Summary
Background
Ruxolitinib, a Janus kinase 1 (JAK1)/JAK2 inhibitor, plus capecitabine improved overall survival (OS) vs capecitabine in a subgroup analysis of patients with metastatic pancreatic cancer and systemic inflammation (C-reactive protein [CRP] >13 mg/dL) in the randomized phase II RECAP study. We report results from two randomized phase III studies, JANUS 1 () and JANUS 2 ().
Patients and Methods
Adults with advanced/metastatic pancreatic cancer, one prior chemotherapy regimen and CRP >10 mg/L were randomized 1:1 (stratified by modified Glasgow Prognostic Score [1 vs 2] and Eastern Cooperative Oncology Group performance status [0/1 vs 2]) to 21-day cycles of ruxolitinib 15 mg twice daily plus capecitabine 2000 mg/m2/day (Days 1–14) or placebo plus capecitabine. The primary endpoint was OS.
Results
Both studies were terminated following a planned interim futility/efficacy analysis of JANUS 1. Overall, 321 and 86 patients were randomized in JANUS 1 (ruxolitinib: n = 161; placebo: n = 160) and JANUS 2 (ruxolitinib: n = 43; placebo: n = 43). There was no significant difference in OS or progression-free survival (PFS) between treatments in JANUS 1 (OS: hazard ratio [HR], 0.969, 95% confidence interval [CI], 0.747–1.256; PFS: HR, 1.056; 95% CI, 0.827–1.348) or JANUS 2 (OS: HR, 1.584; 95% CI, 0.886–2.830; PFS: HR, 1.166; 95% CI, 0.687–1.978). The most common hematologic adverse event was anemia. No new safety signals with ruxolitinib or capecitabine were identified.
Conclusions
Ruxolitinib plus capecitabine was well tolerated in refractory pancreatic cancer patients; this combination did not improve survival.
Keywords: Clinical trial, JAK1 protein tyrosine kinase, JAK2 protein tyrosine kinase, Pancreatic neoplasms
Introduction
Metastatic pancreatic cancer is one of the leading causes of cancer-related deaths, with a 5-year survival rate of <5% [1]. The National Comprehensive Cancer Network guidelines recommend FOLFIRINOX (fluorouracil, leucovorin, irinotecan, oxaliplatin) or nanoparticle albumin-bound paclitaxel (nab-paclitaxel) plus gemcitabine as the current standard of care in the first-line setting for patients with metastatic pancreatic cancer [2]. However, patients eventually develop disease pro-gression on first-line therapy [3], and there is no definitive consensus on standard of care after disease progression [2]. The recent approval of nanoliposomal irinotecan in combination with fluorouracil and leucovorin, for the treatment of patients with metastatic adenocarcinoma of the pancreas who progressed after first-line gemcitabine therapy, represents a modest step forward in the second-line treatment of patients with advanced/metastatic pancreatic cancer [4, 5]. Given the limited options, there is an unmet need for the development of agents beyond first-line therapy.
Ruxolitinib (Jakafi®) is an orally bioavailable, potent, and selective inhibitor of Janus kinase 1 (JAK1) and Janus kinase 2 (JAK2) enzymes [6] approved for the treatment of intermediate-risk or high-risk primary or secondary myelofibrosis, as well as polycythemia vera in patients with an inadequate response or intolerance to hydroxyurea [7, 8]. In genetically engineered murine models of pancreatic cancer, ruxolitinib inhibited tumor angiogenesis, controlled disease progression, and improved survival [9]. Ruxolitinib also has been shown to block tumor growth in a syngeneic murine PAN02 pancreatic model [10]. By selectively inhibiting JAK2V617F, STAT5, and ERK1/2 phosphorylation, ruxolitinib was shown to reduce cellular proliferation and induce apoptosis in JAK2V617F+ Ba/F3 cells [6].
Patients with pancreatic cancer exhibit a systemic inflammatory response, which is partially mediated by the JAK/signal transducer and activator of transcription (STAT) pathway [11]. This systemic inflammatory response has been associated with a more pronounced symptom burden, including cachexia syndrome, and poorer survival outcomes [12]. C-reactive protein (CRP) is a well-characterized and routinely measured inflammatory marker, and elevated levels of CRP are a feature of the systemic inflammatory response in patients with cancer [13]. Low serum albumin concentrations (hypoalbuminemia) also are recognized as part of the systemic inflammatory response [14]. The modified Glasgow Prognostic Score (mGPS) is a risk-stratification tool that evaluates systemic inflammation by incorporating CRP and albumin values into a score to predict clinical outcomes in patients with cancer [15, 16]. Although the mGPS has not been prospectively validated to date, the prognostic significance of mGPS has been extensively reported in retrospective analyses of various tumors, including pancreatic cancer [14–16].
In the randomized double-blind phase II Ruxolitinib in Pancreatic Cancer Patients (RECAP) study, in a prespecified subgroup analysis of patients with inflammation, defined by serum CRP levels greater than the study population median (ie, 13 mg/L), the median overall survival (OS) was significantly greater with ruxolitinib vs placebo [17]. Based on these promising results and driven by the JAK/STAT signaling pathway and its relationship to the mGPS, two randomized, phase III studies, JANUS 1 and JANUS 2, were conducted to evaluate ruxolitinib in combination with capecitabine in patients with advanced/metastatic pancreatic cancer.
Patients and methods
Study design
JANUS 1 and JANUS 2 were randomized, double-blind, placebo-controlled, multinational studies that evaluated ruxolitinib or placebo in combination with capecitabine in patients with advanced/metastatic pancreatic cancer after disease progression or intolerance to first-line therapy (JANUS 1 and JANUS 2 ClinicalTrials.gov registration numbers: and , EudraCT registration numbers: 2014–000293-20 and 2014–000294-39, respectively). Randomization was performed centrally via an interactive response technology system and was stratified by mGPS (1 vs 2) and Eastern Cooperative Oncology Group performance status (ECOG PS; 0 or 1 vs 2). All investigators, investigational site staff, patients, and study personnel were blinded to treatment allocation.
Each study was conducted in accordance with the study protocol, Declaration of Helsinki, Good Clinical Practice guidelines, and applicable regulatory requirements. Study protocols and amendments were approved by the relevant institutional review board or independent ethics committee. All patients provided written, informed consent before study participation.
Patients
Adult patients with radiographically evaluable advanced pancreatic adenocarcinoma that was inoperable/metastatic, according to Response Evaluation Criteria In Solid Tumors (RECIST), version 1.1, were enrolled. All patients must have received one prior chemotherapy regimen for advanced/metastatic disease. Patients who previously received neoadjuvant/adjuvant chemotherapy with fluoropyrimidine-based chemotherapy were eligible. Fluoropyrimidine-containing therapies were allowed as the first-line regimen, provided the patient had discontinued treatment for reasons other than disease progression. Patients were required to have a baseline mGPS of 1 (CRP >10 mg/L and albumin ≥35 g/L) or 2 (CRP >10 mg/L and albumin <35 g/L) on a 3-point (0–2) scale, with higher scores indicating worsening prognosis; and an ECOG PS between 0 and 2, on a 5-point scale, with 0 indicating no symptoms and higher scores indicating a worsening ability to conduct activities of daily living.
Patients were excluded if they had received more than one prior regimen for advanced/metastatic disease or single-agent capecitabine in the first-line setting; had brain/central nervous system metastases; a history of seizures; any chronic/active infection requiring systemic treatment; a history of, or active, viral infection (hepatitis B, hepatitis C, or human immunodeficiency virus); any other malignancy ≤2 years (except protocol-specified skin and genitourinary carcinomas); prior treatment with a JAK inhibitor, recent or ongoing radiation therapy, or concurrent anticancer therapy; recent (≤3 months) or ongoing partial or complete bowel obstruction, unless related to the disease under study and surgically corrected; were unwilling to undergo blood transfusion; or had any clinically significant disease or finding that, in the investigator’s opinion, precluded study participation.
Treatment
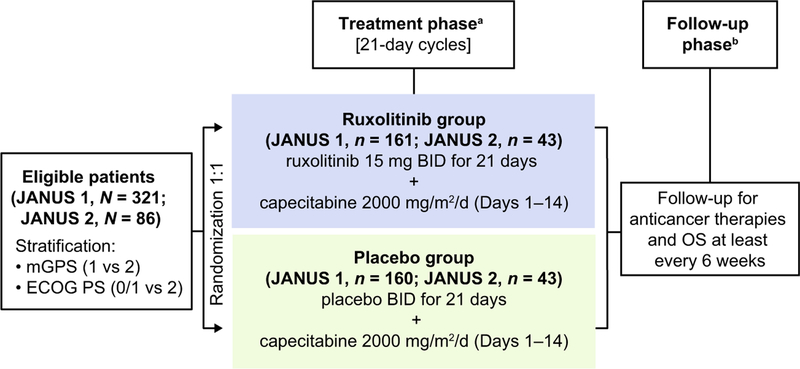
In both studies, patients were randomly assigned, in a 1:1 ratio, to receive ruxolitinib 15 mg twice daily or matching placebo, orally self-administered as three 5-mg tablets in two daily doses approximately 12 h apart for 21 days. In addition, capecitabine 2000 mg/m2 per day was orally self-administered in approximately two equal doses, for the first 14 days of each cycle in both treatment groups (Fig. 1). Treatment was initiated within 3 days of randomization and consisted of continuous 21-day cycles until intolerance or disease progression. Ruxolitinib could be escalated to a maximum dose of 25 mg twice daily at the investigator’s discretion if the patient had stable neutrophil and platelet laboratory parameters (ie, ≤ grade 1) for two complete cycles. Protocol-specified dose interruptions and adjustments also were permitted for safety reasons at the discretion of the investigator. Following withdrawal from the study, patients returned 30 to 35 days later for a safety visit. Survival assessment was planned every 6 weeks until completion of final primary analysis, after which assessments were to occur every 12 weeks.
Fig. 1.
Study design of JANUS 1 and JANUS 2. aTreatment continued until disease progression, withdrawal due to toxicity, or withdrawal of consent; bAfter the final primary analysis, follow-up was to be reduced to every 12 weeks. BID twice daily; ECOG PS Eastern Cooperative Oncology Group performance status; mGPS modified Glasgow Prognostic Score; OS overall survival
Assessments
The primary endpoint in each study was OS, defined as the time from randomization to death from any cause. Secondary endpoints included progression-free survival (PFS), defined as the time from randomization to disease progression (per RECIST 1.1) or death from any cause if earlier; overall response rate (ORR), defined as the proportion of patients who had a partial response or complete response per RECIST 1.1; and safety and tolerability. Radiographic tumor assessments were performed every 6 weeks until progressive disease, and treatment response was assessed according to RECIST 1.1 by blinded investigator radiologic review. Subgroup analyses for OS were also conducted to investigate consistency of treatment effects. Safety and tolerability were assessed by adverse events (graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events [CTCAE], version 4.03, with the exception of death [CTCAE severity grade 5], which was collected as an outcome), laboratory results (hematologic and chemical), physical examination, vital signs, and electrocardiography.
The exploratory endpoints of JANUS 1 and 2 were to evaluate and compare the treatment groups with respect to: (i) body weight, (ii) health-related quality of life (HRQoL), and (iii) pharmacodynamics and tumor markers. HRQoL was assessed using the 45-item Functional Assessment of Cancer Therapy – Hepatobiliary questionnaire (FACT-Hep), version 4, which assessed five areas of quality of life (QoL): physical well-being, social/family well-being, emotional well-being, functional well-being, and additional concerns [18]. Responses were based on a 5-point scale, with a higher score indicating a higher QoL (0 = not at all to 4 = very much). In addition, for both studies, a planned exploratory endpoint was to evaluate the pharmacokinetics of ruxolitinib, capecitabine, and the active metabolite of capecitabine, fluorouracil, when administered alone and in combination. Exploratory analyses also were planned for clinically relevant tumor markers and markers of inflammation, which included but was not limited to CRP, CA19–9, and CA125. Other markers such as vascular cell adhesion molecule-1 and adiponectin that were measured will be reported separately.
Statistical considerations
It was calculated that a total of 262 deaths in JANUS 1 and 227 deaths in JANUS 2 would provide 90% and 85% power, respectively, to detect a 33% reduction in the risk of death (hazard ratio [HR] = 0.67) in the ruxolitinib group (vs placebo) with a two-sided type 1 error of 5%. For each study, an event-driven interim analysis for futility and efficacy was planned when approximately 170 (JANUS 1) or 148 (JANUS 2) total deaths had occurred. In both JANUS 1 and JANUS 2, an interim analysis was planned to be conducted by an external unblinded statistician prior to a review by an independent data monitoring committee, which then made recommendations to the sponsor as to the continuation or early termination of the studies; the planned one-sided p-value threshold for futility was ≥0.7465 for JANUS 1 and ≥0.7672 for JANUS 2. In JANUS 2, the planned interim futility and efficacy analyses were not conducted.
The efficacy analysis was assessed in the intent-to-treat population, which comprised all patients randomized to the study. The safety analysis was based on the safety population, which comprised all patients who received at least one dose of the assigned study treatment.
For both studies, the Kaplan-Meier method was used to estimate OS and PFS. For the OS analyses, patients alive at the time of analysis were censored at the time of last contact. The stratified log-rank test was used to analyze the OS and PFS differences between treatment groups. HRs and associated 95% confidence intervals (CIs) were estimated based on the stratified Cox regression model using Efron’s method of accounting for ties. ORR was compared between treatment groups using the Miettinen and Nurminen method for stratified data. The analyses of OS, PFS, and ORR were stratified by mGPS status (1 vs 2) and ECOG PS (0 or 1 vs 2). For subgroup analyses of OS, the HR and 95% CIs were provided for each subgroup based on the Cox proportional hazards model.
Exploratory analyses were conducted using a two-sided 5% significance level. Changes and percentages in body weight were summarized by visit and treatment group. The proportion of patients achieving a minimally important difference (ie, a 7-point increase from baseline) in FACT-Hep total score was analyzed using a logistic regression model with mGPS and ECOG performance status as covariates. Descriptive statistics, change, and percentage change from baseline in tumor marker levels were measured by treatment group at each scheduled visit. The pharmacokinetics of ruxolitinib, capecitabine, and fluorouracil (the active metabolite of capecitabine) were planned to be evaluated.
Unless otherwise noted, all analyses were conducted with SAS Version 9.0 or later (SAS Institute, Inc., Cary, NC, USA).
Results
JANUS 1 was conducted at 158 centers between March 19, 2014 and April 20, 2016; JANUS 2 was conducted at 51 centers between June 3, 2014 and April 13, 2016. The centers for JANUS 1 were located in Australia, Belgium, Canada, Germany, Italy, New Zealand, the Republic of Korea, Spain, Taiwan, Thailand, United Kingdom, and the United States. The centers for JANUS 2 were located in Argentina, Austria, Chile, Colombia, Denmark, France, Israel, Mexico, the Netherlands, Peru, Portugal, the Republic of Ireland, Sweden, Switzerland, and the United States.
At the planned futility and efficacy interim analysis for OS conducted when 170 deaths were reported in the JANUS 1 study (data cutoff: October 26, 2015), the hazard ratio (HR = 0.969) was very close to the protocol-defined stopping rule for futility; further, no benefits were reported for PFS or ORR for the ruxolitinib plus capecitabine group vs the placebo plus capecitabine group. Based on the findings of the independent data monitoring committee (IDMC), available data from JANUS 2 were reviewed concurrently with the planned interim futility analysis for JANUS 1. The IDMC noted there were no safety issues. However, based on the lack of efficacy demonstrated in JANUS 1, it was recommended that the JANUS 2 trial also should be halted. The sponsor accepted the recommendation to terminate both JANUS 1 and JANUS 2 (February 11, 2016). Final analyses of both studies were conducted, and the results are presented here.
In JANUS 1, 321 patients were randomized, 161 to the ruxolitinib plus capecitabine group and 160 to the placebo plus capecitabine group. In JANUS 2, 86 patients were randomized, 43 to each treatment group. Demographic and baseline clinical characteristics were generally well balanced between treatment groups and across studies (Table 1), although JANUS 1 had a higher proportion of patients with lung metastases in the ruxolitinib group (40.4%) vs the placebo group (31.9%).
Table 1.
Patient demographics and baseline characteristics (intent-to-treat population)
| Characteristics | JANUS 1 (N = 321) |
JANUS 2 (N = 86) |
||
|---|---|---|---|---|
| Ruxolitinib + capecitabine (n = 161) | Placebo + capecitabine (n = 160) | Ruxolitinib + capecitabine (n = 43) | Placebo + capecitabine (n = 43) | |
| Median age (range), years | 68.0 (26.0–91.0) | 67.0 (28.0–83.0) | 68.0 (35.0–88.0) | 69.0 (46.0–86.0) |
| Male, n (%) | 95 (59.0) | 96 (60.0) | 25 (58.1) | 22 (51.2) |
| Race, n (%) | ||||
| White/Caucasian | 126 (78.3) | 129 (80.6) | 35 (81.4) | 35 (81.4) |
| Black/African American | 1 (0.6) | 5 (3.1) | 7 (16.3) | 5 (11.6) |
| Asian | 30 (18.6) | 22 (13.8) | 0 | 1 (2.3) |
| Native Hawaiian/Pacific Islander | 1 (0.6) | 0 | 0 | 0 |
| Other | 3 (1.9) | 4 (2.5) | 1 (2.3) | 1 (2.3) |
| Unknown | 0 | 0 | 0 | 1 (2.3) |
| ECOG PS, n (%) | ||||
| 0 | 35 (21.7) | 27 (16.9) | 10 (23.3) | 9 (20.9) |
| 1 | 107 (66.5) | 109 (68.1) | 27 (62.8) | 29 (67.4) |
| 2 | 19 (11.8) | 24 (15.0) | 6 (14.0) | 5 (11.6) |
| mGPS, n (%) | ||||
| 1 | 108 (67.1) | 105 (65.6) | 26 (60.5) | 28 (65.1) |
| 2 | 53 (32.9) | 55 (34.4) | 17 (39.5) | 15 (34.9) |
| Median duration of 1 L therapy (range), weeks | 22.1 (3.1–91.1)a | 17.3 (2.1–135.1)b | 20.9 (1.0–50.1)c | 19.1 (5.1–74.9)d |
| Prior 1 L regimens, n (%) | ||||
| Gemcitabine monotherapy | 38 (23.6) | 33 (20.6) | 12 (27.9) | 14 (32.6) |
| Gemcitabine + nab-paclitaxel | 80 (49.7) | 82 (51.3) | 18 (41.9) | 16 (37.2) |
| FOLFIRINOXe | 1 (0.6) | 1 (0.6) | 2 (4.7) | 1 (2.3) |
| FOLFOXf | 0 | 0 | 1 (2.3) | 0 |
1 L first-line; ECOG PS Eastern Cooperative Oncology Group performance status; mGPS modified Glasgow Prognostic Score
n = 154
n = 155
n = 42
n = 41
Comprising folic acid, fluorouracil, irinotecan, oxaliplatin
Comprising folic acid, fluorouracil, oxaliplatin
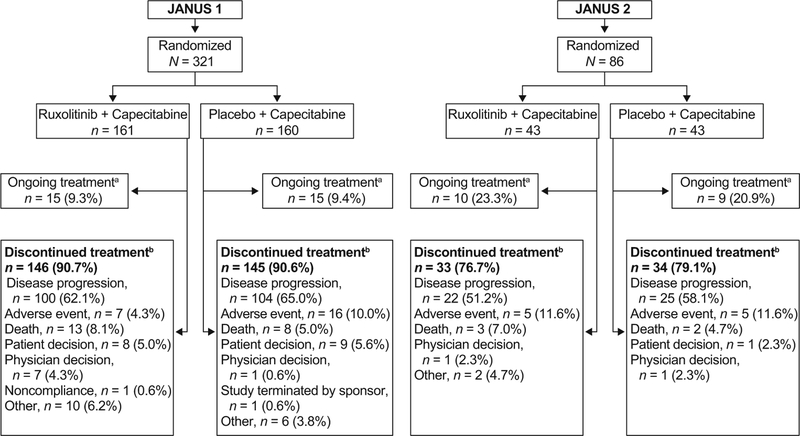
A total of 291 patients (90.7%) discontinued study treatment on or before the termination of JANUS 1 and 67 patients (77.9%) discontinued on or before the termination of JANUS 2. Discontinuations were mainly due to disease progression, adverse event, or death (Fig. 2).
Fig. 2.
Patient disposition (intent-to-treat population). aAt the time of study termination (date of study termination: February 11, 2016); bOn or before study termination
Efficacy
Overall survival
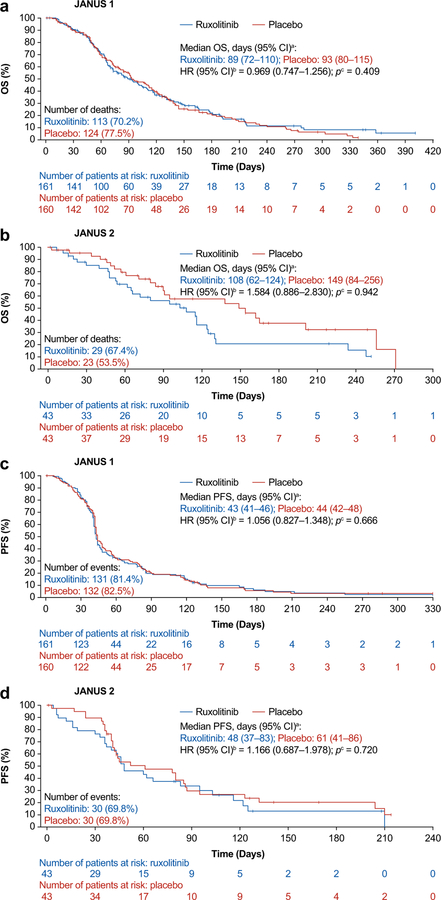
No statistically significant difference was observed between treatment groups for OS or PFS in either study (Table 2; Fig. 3). At the time of study termination, 237 patients had died in JANUS 1 and 52 in JANUS 2. Median OS was similar in the ruxolitinib group (89.0 days) and placebo group (93.0 days) in JANUS 1 (HR [95% CI], 0.969 [0.747–1.256]; p = 0.409), and marginally higher in the placebo group (149.0 days) compared with the ruxolitinib group (108.0 days) in JANUS 2 (HR [95% CI], 1.584 [0.886–2.830]; p = 0.942) (Fig. 3a and b). In subgroup analyses (using an unadjusted Cox proportional hazards model), ruxolitinib compared with placebo showed no significant association (HR [95%CI]) with OS in subgroups of patients with mGPS 0 or 1 (JANUS 1: 0.801 [0.579, 1.110]; JANUS 2: HR: 1.420 [0.694, 2.907]), mGPS 2 (JANUS 1, 1.265 [0.828, 1.933]; JANUS 2: 1.829 [0.728,4.597]), ECOG PS 0 or 1 (JANUS 1, 0.901 [0.682, 1.190]; JANUS 2, 1.487 [0.797, 2.774]), or ECOG PS 2 (JANUS 1, 1.715 [0.862, 3.412]; JANUS 2, 2.599 [0.722, 9.354]).
Table 2.
Summary of survival and overall response (intent-to-treat population)
| Variable | JANUS 1 (N = 321) |
JANUS 2 (N = 86) |
||
|---|---|---|---|---|
| Ruxolitinib + capecitabine (n = 161) | Placebo + capecitabine (n = 160) | Ruxolitinib + capecitabine (n = 43) | Placebo + capecitabine (n = 43) | |
| OS | ||||
| Deaths, n (%) | 113 (70.2) | 124 (77.5) | 29 (67.4) | 23 (53.5) |
| Hazard ratio (95% CI)a | 0.969 (0.747–1.256) | 1.584 (0.886–2.830) | ||
| One-sided p-valueb | p = 0.409 | p = 0.942 | ||
| Median (95% CI), days | 89.0 (72.0–110.0) | 93.0 (80.0–115.0) | 108.0 (62.0–124.0) | 149.0 (84.0–256.0) |
| Probability at 3 months (95% CI) | 49.8 (41.3–57.7) | 52.8 (44.3–60.5) | 56.1 (39.1–70.1) | 60.9 (42.7–74.9) |
| Probability at 6 months (95% CI) | 20.7 (13.5–28.9) | 20.1 (13.4–27.8) | 20.7 (8.3–37.0) | 37.7 (20.4–54.9) |
| Probability at 9 months (95% CI) | 11.2 (5.6–18.9) | 6.2 (2.5–12.2) | 10.4 (2.1–26.6) | Not evaluable |
| Probability at 12 months (95% CI) | 5.3 (1.4–13.4) | Not evaluable | Not evaluable | Not evaluable |
| PFS | ||||
| Earlier event of disease progression or death, n (%) | 131 (81.4) | 132 (82.5) | 30 (69.8) | 30 (69.8) |
| Hazard ratio (95% CI)a | 1.056 (0.827–1.348) | 1.166 (0.687–1.978) | ||
| One-sided p-valueb | p = 0.666 | p = 0.720 | ||
| Median (95% CI), days | 43.0 (41.0–46.0) | 44.0 (42.0–48.0) | 48.0 (37.0–83.0) | 61.0 (41.0–86.0) |
| Probability at 3 months (95% CI) | 18.9 (12.7–25.9) | 19.8 (13.6–26.8) | 33.7 (18.7–49.4) | 29.7 (15.7–45.1) |
| Probability at 6 months (95% CI) | 6.1 (2.5–12.2) | 5.7 (2.3–11.3) | 13.1 (3.7–28.7) | 20.4 (8.8–35.2) |
| Probability at 9 months (95% CI) | 2.5 (0.5–7.5) | 3.4 (1.0–8.5) | Not evaluable | Not evaluable |
| Probability at 12 months (95% CI) | 2.5 (0.5–7.5) | Not evaluable | Not evaluable | Not evaluable |
| Response | ||||
| ORR (CR + PR) n (%) | 6 (3.7) | 3 (1.9) | 2 (4.7) | 1 (2.3) |
| (95% CI)c | (1.8–10.3) | (0.5–6.7) | (0.8–22.1) | (0.1–15.3) |
| Odds ratio (95% CI) | 2.13 (0.44–13.48) | 2.39 (0.11–162.0) | ||
| Two-sided p-valued | p = 0.460 | p = 0.925 | ||
| CR, n (%) | 0 | 0 | 1 (2.3) | 0 |
| PR, n (%) | 6 (3.7) | 3 (1.9) | 1 (2.3) | 1 (2.3) |
| SD, n (%) | 44 (27.3) | 55 (34.4) | 15 (34.9) | 18 (41.9) |
| PD, n (%) | 70 (43.5) | 67 (41.9) | 13 (30.2) | 14 (32.6) |
| Not evaluable,e n (%) | 3 (1.9) | 2 (1.3) | 0 | 1 (2.3) |
| Not assessed,e n (%) | 38 (23.6) | 33 (20.6) | 13 (30.2) | 9 (20.9) |
CI confidence interval; CR complete response; ECOG PS Eastern Cooperative Oncology Group performance status; mGPS modified Glasgow Prognostic Score; ORR overall response rate; OS overall survival; PD progressive disease; PFS progression-free survival; PR partial response; SD stable disease
Calculated using a stratified Cox regression model with Efron’s method to account for ties
Calculated based on the log-rank test, stratified by mGPS score and ECOG PS
Calculated based on the exact method for binomial distributions
Calculated using conditional exact logistic regression, stratified by mGPS score and ECOG PS
Not evaluable/missing patients included in denominator even if these patients did not have a response assessment
Fig. 3.
OS and PFS Kaplan-Meier of JANUS 1 and JANUS 2 (intent-to-treat population). aCalculated using the method of Brookmeyer and Crowley (1982) [34]; bEstimated using a Cox regression model with Efron’s method used for ties, stratified by mGPS score and ECOG PS; cOne-sided p-value calculated from log-rank test stratified by mGPS score and ECOG PS. CI confidence interval; ECOG PS Eastern Cooperative Oncology Group performance status; HR hazard ratio; mGPS modified Glasgow Prognostic Score; OS overall survival; PFS progression-free survival
Progression-free survival
Median PFS was similar in patients receiving ruxolitinib plus capecitabine (43 days) and placebo plus capecitabine (44 days) in JANUS 1 (HR [95% CI], 1.056 [0.827–1.348]; p = 0.666), and marginally higher in the placebo plus capecitabine group (61 days) vs the ruxolitinib plus capecitabine group (48 days) in JANUS 2 (HR [95% CI], 1.166 [0.687–1.978]; p = 0.720) (Fig. 3c and d). Probabilities of OS and PFS at 3, 6, 9, and 12 months are reported in Table 2.
ORR and duration of response
ORR was not improved in patients in the ruxolitinib plus capecitabine group vs the placebo plus capecitabine group in either JANUS 1 or JANUS 2 (Table 2), and was less than 5% in all treatment groups. In JANUS 1, three patients (1.9%) treated with ruxolitinib plus capecitabine and one patient (0.6%) treated with placebo plus capecitabine had a durable response (ie, a response of at least partial response at two subsequent measurements that were ≥4 weeks apart). In JANUS 2, two patients (4.7%), both in the ruxolitinib plus capecitabine group, had a durable response. The median duration of response was not calculated due to small number of responders and instability of the estimation method.
Exploratory analyses
No clinically relevant difference was seen between treatment groups in the largest confirmed percentage change in baseline body weight (mean in kilograms [standard deviation]) in both studies (JANUS 1: ruxolitinib group, −1.4 [5.45]; placebo group, −2.3 [5.60]; p = 0.4308; JANUS 2: ruxolitinib group, −0.2 [6.09]; placebo group, −2.4 [5.00]; p = 0.9218). The proportions of patients achieving a minimally important difference (ie, a 7-point increase from baseline) in FACT-Hep total score were similar between treatment groups and both studies (JANUS 1: ruxolitinib group, 28.6%; placebo group, 32.5%; odds ratio, 1.22; 95% CI, 0.735–2.031; p = 0.48; JANUS 2: ruxolitinib group, 25.6%; placebo group, 39.5%; odds ratio, 1.737; 95% CI, 0.587–5.331; p = 0.38).
The levels of the pharmacodynamic biomarker, CRP, were similar between groups at baseline (mean CRP [mg/L]: JANUS 1: ruxolitinib group, 39.5; placebo group, 38.5; JANUS 2: ruxolitinib group, 36.0; placebo group, 32.1). Cycle 2 Day 1 median CRP reductions from baseline were larger for the ruxolitinib group vs the placebo group in both studies (JANUS 1: −57.4% vs −33.3%; JANUS 2: −55.2% vs −33.3%). The number of patients with reductions in tumor markers CA19–9 and CA125 from baseline at Cycle 2 Day 1, the last visit where patients were reliably measured, were similar between groups in JANUS 1 and numerically higher in the placebo group in JANUS 2 (CA19–9: JANUS 1: ruxolitinib group, 53/114 [46.5%], placebo group, 53/110 [48.2%]; JANUS 2: ruxolitinib group, 12/26 [46.2%], placebo group, 17/30 [56.7%]) (CA125: JANUS 1: ruxolitinib group, 29/114 [25.4%]; placebo group, 27/110 [24.5%]; JANUS 2: ruxolitinib group, 7/26 [26.9%], placebo group, 12/30 [40.0%]). As the studies were terminated, pharmacokinetic analyses were not performed.
Safety and tolerability
Safety population included 307 patients in JANUS 1 (ruxolitinib plus capecitabine, 153; placebo plus capecitabine, 154) and 85 patients in JANUS 2 (ruxolitinib plus capecitabine, 42; placebo plus capecitabine, 43). Median treatment durations were 42 days (range, 1–400) for patients receiving ruxolitinib plus capecitabine and 42 days (1–392) for patients receiving placebo plus capecitabine in JANUS 1; the corresponding treatment durations were 40 days (1–251) and 43 days (1–247), respectively, in JANUS 2. Median dose intensity was 97.5% (range, 10.9–1000.0) for patients receiving ruxolitinib plus capecitabine (one patient was dosed for 3 days only and was treated as received all of the study treatment, which led to the high dose intensity) and 97.4% (12.6–425.0) for patients receiving placebo plus capecitabine in JANUS 1; the corresponding median dose intensity was 97.0% (54.9– 125.8) and 97.7% (48.0–109.0), respectively, in JANUS 2. Over 90% of patients had at least one treatment-emergent adverse event (TEAE) in each of the treatment groups in both studies; over 70% had at least one grade 3/4 TEAE (Table 3). The most common nonhematologic and hematologic TEAEs are summarized in Tables 4 and 5, respectively.
Table 3.
Safety summary of TEAEs (safety population)
| n (%) | JANUS 1 (N = 307) |
JANUS 2 (N = 85) |
||
|---|---|---|---|---|
| Ruxolitinib + capecitabine (n = 153) | Placebo + capecitabine (n = 154) | Ruxolitinib + capecitabine (n = 42) | Placebo + capecitabine (n = 43) | |
| Patients with any TEAEs | 152 (99.3) | 152 (98.7) | 41 (97.6) | 40 (93.0) |
| Patients with grade 3/4 TEAEs | 112 (73.2) | 113 (73.4) | 31 (73.8) | 31 (72.1) |
| Patients with any serious TEAEs | 94 (61.4) | 83 (53.9) | 28 (66.7) | 20 (46.5) |
| Patients with a fatal AE | 19 (12.4)a | 15 (9.7)a | 8 (19.0)b | 2 (4.7)b |
| Patients who discontinued treatment due to a TEAE | 12 (7.8) | 22 (14.3) | 7 (16.7) | 5 (11.6) |
AE adverse event; TEAE treatment-emergent adverse event
Fatal AEs occurring in ≥1 patient in the ruxolitinib group in JANUS 1 were sepsis (ruxolitinib plus capecitabine, 3; placebo plus capecitabine, 1), myocardial infarction (ruxolitinib plus capecitabine, 3; placebo plus capecitabine, 0), cardiac arrest, pulmonary embolism (ruxolitinib plus capecitabine, 2 for each AE; placebo plus capecitabine, 0 for each AE), dyspnea (ruxolitinib plus capecitabine, 2; placebo plus capecitabine, 1), pneumonia (ruxolitinib plus capecitabine, 1; placebo plus capecitabine, 2), septic shock, and upper gastrointestinal hemorrhage (ruxolitinib plus capecitabine, 0 for each AE; placebo plus capecitabine, 2 for each AE)
Fatal AEs in JANUS 2 were pulmonary embolism (ruxolitinib plus capecitabine, 2; placebo plus capecitabine, 0), cardiac failure, ileus, general physical health deterioration, sudden cardiac death, sudden death, dyspnea (ruxolitinib plus capecitabine, 1 for each AE; placebo plus capecitabine, 0 for each AE), cardiac arrest, and portal vein thrombosis (ruxolitinib plus capecitabine, 0 for each AE; placebo plus capecitabine, 1 for each AE)
Table 4.
Most common treatment-emergent nonhematologic adverse eventsa (safety population)
| n (%) | JANUS 1 (N = 307) |
JANUS 2 (N = 85) |
||||||
|---|---|---|---|---|---|---|---|---|
| Ruxolitinib + capecitabine (n = 153) |
Placebo + capecitabine (n = 154) |
Ruxolitinib + capecitabine (n = 42) |
Placebo + capecitabine (n = 43) |
|||||
| All grade | Grade 3/4 | All grade | Grade 3/4 | All grade | Grade 3/4 | All grade | Grade 3/4 | |
| Nausea | 48 (31.4) | 8 (5.2) | 52 (33.8) | 3 (1.9) | 13 (31.0) | 2 (4.8) | 12 (27.9) | 4 (9.3) |
| Fatigue | 47 (30.7) | 8 (5.2) | 51 (33.1) | 12 (7.8) | 9 (21.4) | 1 (2.4) | 15 (34.9) | 2 (4.7) |
| Diarrhea | 44 (28.8) | 9 (5.9) | 38 (24.7) | 7 (4.5) | 11 (26.2) | 3 (7.1) | 11 (25.6) | 1 (2.3) |
| Abdominal pain | 40 (26.1) | 10 (6.5) | 59 (38.3) | 22 (14.3) | 15 (35.7) | 6 (14.3) | 6 (14.0) | 2 (4.7) |
| PPE syndrome | 37 (24.2) | 10 (6.5) | 26 (16.9) | 6 (3.9) | 10 (23.8) | 0 | 10 (23.3) | 5 (11.6) |
| Vomiting | 35 (22.9) | 8 (5.2) | 48 (31.2) | 7 (4.5) | 11 (26.2) | 1 (2.4) | 12 (27.9) | 5 (11.6) |
| Constipation | 34 (22.2) | 3 (2.0) | 30 (19.5) | 5 (3.2) | 8 (19.0) | 0 | 11 (25.6) | 1 (2.3) |
| Stomatitis | 32 (20.9) | 8 (5.2) | 18 (11.7) | 2 (1.3) | 6 (14.3) | 0 | 4 (9.3) | 1 (2.3) |
| Decreased appetite | 31 (20.3) | 6 (3.9) | 48 (31.2) | 5 (3.2) | 11 (26.2) | 1 (2.4) | 12 (27.9) | 1 (2.3) |
| Pyrexia | 31 (20.3) | 3 (2.0) | 14 (9.1) | 1 (0.6) | 7 (16.7) | 1 (2.4) | 5 (11.6) | 0 |
| Peripheral edema | 18 (11.8) | 0 | 35 (22.7) | 1 (0.6) | 7 (16.7) | 0 | 8 (18.6) | 1 (2.3) |
| Abdominal distension | 15 (9.8) | 0 | 12 (7.8) | 1 (0.6) | 10 (23.8) | 0 | 4 (9.3) | 0 |
| Dehydration | 15 (9.8) | 8 (5.2) | 13 (8.4) | 5 (3.2) | 6 (14.3) | 3 (7.1) | 4 (9.3) | 2 (4.7) |
| Hypokalemia | 17 (11.1) | 8 (5.2) | 14 (9.1) | 5 (3.2) | 7 (16.7) | 1 (2.4) | 10 (23.3) | 4 (9.3) |
| Hyponatremia | 13 (8.5) | 8 (5.2) | 6 (3.9) | 5 (3.2) | 5 (11.9) | 4 (9.5) | 2 (4.7) | 1 (2.3) |
| Sepsis | 7 (4.6) | 7 (4.6) | 5 (3.2) | 5 (3.2) | 2 (4.8) | 2 (4.8) | 4 (9.3) | 3 (7.0) |
| Pulmonary embolism | 7 (4.6) | 6 (3.9) | 5 (3.2) | 4 (2.6) | 4 (9.5) | 4 (9.5) | 3 (7.0) | 2 (4.7) |
| Dyspnea | 12 (7.8) | 3 (2.0) | 22 (14.3) | 3 (1.9) | 5 (11.9) | 4 (9.5) | 2 (4.7) | 1 (2.3) |
PPE palmar-plantar erythrodysesthesia
Events occurring in ≥20% of patients (all grade) or in ≥7% of patients (grade 3/4) in any treatment group
Table 5.
Worsening of hematologic toxicity (safety population)a
| n (%) | JANUS 1 (N = 292) |
JANUS 2 (N = 77) |
||||||
|---|---|---|---|---|---|---|---|---|
| Ruxolitinib + capecitabine (n = 148) |
Placebo + capecitabine (n = 144) |
Ruxolitinib + capecitabine (n = 38) |
Placebo + capecitabine (n = 39) |
|||||
| All grade | Grade 3/4 | All grade | Grade 3/4 | All grade | Grade 3/4 | All grade | Grade 3/4 | |
| Anemia | 93 (62.8) | 29 (19.6) | 51 (35.4) | 9 (6.3) | 24 (63.2) | 4 (10.5) | 14 (35.9) | 3 (7.7) |
| Lymphopenia | 59 (39.9) | 25 (16.9) | 50 (34.7) | 13 (9.0) | 14 (36.8) | 6 (15.8) | 10 (25.6) | 3 (7.7) |
| Thrombocytopenia | 43 (29.1) | 4 (2.7) | 32 (22.2) | 0 | 9 (23.7) | 1 (2.6) | 10 (25.6) | 1 (2.6) |
| Leukopenia | 34 (23.0) | 2 (1.4) | 23 (16.0) | 1 (0.7) | 11 (28.9) | 0 | 11 (28.2) | 1 (2.6) |
| Neutropenia | 17 (11.5) | 3 (2.0) | 10 (6.9) | 1 (0.7) | 9 (23.7) | 1 (2.6) | 8 (20.5) | 1 (2.6) |
Laboratory abnormalities (includes patients with baseline and postbaseline laboratory values)
Over 60% of patients in the ruxolitinib plus capecitabine groups and over 45% of patients in the placebo plus capecitabine groups of both studies had serious TEAEs (Table 3). Serious TEAEs occurring in ≥6% of patients in the ruxolitinib plus capecitabine group or the placebo plus capecitabine group, respectively, in either study were abdominal pain (JANUS 1: 4% vs 12%; JANUS 2: 10% vs 5%), dyspnea (JANUS 1: 3% vs 3%; JANUS 2: 10% vs 2%), nausea (JANUS 1: 3% vs 3%; JANUS 2: 7% vs 9%), dehydration (JANUS 1: 5% vs 3%; JANUS 2: 7% vs 2%), and vomiting (JANUS 1: 5% vs 3%; JANUS 2: 2% vs 12%). A total of 34 and 10 fatal TEAEs were reported in JANUS 1 and JANUS 2, respectively.
Discussion
In the randomized phase III JANUS 1 and JANUS 2 studies, the addition of ruxolitinib to capecitabine did not improve clinical outcomes for the second-line treatment of patients with advanced/metastatic pancreatic cancer and evidence of systemic inflammation. Based on the results of the planned interim futility analysis for JANUS 1, both studies were terminated as the addition of ruxolitinib to capecitabine did not improve OS, PFS, or ORR when compared with placebo plus capecitabine. Furthermore, changes in body weight and HRQoL were not significantly different between treatment groups in both studies. No new safety signals with ruxolitinib were identified. Anemia was the most frequently reported hematologic TEAE, consistent with results from the RECAP study [10] and the known safety profile of ruxolitinib [2].
Despite advances in the understanding of the molecular biology of pancreatic cancer, second-line treatment options remain a clear unmet need in patients with disease progression following first-line treatment for advanced/metastatic pancreatic cancer [19–21]. The rationale for evaluating the therapeutic utility of JAK inhibition in patients with pancreatic cancer and evidence of systemic inflammation has strong scientific merit [17, 22]. A substantial body of evidence confirms the existence of a systemic inflammatory response, measured by an elevated serum CRP level, in patients with cancer [11, 12, 14, 22]. The mGPS is an extensively reported prognostic marker used to stratify cancer outcomes based on CRP and albumin levels [14–16]. In two studies conducted in patients with resectable pancreatic ductal adenocarcinoma, patients with mGPS 0 had improved OS (27–37 months) vs patients with mGPS 1 and 2 (<18 months) [22, 23]. Interestingly, ruxolitinib was found to favorably modify pancreatic ductal carcinoma only in those patients with mGPS 1 or 2 [17]. Given the role of the JAK/STAT pathway in the development and perpetuation of the systemic inflammatory response, it was anticipated that treatment with the JAK 1/2 inhibitor ruxolitinib may be of benefit in these patients.
The current phase III studies were based on promising preliminary findings from the phase II RECAP study in a prespecified subgroup of patients with metastatic pancreatic cancer who had failed gemcitabine and had evidence of a systemic inflammatory response [17]. In the RECAP study, patients with a CRP level higher than the study population median (>13 mg/L) had a significantly greater improvement in OS with ruxolitinib plus capecitabine compared with placebo plus capecitabine. Furthermore, benefits across multiple endpoints, including PFS, reduction in tumor burden, and the composite endpoint of clinical benefit response also were observed [17]. While the effectiveness of ruxolitinib plus capecitabine across the two JANUS studies was less than that reported in the RECAP subgroup analysis in patients with CRP levels >13 mg/dL, our findings are in accordance with the primary analysis of the RECAP study, which reported only modest, statistically nonsignificant, improvements in survival outcomes with ruxolitinib in patients with metastatic pancreatic cancer [17]. Based on multiple retrospective studies in solid tumors, the prognostic significance of mGPS has been extensively reported. Unfortunately, the current larger phase III studies did not support the hypothesis of therapeutic utility of JAK1/2 inhibition in patients with pancreatic cancer and high levels of systemic inflammation that was initially supported by a post hoc subgroup analysis of the phase II RECAP study. In light of this, an unmet need remains to improve outcomes for patients with metastatic pancreatic cancer, and alternative systemic therapies need to be explored.
Our results are supported by a robust study design (prospective, randomized, phase III, placebo-controlled, multicenter) with sufficient power to meet the primary endpoint. JANUS 1 and JANUS 2 were conducted concurrently based on feedback from the United States Food Drug Administration to support the registration of ruxolitinib in this setting. However, both studies were terminated following recommendation by the IDMC on the basis of an interim futility analysis in JANUS 1. Further, ruxolitinib was only evaluated in those patients with a high baseline CRP level (>10 mg/L) and in patients with refractory disease. Given the myelosuppression associated with first-line therapies including FOLFIRINOX and nab-paclitaxel plus gemcitabine, investigating the addition of ruxolitinib, also associated with myelosuppression, to these therapies will be challenging [24, 25].
The lack of efficacy of the JANUS 1 and 2 studies highlight the complexity of the JAK/STAT pathway and tumor inflammation in pancreatic cancer. Ruxolitinib has activity against several in vitro and in vivo preclinical models of pancreatic cancer [9–11, 26, 27]. Ruxolitinib also has been shown to suppress serum CRP levels, a marker of JAK pathway–mediated inflammation, in multiple clinical trials (including in the JANUS studies) [28–30]. However, the exact effects of ruxolitinib on different JAK receptors, and more importantly on different STATs, within the tumor microenvironment of patients with pancreatic cancer are not known. Numerous signaling bypass pathways and compensatory feedback loops may affect the activity of JAK inhibitors [31]. Given the importance of the JAK/STAT pathway in pancreatic cancer, it is possible other molecules targeting this signaling axis may prove useful. Given the importance of JAK/STAT signaling in myeloid and lymphoid compartments, these pathways are also likely to have important roles for cancer immunotherapies [32, 33].
In conclusion, in the JANUS 1 and JANUS 2 studies, ruxolitinib did not improve the clinical outcomes of patients with refractory pancreatic cancer when added to capecitabine. The development of novel mechanism-based therapies for these patients remains an ongoing challenge.
Acknowledgments
The authors wish to thank the patients and their families, the investigators, and the site personnel who participated in this study. This study was sponsored by Incyte Corporation (Wilmington, DE, USA). Medical writing assistance was provided by Sneha D’Silva, MD, on behalf of Evidence Scientific Solutions Inc. (Philadelphia, PA, USA), and funded by Incyte.
Footnotes
Conflicts of interest Hurwitz: Honoraria – Genentech/Roche, Lilly/ImClone; Consulting or advisory role – Acceleron Pharma, Bristol-Myers Squibb, Genentech/Roche, GlaxoSmithKline, Incyte, Lilly, Novartis, OncoMed, TRACON Pharma; Research funding – Bristol-Myers Squibb (Inst), Genentech/Roche (Inst), GlaxoSmithKline (Inst), Lilly (Inst), Macrogenics (Inst), NCI (Inst), Novartis (Inst), Regeneron (Inst), TRACON Pharma (Inst); Employment – Genentech/Roche as of 23 October 2017. Van Cutsem: Research funding – Amgen (Inst), Bayer (Inst), Boehringer Ingelheim (Inst), Celgene (Inst), Ipsen (Inst), Lilly (Inst), Merck Serono (Inst), Novartis (Inst), Roche (Inst), Sanofi (Inst). Bendell: Research funding – Incyte. Sahai: Consulting – Celgene, Halozyme, NewLink; Research funding – Agios (Inst), Bristol-Myers Squibb (Inst), Celgene (Inst), Incyte (Inst). Sama: Honoraria – OncoPlex Diagnostics; Research funding – Bristol-Myers Squibb (Inst), Incyte (Inst), MedImmune (Inst), OncoMed (Inst), Precision Biologics (Inst); Employment – Bristol-Myers Squibb (Inst) as of March 20, 2017. Yu: Consulting or advisory role – Celgene, Merck Serono, Merrimack. Dawkins, Walker, Clark: Employment – Incyte; Stock and other ownership interests – Incyte. O’Reilly: Consulting or advisory role – Aduro Biotech, Astellas Pharma (Inst), Celgene, Celsion (Inst), Cipla, Exelixis (Inst), Gilead Sciences, Hexal (Inst), IntegraGen (Inst), Jennerex (Inst), Lilly (Inst), MedImmune, Merrimack, Novartis (Inst), Pharmacyclics, Sanofi, Silenseed, Vicus Therapeutics; Research funding – AstraZeneca/MedImmune (Inst), Bristol-Myers Squibb (Inst), Celgene (Inst), Immunomedics (Inst), Incyte (Inst), Momenta Pharmaceuticals (Inst), Myriad Genetics (Inst), OncoMed (Inst), Polaris (Inst), Sanofi (Inst). Hidalgo, Li, Garrido, Macarulla, Greeno, Verslype: Nothing to disclose.
Ethical approval All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent Informed consent was obtained from all individual participants included in the study.
References
- 1.Siegel RL, Miller KD, Jemal A (2017) Cancer statistics, 2017. CA Cancer J Clin 67:7–30. 10.3322/caac.21387 [DOI] [PubMed] [Google Scholar]
- 2.NCCN clinical practice guidelines in oncology: Pancreatic adenocarcinoma. Version 2.2017. National Comprehensive Cancer Network website http://www.nccn.org/professionals/physician_gls/pdf/pancreatic.pdf. Accessed 16 August 2017 [DOI] [PubMed]
- 3.Walker EJ, Ko AH (2014) Beyond first-line chemotherapy for advanced pancreatic cancer: an expanding array of therapeutic options? World J Gastroenterol 20:2224–2236. 10.3748/wjg.v20.i9.2224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang-Gillam A, Li CP, Bodoky G, Dean A, Shan YS, Jameson G, Macarulla T, Lee KH, Cunningham D, Blanc JF, Hubner RA, Chiu CF, Schwartsmann G, Siveke JT, Braiteh F, Moyo V, Belanger B, Dhindsa N, Bayever E, Von Hoff DD, Chen LT (2016) Nanoliposomal irinotecan with fluorouracil and folinic acid in metastatic pancreatic cancer after previous gemcitabine-based therapy (NAPOLI-1): a global, randomised, open-label, phase 3 trial. Lancet 387:545–557. 10.1016/S0140-6736(15)00986-1 [DOI] [PubMed] [Google Scholar]
- 5.ONIVYDE® (irinotecan liposome injection) [package insert] (2015) Cambridge, Merrimack Pharmaceuticals, Inc [Google Scholar]
- 6.Quintás-Cardama A, Vaddi K, Liu P, Manshouri T, Li J, Scherle PA, Caulder E, Wen X, Li Y, Waeltz P, Rupar M, Burn T, Lo Y, Kelley J, Covington M, Shepard S, Rodgers JD, Haley P, Kantarjian H, Fridman JS, Verstovsek S (2010) Preclinical characterization of the selective JAK1/2 inhibitor INCB018424: therapeutic implications for the treatment of myeloproliferative neoplasms. Blood 115: 3109–3117. 10.1182/blood-2009-04-214957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.JAKAVI® (ruxolitinib) [summary of product characteristics] (2016) Camberley, UK, Novartis Europharm Limited; www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002464/WC500133223.pdf. Accessed 8 May 2017 [Google Scholar]
- 8.JAKAFI® (ruxolitinib) [package insert] (2016) Wilmington, DE, Incyte Corporation; http://www.jakafi.com/pdf/prescribinginformation.pdf. Accessed 8 May 2017 [Google Scholar]
- 9.Gore J, Craven KE, Wilson JL, Cote GA, Cheng M, Nguyen HV, Cramer HM, Sherman S, Korc M (2015) TCGA data and patientderived orthotopic xenografts highlight pancreatic cancerassociated angiogenesis. Oncotarget 6:7504–7521. 10.18632/oncotarget.3233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koblish HK, Hansbury M, Wang L-CS, Yang G, Huang T, Xue C-B, Li Y-L, Yue E, Combs A, Yao W, Huber R, Scherle P (2015) Novel immunotherapeutic activity of JAK and PI3Kδ inhibitors in a model of pancreatic cancer [abstract]. Cancer Res 75:1336 10.1158/1538-7445.am2015-1336 [DOI] [Google Scholar]
- 11.Lesina M, Kurkowski MU, Ludes K, Rose-John S, Treiber M, Klöppel G, Yoshimura A, Reindl W, Sipos B, Akira S, Schmid RM, Algül H (2011) Stat3/Socs3 activation by IL-6 transsignaling promotes progression of pancreatic intraepithelial neoplasia and development of pancreatic cancer. Cancer Cell 19:456–469. 10.1016/j.ccr.2011.03.009 [DOI] [PubMed] [Google Scholar]
- 12.Tan CR, Yaffee PM, Jamil LH, Lo SK, Nissen N, Pandol SJ, Tuli R, Hendifar AE (2014) Pancreatic cancer cachexia: a review of mechanisms and therapeutics. Front Physiol 5(88). 10.3389/fphys.2014.00088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Allin KH, Nordestgaard BG (2011) Elevated C-reactive protein in the diagnosis, prognosis, and cause of cancer. Crit Rev Clin Lab Sci 48:155–170. 10.3109/10408363.2011.599831 [DOI] [PubMed] [Google Scholar]
- 14.Salmiheimo A, Mustonen H, Stenman U-H, Puolakkainen P, Kemppainen E, Seppänen H, Haglund C (2016) Systemic inflammatory response and elevated tumour markers predict worse survival in resectable pancreatic ductal adenocarcinoma. PLoS One 11:e0163064 10.1371/journal.pone.0163064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McMillan DC, Crozier JE, Canna K, Angerson WJ, McArdle CS (2007) Evaluation of an inflammation-based prognostic score (GPS) in patients undergoing resection for colon and rectal cancer. Int J Color Dis 22:881–886. 10.1007/s00384-006-0259-6 [DOI] [PubMed] [Google Scholar]
- 16.McMillan DC (2013) The systemic inflammation-based Glasgow prognostic score: a decade of experience in patients with cancer. Cancer Treat Rev 39:534–540. 10.1016/j.ctrv.2012.08.003 [DOI] [PubMed] [Google Scholar]
- 17.Hurwitz HI, Uppal N, Wagner SA, Bendell JC, Beck JT, Wade SM 3rd, Nemunaitis JJ, Stella PJ, Pipas JM, Wainberg ZA, Manges R, Garrett WM, Hunter DS, Clark J, Leopold L, Sandor V, Levy RS (2015) Randomized, double-blind, phase II study of ruxolitinib or placebo in combination with capecitabine in patients with metastatic pancreatic cancer for whom therapy with gemcitabine has failed. J Clin Oncol 33:4039–4047. 10.1200/JCO.2015.61.4578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heffernan N, Cella D, Webster K, Odom L, Martone M, Passik S, Bookbinder M, Fong Y, Jarnagin W, Blumgart L (2002) Measuring health-related quality of life in patients with hepatobiliary cancers: the functional assessment of cancer therapy-hepatobiliary questionnaire. J Clin Oncol 20: 2229–2239. 10.1200/JCO.2002.07.093 [DOI] [PubMed] [Google Scholar]
- 19.Sonbol MB, Firwana B, Wang Z, Almader-Douglas D, Borad MJ, Makhoul I, Ramanathan RK, Ahn DH, Bekaii-Saab T (2017) Second-line treatment in patients with pancreatic ductal adenocarcinoma: a meta-analysis. Cancer 123:4680–4686. 10.1002/cncr.30927 [DOI] [PubMed] [Google Scholar]
- 20.Cinar P, Ko AH (2017) Best practices for the treatment of metastatic pancreatic adenocarcinoma: the therapeutic landscape in 2017. Chin Clin Oncol 6:29 10.21037/cco.2017.06.13 [DOI] [PubMed] [Google Scholar]
- 21.Aprile G, Negri FV, Giuliani F, De Carlo E, Melisi D, Simionato F, Silvestris N, Brunetti O, Leone F, Marino D, Santini D, Dell’Aquila E, Zeppola T, Puzzoni M, Scartozzi M (2017) Second-line chemotherapy for advanced pancreatic cancer: which is the best option? Crit Rev Oncol Hematol 115:1–12. 10.1016/j.critrevonc.2017.03.025 [DOI] [PubMed] [Google Scholar]
- 22.Imrie CW (2015) Host systemic inflammatory response influences outcome in pancreatic cancer. Pancreatology 15:327–330. 10.1016/j.pan.2015.04.004 [DOI] [PubMed] [Google Scholar]
- 23.Jamieson NB, Denley SM, Logue J, MacKenzie DJ, Foulis AK, Dickson EJ, Imrie CW, Carter R, McKay CJ, McMillan DC (2011) A prospective comparison of the prognostic value of tumor- and patient-related factors in patients undergoing potentially curative surgery for pancreatic ductal adenocarcinoma. Ann Surg Oncol 18:2318–2328. 10.1245/s10434-011-1560-3 [DOI] [PubMed] [Google Scholar]
- 24.Muranaka T, Kuwatani M, Komatsu Y, Sawada K, Nakatsumi H, Kawamoto Y, Yuki S, Kubota Y, Kubo K, Kawahata S, Kawakubo K, Kawakami H, Sakamoto N (2017) Comparison of efficacy and toxicity of FOLFIRINOX and gemcitabine with nab-paclitaxel in unresectable pancreatic cancer. J Gastrointest Oncol 8:566–571. 10.21037/jgo.2017.02.02 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ostojic A, Vrhovac R, Verstovsek S (2012) Ruxolitinib for the treatment of myelofibrosis: its clinical potential. Ther Clin Risk Manag 8:95–103. 10.2147/TCRM.S23277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wörmann SM, Song L, Ai JY, Diakopoulos KN, Kurkowski MU, Görgülü K, Ruess D, Campbell A, Doglioni C, Jodrell D, Neesse A, Demir IE, Karpathaki AP, Barenboim M, Hagemann T, Rose-John S, Sansom O, Schmid RM, Protti MP, Lesina M, Algül H (2016) Loss of P53 function activates JAK2-STAT3 signaling to promote pancreatic tumor growth, stroma modification, and gemcitabine resistance in mice and is associated with patient survival. Gastroenterology 151:180–193. 10.1053/j.gastro.2016.03.010 [DOI] [PubMed] [Google Scholar]
- 27.Smigiel JM, Parameswaran N, Jackson MW (2017) Potent EMT and CSC phenotypes are induced by oncostatin-M in pancreatic cancer. Mol Cancer Res 15:478–488. 10.1158/1541-7786.mcr-16-0337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mascarenhas J, Hoffman R (2012) Ruxolitinib: the first FDA approved therapy for the treatment of myelofibrosis. Clin Cancer Res 18:3008–3014. 10.1158/1078-0432.CCR-11-3145 [DOI] [PubMed] [Google Scholar]
- 29.Kremyanskaya M, Atallah EL, Hoffman R, Mascarenhas JO (2013) Clarifying the use of ruxolitinib in patients with myelofibrosis. Oncology 27:706–714 [PubMed] [Google Scholar]
- 30.Mascarenhas J, Mughal TI, Verstovsek S (2012) Biology and clinical management of myeloproliferative neoplasms and development of the JAK inhibitor ruxolitinib. Curr Med Chem 19:4399–4413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.von Manstein V, Yang CM, Richter D, Delis N, Vafaizadeh V, Groner B (2013) Resistance of cancer cells to targeted therapies through the activation of compensating signaling loops. Curr Signal Transduct Ther 8:193–202. 10.2174/1574362409666140206221931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zaretsky JM, Garcia-Diaz A, Shin DS, Escuin-Ordinas H, Hugo W, Hu-Lieskovan S, Torrejon DY, Abril-Rodriguez G, Sandoval S, Barthly L, Saco J, Homet Moreno B, Mezzadra R, Chmielowski B, Ruchalski K, Shintaku IP, Sanchez PJ, PuigSaus C, Cherry G, Seja E, Kong X, Pang J, Berent-Maoz B, Comin-Anduix B, Graeber TG, Tumeh PC, Schumacher TN, Lo RS, Ribas A (2016) Mutations associated with acquired resistance to PD-1 blockade in melanoma. N Engl J Med 375:819–829. 10.1056/NEJMoa1604958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yu H, Kortylewski M, Pardoll D (2007) Crosstalk between cancer and immune cells: role of STAT3 in the tumour microenvironment. Nat Rev Immunol 7:41–51. 10.1038/nri1995 [DOI] [PubMed] [Google Scholar]
- 34.Brookmeyer R, Crowley JJ (1982) A confidence interval for the median survival time. Biometrics 38:29–41 [Google Scholar]