated E-cadherin repression in human breast cancer. The Journal of clinical investigation 122, 1469–1486, doi: 10.1172/JCI57349 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pattabiraman DR et al. Activation of PKA leads to mesenchymal-to-epithelial transition and loss of tumor-initiating ability. Science 351, aad3680, doi: 10.1126/science.aad3680 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Park ES et al. Cross-species hybridization of microarrays for studying tumor transcriptome of brain metastasis. Proceedings of the National Academy of Sciences of the United States of America 108, 17456–17461, doi: 10.1073/pnas.1114210108 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sato R et al. RNA Sequencing Analysis Reveals Interactions between Breast Cancer or Melanoma Cells and the Tissue Microenvironment during Brain Metastasis. Biomed Res Int 2017, 8032910, doi: 10.1155/2017/8032910 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wingrove E et al. Transcriptomic Hallmarks of Tumor Plasticity and Stromal Interactions in Brain Metastasis. Cell Rep 27, 1277–1292 e1277, doi: 10.1016/j.celrep.2019.03.085 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kiss M, Van Gassen S, Movahedi K, Saeys Y & Laoui D Myeloid cell heterogeneity in cancer: not a single cell alike. Cell Immunol 330, 188–201, doi: 10.1016/j.cellimm.2018.02.008 (2018). [DOI] [PubMed] [Google Scholar]
- 35.Tata PR et al. Developmental History Provides a Roadmap for the Emergence of Tumor Plasticity. Dev Cell 44, 679–693 e675, doi: 10.1016/j.devcel.2018.02.024 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang H et al. Lkb1 inactivation drives lung cancer lineage switching governed by Polycomb Repressive Complex 2. Nat Commun 8, 14922, doi: 10.1038/ncomms14922 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mukhopadhyay A et al. Sox2 cooperates with Lkb1 loss in a mouse model of squamous cell lung cancer. Cell Rep 8, 40–49, doi: 10.1016/j.celrep.2014.05.036 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ferone G et al. SOX2 Is the Determining Oncogenic Switch in Promoting Lung Squamous Cell Carcinoma from Different Cells of Origin. Cancer Cell 30, 519–532, doi: 10.1016/j.ccell.2016.09.001 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim W et al. Targeted disruption of the EZH2-EED complex inhibits EZH2-dependent cancer. Nat Chem Biol 9, 643–650, doi: 10.1038/nchembio.1331 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kwon OJ, Zhang L, Ittmann MM & Xin L Prostatic inflammation enhances basal-to-luminal differentiation and accelerates initiation of prostate cancer with a basal cell origin. Proc Natl Acad Sci U S A 111, E592–600, doi: 10.1073/pnas.1318157111 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tata PR & Rajagopal J Plasticity in the lung: making and breaking cell identity. Development 144, 755–766, doi: 10.1242/dev.143784 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yu HA et al. Analysis of tumor specimens at the time of acquired resistance to EGFR-TKI therapy in 155 patients with EGFR-mutant lung cancers. Clin Cancer Res 19, 2240–2247, doi: 10.1158/1078-0432.CCR-12-2246 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huang Y, Jiang X, Liang X & Jiang G Molecular and cellular mechanisms of castration resistant prostate cancer. Oncol Lett 15, 6063–6076, doi: 10.3892/ol.2018.8123 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zou M et al. Transdifferentiation as a Mechanism of Treatment Resistance in a Mouse Model of Castration-Resistant Prostate Cancer. Cancer Discov 7, 736–749, doi: 10.1158/2159-8290.CD-16-1174 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bluemn EG et al. Androgen Receptor Pathway-Independent Prostate Cancer Is Sustained through FGF Signaling. Cancer Cell 32, 474–489 e476, doi: 10.1016/j.ccell.2017.09.003 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sehrawat A et al. LSD1 activates a lethal prostate cancer gene network independently of its demethylase function. Proc Natl Acad Sci U S A 115, E4179–E4188, doi: 10.1073/pnas.1719168115 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Welti J et al. Targeting Bromodomain and Extra-Terminal (BET) Family Proteins in Castration-Resistant Prostate Cancer (CRPC). Clin Cancer Res 24, 3149–3162, doi: 10.1158/1078-0432.CCR-17-3571 (2018). [DOI] [PubMed] [Google Scholar]
- 48.Biehs B et al. A cell identity switch allows residual BCC to survive Hedgehog pathway inhibition. Nature 562, 429–433, doi: 10.1038/s41586-018-0596-y (2018). [DOI] [PubMed] [Google Scholar]
- 49.Sanchez-Danes A et al. A slow-cycling LGR5 tumour population mediates basal cell carcinoma relapse after therapy. Nature 562, 434–438, doi: 10.1038/s41586-018-0603-3 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tsoi J et al. Multi-stage Differentiation Defines Melanoma Subtypes with Differential Vulnerability to Drug-Induced Iron-Dependent Oxidative Stress. Cancer Cell 33, 890–904 e895, doi: 10.1016/j.ccell.2018.03.017 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rambow F et al. Toward Minimal Residual Disease-Directed Therapy in Melanoma. Cell 174, 843–855 e819, doi: 10.1016/j.cell.2018.06.025 (2018). [DOI] [PubMed] [Google Scholar]
- 52.Fallahi-Sichani M et al. Adaptive resistance of melanoma cells to RAF inhibition via reversible induction of a slowly dividing de-differentiated state. Mol Syst Biol 13, 905, doi: 10.15252/msb.20166796 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Landsberg J et al. Melanomas resist T-cell therapy through inflammation-induced reversible dedifferentiation. Nature 490, 412–416, doi: 10.1038/nature11538 (2012). [DOI] [PubMed] [Google Scholar]
- 54.Offin M et al. Concurrent RB1 and TP53 Alterations Define a Subset of EGFR-Mutant Lung Cancers at risk for Histologic Transformation and Inferior Clinical Outcomes. J Thorac Oncol, doi: 10.1016/j.jtho.2019.06.002 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lee JK et al. Clonal History and Genetic Predictors of Transformation Into Small-Cell Carcinomas From Lung Adenocarcinomas. J Clin Oncol 35, 3065–3074, doi: 10.1200/JCO.2016.71.9096 (2017). [DOI] [PubMed] [Google Scholar]
- 56.Aggarwal R et al. Clinical and Genomic Characterization of Treatment-Emergent Small-Cell Neuroendocrine Prostate Cancer: A Multi-institutional Prospective Study. J Clin Oncol 36, 2492–2503, doi: 10.1200/JCO.2017.77.6880 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Niederst MJ et al. RB loss in resistant EGFR mutant lung adenocarcinomas that transform to small-cell lung cancer. Nat Commun 6, 6377, doi: 10.1038/ncomms7377 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Takahashi K & Yamanaka S Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126, 663–676, doi: 10.1016/j.cell.2006.07.024 (2006). [DOI] [PubMed] [Google Scholar]
- 59.Lin T et al. p53 induces differentiation of mouse embryonic stem cells by suppressing Nanog expression. Nat Cell Biol 7, 165–171, doi: 10.1038/ncb1211 (2005). [DOI] [PubMed] [Google Scholar]
- 60.Lim ST et al. Nuclear FAK promotes cell proliferation and survival through FERM-enhanced p53 degradation. Mol Cell 29, 9–22, doi: 10.1016/j.molcel.2007.11.031 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gastaldi S et al. Met signaling regulates growth, repopulating potential and basal cell-fate commitment of mammary luminal progenitors: implications for basal-like breast cancer. Oncogene 32, 1428–1440, doi: 10.1038/onc.2012.154 (2013). [DOI] [PubMed] [Google Scholar]
- 62.Chiche A et al. p53 controls the plasticity of mammary luminal progenitor cells downstream of Met signaling. Breast Cancer Res 21, 13, doi: 10.1186/s13058-019-1101-8 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ku SY et al. Rb1 and Trp53 cooperate to suppress prostate cancer lineage plasticity, metastasis, and antiandrogen resistance. Science 355, 78–83, doi: 10.1126/science.aah4199 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Offin M et al. Concurrent RB1 and TP53 Alterations Define a Subset of EGFR-Mutant Lung Cancers at risk for Histologic Transformation and Inferior Clinical Outcomes. J Thorac Oncol 14, 1784–1793, doi: 10.1016/j.jtho.2019.06.002 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Park JW et al. Reprogramming normal human epithelial tissues to a common, lethal neuroendocrine cancer lineage. Science 362, 91–95, doi: 10.1126/science.aat5749 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nakagawa M, Takizawa N, Narita M, Ichisaka T & Yamanaka S Promotion of direct reprogramming by transformation-deficient Myc. Proc Natl Acad Sci U S A 107, 14152–14157, doi: 10.1073/pnas.1009374107 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dardenne E et al. N-Myc Induces an EZH2-Mediated Transcriptional Program Driving Neuroendocrine Prostate Cancer. Cancer Cell 30, 563–577, doi: 10.1016/j.ccell.2016.09.005 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Beltran H et al. Molecular characterization of neuroendocrine prostate cancer and identification of new drug targets. Cancer Discov 1, 487–495, doi: 10.1158/2159-8290.CD-11-0130 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Beltran H et al. Divergent clonal evolution of castration-resistant neuroendocrine prostate cancer. Nat Med 22, 298–305, doi: 10.1038/nm.4045 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Berger A et al. N-Myc-mediated epigenetic reprogramming drives lineage plasticity in advanced prostate cancer. The Journal of clinical investigation 130, doi: 10.1172/JCI127961 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chen Z et al. Diverse AR-V7 cistromes in castration-resistant prostate cancer are governed by HoxB13. Proceedings of the National Academy of Sciences of the United States of America 115, 6810–6815, doi: 10.1073/pnas.1718811115 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang J et al. Pim1 kinase synergizes with c-MYC to induce advanced prostate carcinoma. Oncogene 29, 2477–2487, doi: 10.1038/onc.2010.10 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ellwood-Yen K et al. Myc-driven murine prostate cancer shares molecular features with human prostate tumors. Cancer Cell 4, 223–238 (2003). [DOI] [PubMed] [Google Scholar]
- 74.Kim J et al. A mouse model of heterogeneous, c-MYC-initiated prostate cancer with loss of Pten and p53. Oncogene 31, 322–332, doi: 10.1038/onc.2011.236 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Farrell AS et al. MYC regulates ductal-neuroendocrine lineage plasticity in pancreatic ductal adenocarcinoma associated with poor outcome and chemoresistance. Nat Commun 8, 1728, doi: 10.1038/s41467-017-01967-6 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chen Y et al. ETS factors reprogram the androgen receptor cistrome and prime prostate tumorigenesis in response to PTEN loss. Nat Med 19, 1023–1029, doi: 10.1038/nm.3216 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lee DK et al. Neuroendocrine prostate carcinoma cells originate from the p63-expressing basal cells but not the pre-existing adenocarcinoma cells in mice. Cell Res 29, 420–422, doi: 10.1038/s41422-019-0149-4 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yao JC et al. Everolimus for advanced pancreatic neuroendocrine tumors. N Engl J Med 364, 514–523, doi: 10.1056/NEJMoa1009290 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Schaefer T & Lengerke C SOX2 protein biochemistry in stemness, reprogramming, and cancer: the PI3K/AKT/SOX2 axis and beyond. Oncogene, doi: 10.1038/s41388-019-0997-x (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rudin CM et al. Comprehensive genomic analysis identifies SOX2 as a frequently amplified gene in small-cell lung cancer. Nat Genet 44, 1111–1116, doi: 10.1038/ng.2405 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bass AJ et al. SOX2 is an amplified lineage-survival oncogene in lung and esophageal squamous cell carcinomas. Nat Genet 41, 1238–1242, doi: 10.1038/ng.465 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mu P et al. SOX2 promotes lineage plasticity and antiandrogen resistance in TP53- and RB1-deficient prostate cancer. Science 355, 84–88, doi: 10.1126/science.aah4307 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bhattaram P et al. Organogenesis relies on SoxC transcription factors for the survival of neural and mesenchymal progenitors. Nat Commun 1, 9, doi: 10.1038/ncomms1008 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sock E et al. Gene targeting reveals a widespread role for the high-mobility-group transcription factor Sox11 in tissue remodeling. Molecular and cellular biology 24, 6635–6644, doi: 10.1128/MCB.24.15.6635-6644.2004 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mosquera JM et al. Concurrent AURKA and MYCN gene amplifications are harbingers of lethal treatment-related neuroendocrine prostate cancer. Neoplasia 15, 1–10, doi: 10.1593/neo.121550 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gong X et al. Aurora A Kinase Inhibition Is Synthetic Lethal with Loss of the RB1 Tumor Suppressor Gene. Cancer Discov 9, 248–263, doi: 10.1158/2159-8290.CD-18-0469 (2019). [DOI] [PubMed] [Google Scholar]
- 87.Oser MG et al. Cells Lacking the RB1 Tumor Suppressor Gene Are Hyperdependent on Aurora B Kinase for Survival. Cancer Discov 9, 230–247, doi: 10.1158/2159-8290.CD-18-0389 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kim J et al. FOXA1 inhibits prostate cancer neuroendocrine differentiation. Oncogene 36, 4072–4080, doi: 10.1038/onc.2017.50 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Adams EJ et al. FOXA1 mutations alter pioneering activity, differentiation and prostate cancer phenotypes. Nature, doi: 10.1038/s41586-019-1318-9 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Barbieri CE et al. Exome sequencing identifies recurrent SPOP, FOXA1 and MED12 mutations in prostate cancer. Nat Genet 44, 685–689, doi: 10.1038/ng.2279 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Robinson D et al. Integrative Clinical Genomics of Advanced Prostate Cancer. Cell 162, 454, doi: 10.1016/j.cell.2015.06.053 (2015). [DOI] [PubMed] [Google Scholar]
- 92.Blee AM et al. TMPRSS2-ERG Controls Luminal Epithelial Lineage and Antiandrogen Sensitivity in PTEN and TP53-Mutated Prostate Cancer. Clin Cancer Res 24, 4551–4565, doi: 10.1158/1078-0432.CCR-18-0653 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.McGranahan N et al. Clonal status of actionable driver events and the timing of mutational processes in cancer evolution. Sci Transl Med 7, 283ra254, doi: 10.1126/scitranslmed.aaa1408 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sequist LV et al. Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci Transl Med 3, 75ra26, doi: 10.1126/scitranslmed.3002003 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Beltran H et al. The Initial Detection and Partial Characterization of Circulating Tumor Cells in Neuroendocrine Prostate Cancer. Clin Cancer Res 22, 1510–1519, doi: 10.1158/1078-0432.CCR-15-0137 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Roca E et al. Outcome of patients with lung adenocarcinoma with transformation to small-cell lung cancer following tyrosine kinase inhibitors treatment: A systematic review and pooled analysis. Cancer Treat Rev 59, 117–122, doi: 10.1016/j.ctrv.2017.07.007 (2017). [DOI] [PubMed] [Google Scholar]
- 97.Marcoux N et al. EGFR-Mutant Adenocarcinomas That Transform to Small-Cell Lung Cancer and Other Neuroendocrine Carcinomas: Clinical Outcomes. J Clin Oncol 37, 278–285, doi: 10.1200/JCO.18.01585 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Foster NR et al. Tumor response and progression-free survival as potential surrogate endpoints for overall survival in extensive stage small-cell lung cancer: findings on the basis of North Central Cancer Treatment Group trials. Cancer 117, 1262–1271, doi: 10.1002/cncr.25526 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Jiang SY et al. Small-Cell Lung Cancer Transformation in Patients With Pulmonary Adenocarcinoma: A Case Report and Review of Literature. Medicine (Baltimore) 95, e2752, doi: 10.1097/MD.0000000000002752 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Soo RA et al. Immune checkpoint inhibitors in epidermal growth factor receptor mutant non-small cell lung cancer: Current controversies and future directions. Lung Cancer 115, 12–20, doi: 10.1016/j.lungcan.2017.11.009 (2018). [DOI] [PubMed] [Google Scholar]
- 101.Davies AH, Beltran H & Zoubeidi A Cellular plasticity and the neuroendocrine phenotype in prostate cancer. Nat Rev Urol 15, 271–286, doi: 10.1038/nrurol.2018.22 (2018). [DOI] [PubMed] [Google Scholar]
- 102.Shah RB et al. Androgen-independent prostate cancer is a heterogeneous group of diseases: lessons from a rapid autopsy program. Cancer Res 64, 9209–9216, doi: 10.1158/0008-5472.CAN-04-2442 (2004). [DOI] [PubMed] [Google Scholar]
- 103.Turbat-Herrera EA et al. Neuroendocrine differentiation in prostatic carcinomas. A retrospective autopsy study. Arch Pathol Lab Med 112, 1100–1105 (1988). [PubMed] [Google Scholar]
- 104.Gilani S, Guo CC, Li-Ning EM, Pettaway C & Troncoso P Transformation of prostatic adenocarcinoma to well-differentiated neuroendocrine tumor after hormonal treatment. Hum Pathol 64, 186–190, doi: 10.1016/j.humpath.2017.01.006 (2017). [DOI] [PubMed] [Google Scholar]
- 105.Volta AD et al. Transformation of Prostate Adenocarcinoma Into Small-Cell Neuroendocrine Cancer Under Androgen Deprivation Therapy: Much Is Achieved But More Information Is Needed. J Clin Oncol 37, 350–351, doi: 10.1200/JCO.18.01055 (2019). [DOI] [PubMed] [Google Scholar]
- 106.Aparicio AM et al. Platinum-based chemotherapy for variant castrate-resistant prostate cancer. Clin Cancer Res 19, 3621–3630, doi: 10.1158/1078-0432.CCR-12-3791 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Flechon A et al. Phase II study of carboplatin and etoposide in patients with anaplastic progressive metastatic castration-resistant prostate cancer (mCRPC) with or without neuroendocrine differentiation: results of the French Genito-Urinary Tumor Group (GETUG) P01 trial. Ann Oncol 22, 2476–2481, doi: 10.1093/annonc/mdr004 (2011). [DOI] [PubMed] [Google Scholar]
- 108.Papandreou CN et al. Results of a phase II study with doxorubicin, etoposide, and cisplatin in patients with fully characterized small-cell carcinoma of the prostate. J Clin Oncol 20, 3072–3080, doi: 10.1200/JCO.2002.12.065 (2002). [DOI] [PubMed] [Google Scholar]
- 109.Shukuya T et al. Efficacy of gefitinib for non-adenocarcinoma non-small-cell lung cancer patients harboring epidermal growth factor receptor mutations: a pooled analysis of published reports. Cancer Sci 102, 1032–1037, doi: 10.1111/j.1349-7006.2011.01887.x (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Park S, Han J & Sun JM Histologic transformation of ALK-rearranged adenocarcinoma to squamous cell carcinoma after treatment with ALK inhibitor. Lung Cancer 127, 66–68, doi: 10.1016/j.lungcan.2018.11.027 (2019). [DOI] [PubMed] [Google Scholar]
- 111.Gong J et al. Squamous Cell Transformation of Primary Lung Adenocarcinoma in a Patient With EML4-ALK Fusion Variant 5 Refractory to ALK Inhibitors. J Natl Compr Canc Netw 17, 297–301, doi: 10.6004/jnccn.2019.7291 (2019). [DOI] [PubMed] [Google Scholar]
- 112.Scagliotti G et al. The differential efficacy of pemetrexed according to NSCLC histology: a review of two Phase III studies. Oncologist 14, 253–263, doi: 10.1634/theoncologist.2008-0232 (2009). [DOI] [PubMed] [Google Scholar]
- 113.Izumi H et al. Squamous Cell Carcinoma Transformation from EGFR-mutated Lung Adenocarcinoma: A Case Report and Literature Review. Clin Lung Cancer 19, e63–e66, doi: 10.1016/j.cllc.2017.10.005 (2018). [DOI] [PubMed] [Google Scholar]
- 114.Balanis NG et al. Pan-cancer Convergence to a Small-Cell Neuroendocrine Phenotype that Shares Susceptibilities with Hematological Malignancies. Cancer Cell 36, 17–34 e17, doi: 10.1016/j.ccell.2019.06.005 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Fujita S, Masago K, Katakami N & Yatabe Y Transformation to SCLC after Treatment with the ALK Inhibitor Alectinib. J Thorac Oncol 11, e67–72, doi: 10.1016/j.jtho.2015.12.105 (2016). [DOI] [PubMed] [Google Scholar]
- 116.Balla A, Khan F, Hampel KJ, Aisner DL & Sidiropoulos N Small-cell transformation of ALK-rearranged non-small-cell adenocarcinoma of the lung. Cold Spring Harb Mol Case Stud 4, doi: 10.1101/mcs.a002394 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Le X et al. De novo pulmonary small cell carcinomas and large cell neuroendocrine carcinomas harboring EGFR mutations: Lack of response to EGFR inhibitors. Lung Cancer 88, 70–73, doi: 10.1016/j.lungcan.2015.02.003 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Schartinger VH et al. Neuroendocrine differentiation in head and neck squamous cell carcinoma. J Laryngol Otol 126, 1261–1270, doi: 10.1017/S0022215112002265 (2012). [DOI] [PubMed] [Google Scholar]
- 119.Yamagata K et al. A Case of Primary Combined Squamous Cell Carcinoma with Neuroendocrine (Atypical Carcinoid) Tumor in the Floor of the Mouth. Case Rep Dent 2016, 7532805, doi: 10.1155/2016/7532805 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Mangum MD, Greco FA, Hainsworth JD, Hande KR & Johnson DH Combined small-cell and non-small-cell lung cancer. J Clin Oncol 7, 607–612, doi: 10.1200/JCO.1989.7.5.607 (1989). [DOI] [PubMed] [Google Scholar]
- 121.Rudin CM et al. Molecular subtypes of small cell lung cancer: a synthesis of human and mouse model data. Nat Rev Cancer 19, 289–297, doi: 10.1038/s41568-019-0133-9 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Moriguchi S et al. Transformation of epidermal growth factor receptor T790M mutation-positive adenosquamous carcinoma of the lung to small cell carcinoma and large-cell neuroendocrine carcinoma following osimertinib therapy: an autopsy case report. Respirol Case Rep 7, e00402, doi: 10.1002/rcr2.402 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Priftakis D, Kritikos N, Stavrinides S, Kleanthous S & Baziotis N Neuroendocrine differentiation in castration-resistant prostate cancer: A case report. Mol Clin Oncol 3, 1392–1394, doi: 10.3892/mco.2015.645 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Gluck G, Mihai M, Stoica R, Andrei R & Sinescu I Prostate cancer with neuroendocrine differentiation--case report. J Med Life 5, 101–104 (2012). [PMC free article] [PubMed] [Google Scholar]
- 125.Rudin CM et al. Phase II study of single-agent navitoclax (ABT-263) and biomarker correlates in patients with relapsed small cell lung cancer. Clin Cancer Res 18, 3163–3169, doi: 10.1158/1078-0432.CCR-11-3090 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Saunders LR et al. A DLL3-targeted antibody-drug conjugate eradicates high-grade pulmonary neuroendocrine tumor-initiating cells in vivo. Sci Transl Med 7, 302ra136, doi: 10.1126/scitranslmed.aac9459 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Rudin CM et al. Rovalpituzumab tesirine, a DLL3-targeted antibody-drug conjugate, in recurrent small-cell lung cancer: a first-in-human, first-in-class, open-label, phase 1 study. Lancet Oncol 18, 42–51, doi: 10.1016/S1470-2045(16)30565-4 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Takagi S et al. LSD1 Inhibitor T-3775440 Inhibits SCLC Cell Proliferation by Disrupting LSD1 Interactions with SNAG Domain Proteins INSM1 and GFI1B. Cancer Res 77, 4652–4662, doi: 10.1158/0008-5472.CAN-16-3502 (2017). [DOI] [PubMed] [Google Scholar]
- 129.Asangani IA et al. Therapeutic targeting of BET bromodomain proteins in castration-resistant prostate cancer. Nature 510, 278–282, doi: 10.1038/nature13229 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Aggarwal RR et al. Whole-Genome and Transcriptional Analysis of Treatment-Emergent Small-Cell Neuroendocrine Prostate Cancer Demonstrates Intraclass Heterogeneity. Mol Cancer Res 17, 1235–1240, doi: 10.1158/1541-7786.MCR-18-1101 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]