Abstract
Qinghaosu and its derivatives are widely used in the world as a new generation of antimalarial drug. Up to now, some important progresses of Qinghaosu research have been made, including synthesis of new qinghaosu derivatives and analogs, investigation on their bioactivities and mode of actions. The present review briefly describes these efforts made by researchers in China, particularly in this Institute.
Keywords: Qinghaosu, artemisinin, structure modification, antimalarial activity, anticancer activity, immunosuppressive activity, mode of action
On 23rd May 1967, China established National Steering Group on antimalarial drug research, more than 60 institutes and 500 researchers joined in this project. After screening of over 5000 traditional Chinese medicines, Qinghaosu (QHS), an antimalarial principle, was isolated from Artemisia annua L in 19721, 2. At the end of 1975, its unique chemical structure was elucidated, as a sesquiterpene lactone bearing a peroxy group, quite different from that of all known antimalarial drugs3.
Early pharmacological and clinic studies showed QHS have rapid onset of action, low toxicity and high effect on both drug-resistant and drug-sensitive malaria. However, its shortcomings (poor solubility in water or oil; high rate of parasite recrudescence) needed to be overcome. In 1976, our Institute was charged with this important mission, and a research in relationship between chemical structure and activity immediately started.
First of all, the function of peroxy group for antimalarial activity was examined. The negative result of deoxyqinghaosu (Scheme 1) against P berghei in mice demonstrated that the peroxy group was essential. Soon afterwards, it was found that some other simple peroxides including monoterpene ascaridol had no antimalarial activity. These experimental results proved peroxy group to be an essential but not a sufficient factor. At that time, we noted the molecule contained a rare segment -O-C-O-C-O-C=O, and realized that whole molecular skeleton might play an important role for antimalarial activity.
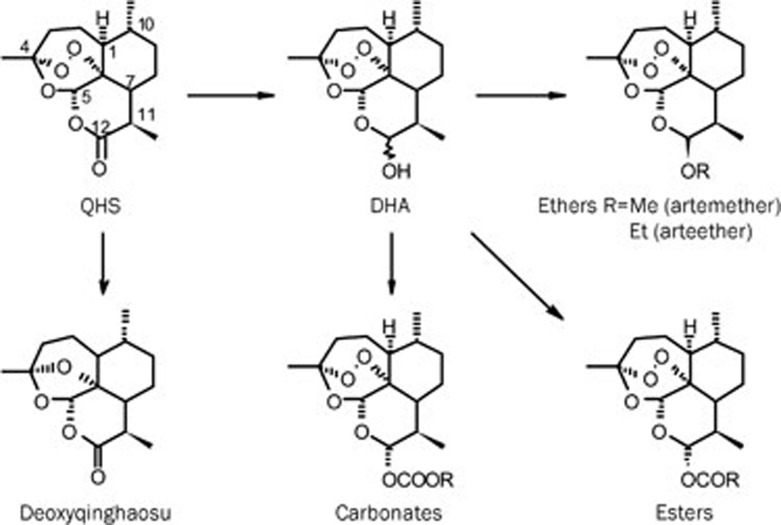
Scheme 1.
Synthesis of Qinghaosu derivatives.
When dihydroartemisinin (DHA) was found to be more active than QHS, but was still with poor solubility and lower stability (as a lactol) than QHS, we decided to prepare its derivatives. In 1976–1977, over 50 derivatives of DHA were synthesized (Scheme 1) and evaluated4, 5.
The first 25 compounds (in oil solution) were tested in mice infected chloroquine-resistant P berghei though intramuscular injection6. Most of these derivatives showed higher activity than QHS (SD50 6.20 mg/kg) and DHA (SD50 3.65 mg/kg). In the ether series, SM 224 (R=CH3, SD50 1.02 mg/kg) is more active than SM 227 (R=C2H5, SD50 1.95 mg/kg) and others. Then, SM 224, SM 108 (ester, R=C2H5,SD50 0.66 mg/kg) and SM 242 (carbonate, R=n-C3H7, SD50 0.50 mg/kg) were compared with regard to activity, stability, toxicity and cost. Because SM 224 was highly soluble in oil and more stable than others, it was selected as candidate and named as artemether.
At the same period, water-soluble sodium artesunate (ester, R=COCH2CH2COONa) was developed by the Guilin Pharmaceutical Factory7. QHS suppository in 1986; artemether oil injection and sodium artesunate aqueous injection in 1987 were approved as new antimalarial drugs. Since then, other new antimalarial drugs such as dihydroartemisinin, coartem (artemether and benflumetol), co-naphthoquine (naphthoquine phosphate and QHS), compound-dihydroartemisinin were successively developed in China1, 2.
Since 1980s, millions malaria patients in the world (mainly in China, Southeast Asia and Africa) were saved by administration of QHS, QHS derivatives and their combinations. Artemether, artesunate and coartem have been enrolled in WHO's “List of Essential Medicines”.
Artemisinins can kill drug-resistant P falciparum, its antimalarial mechanism must be different from that of the previously-used antimalarial drugs. Elucidation of its mode of action is really an interesting project. Up to now, many research papers have been published8, 9, 10, 11, 12, 13, 14. Chinese researchers first reported in 1979 that artemisinin drugs had a direct parasiticidal action against P falciparum in the erythrocytic stage both in vitro and in vivo, and observed their morphologic changes under the electron microscope15, 16. The main pathological ultrastructural changes caused by QHS were quick damage of the membrane system of the asexual forms of parasites, the swelling and spiral deformation of the membrane of food vacuoles, limiting membrane and the membrane of mitochondria, followed by swelling of the nuclear membrane and endoplasmic reticulum. Among the latter results made in China17, 18, 19, 20, 21, a series of reaction of artemisinins and Fe2+, which involes the intermediate of oxygen-centered or carbon-centered free radicals, is noteworthly.
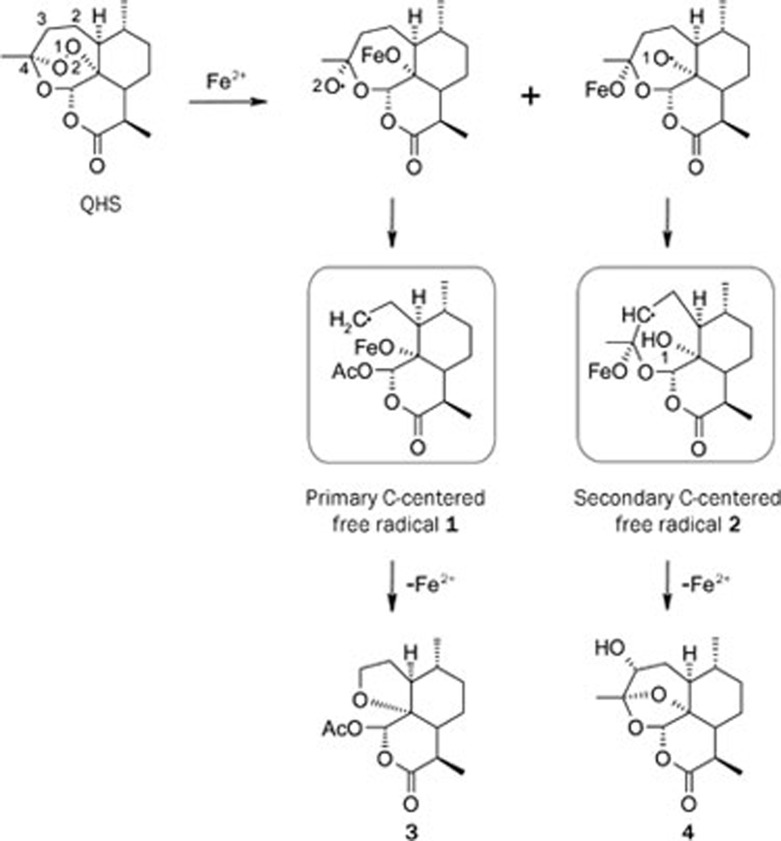
For exploring the mode of action, reaction of QHS and Fe2+ was carefully studied by Chinese chemists22, 23. The major products tetrahydrofuran 3 and 3-hydroxy deoxyqinghaosu 4 were proposed to be derived from primary C-centered free radical 1 and secondary C-centered free radical 2, respectively, as shown in Scheme 2. In addition, the electron spin resonance (ESR) signals of the C-centered free radicals were detected23, 24.
Scheme 2.
Formation of Carbon-centered free radicals.
It is worthy to note that compound 3 and 4 are also the major metabolites of QHS in vivo or in human25, 26.
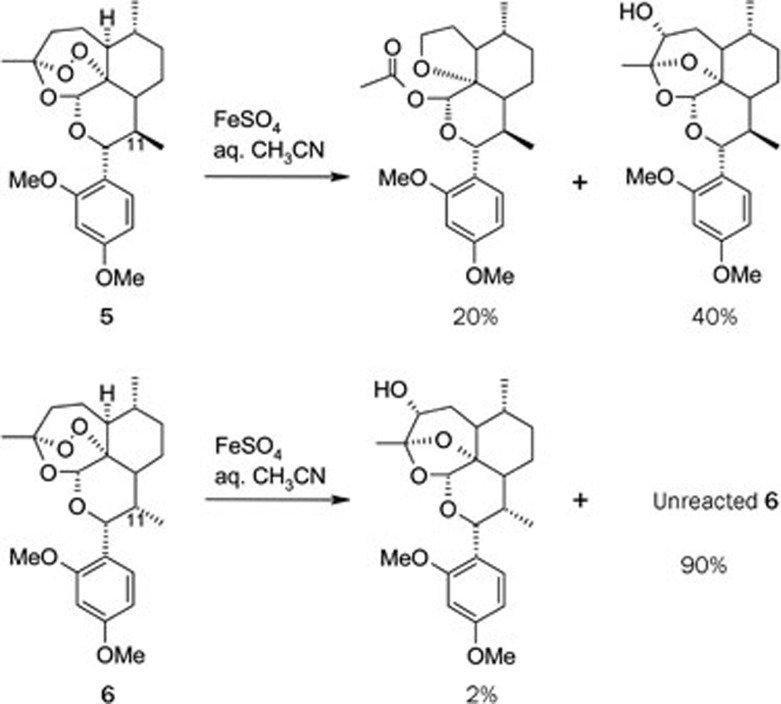
With the clarification on the C-centered free radicals participation mechanism, it is then interested whether this free radical mechanism is related to its antimalarial activity. Thus, the stable and UV-detectable C-12 aromatic substituted derivatives of QHS were synthezied27. Using the usual Lewis acid as the catalyst, the Friedel-Crafts alkylation gave the desired product 2′,4′-dimethoxyphenyl deoxoartemisinin 5 and also 11α-methyl epimer 6 as the by-product (Scheme 3). These products were separated and subjected to both bioassay and chemical reaction with ferrous ion. It is interesting to find that the derivative with normal configuration at C-11 showed higher antimalarial activity and also higher chemical reactivity in the reaction with ferrous ion. On the contrary, the 11α-methyl epimer 6 was obviously less active and almost inert to the reaction with ferrous ion (Scheme 3, Table 1).
Scheme 3.
Reaction of QHS derivatives and FeSO4.
Table 1. The ED50- and ED90-values against P berghei K173 strain (administered orally to mice as suspensions in Tween 80).
| Compound | ED50 (mg/kg) | ED90 (mg/kg) |
|---|---|---|
| Artemether | 1 | 3.1 |
| 5 | 1.27 | 5.27 |
| 6 | 4.18 | 76.27 |
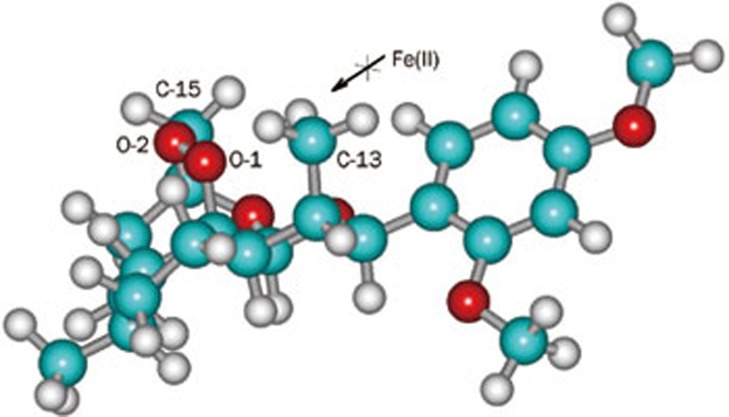
In the case of 11α and 11β-epimers of α-hydroxy naphthyl deoxoqinghaosu, similar result was also obtained28. Their lower activity may be attributed to the steric hindrance around O-1 atom in 11α-epimer, which blocks the way for Fe2+ to attack O-1 (Figure 1).
Figure 1.
11α-methyl group blocks the attack of Fe2+ on O-1.
The close relationship between antimalarial activity and the primary C-centered free radicals strongly suggests that the Fe2+-induced cleavage of peroxide bond in artemisinins leads to C-centered free radicals, a highly potent alkylating species. Then, what targets would be alkylated? Up to now, a growing list shows that heme, heme-containing protein, translationally controlled tumor-associated protein (TCTP), sarcoendoplasmic reticulum Ca2+ ATPase (SERCA)-type protein encoded by PfATP6 might be the targets14. Recently, Chinese researchers reported that artemisinins are distributed to malarial mitochondria and directly impair their functions29.
As for process of alkylation of targets, there was another interesting topic. Considering that malaria-parasite-infected red blood cells have a high concentration of the reducing glutathione (GSH) and excess GSH might be responsible for protecting the parasite from the toxicity of heme, Chinese chemists have performed some reactions, such as artemisinins reacted with cysteine or GSH in the present of a catalytic amount of ferrous ion27, 30, 31, 32, 33. Successful isolation and identification of the cysteine-artemisinins or GSH-artemisinins adducts proved the formation of covalent bond in these adducts. It is instructive that the C-centered radicals derived from QHS and their derivatives could attack free cysteine and cysteine rasidue not only in peptide but also probably in protein.
The outstanding characteristics of QHS, including high antimalarial action, less significant side effects and less clinical resistance, impelled researchers to extend its medical application. As a result, hundreds of artemisinin derivatives and analogs were synthesized and widely assayed in China. So far, the successful examples are artemether and artesunate, which were found to be effective in the treatment of Schistosomiasis34, 35. From 1993, artemether and artesunate were studied in randomized, double-blind, placebo-controlled trials in China, and approved as the prophylactic drugs for schistosomiasis japonica in 19962. Afterwards, they were found to have similar activity against S mansoni and S haematobium in other countries36.
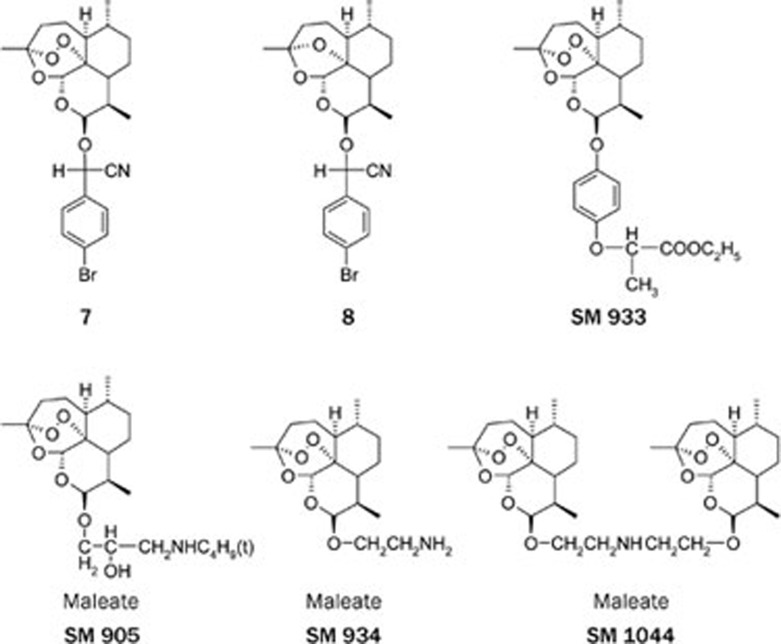
Since 1992, when this Institute first reported that some components of Artemisia annua L (such as artemisinic acid, artemisinin B) have antitumor activity in vitro37, 38, hundreds of papers have described the cytotoxicity of QHS and related compounds against tumor cells. Artesunate and DHA are most favorable molecules. In 2001 Efferth et al reported that artesunate showed antitumor activity against 55 tumor cell lines, and it was most active against leukemia and colon cancer cell lines. It is notable that none of CEM leukemia sublines, resistant to doxorubicin, vincristin, methotrexate or hydroxyurea showed cross-resistance to artesunate39. Since then, more new artemisinin derivatives and analogs were synthesized and tested against human cancer cells. Some compounds showed high activity at the nano- to micromolar range40. For example, this Institute in collaboration with French cooperator found that a dihydroartemisinin ether containing cyanoarylmethyl group 7 (Figure 2) was very active against P388 and A549 cell lines, as comparable to VCR, whereas its deoxy-analog 8 (Figure 2) was inactive. Thus peroxy group was proved to be essential for antitumor activity, like for antimalarial activity41, 42. In addition, the type of derivative was proved to target G1 phase of the cell cycle41. Thus far, antitumor activity of artemisinins and the underlying mechanisms have been widely studied43, 44, 45, 46, 47, 48, 49, however, few clinical trials were reported.
Figure 2.
Some compounds with anticancer or immunosuppressive activity.
Antimalarial chloroquine was used as immunosuppressive agent. Therefore, the research on immunological activity of QHS and its derivatives became an attractive program in China in the 1980'. At the begining, mainly new antimalarial drugs (QHS, artemether and artesunate) were studied in laboratories in China. Afterwards, the clinical trials of artesunate for the treatment of DLE, SLE, rheumatoid arthritis, polymorphous light eruption and chronic actinic dermatitis were conducted. Some clinical data were promising, for example, 56 patients with lupus erythematosus (DLE 16, SCLE 10, and SLE 30) were treated by sodium artesunate (iv 60 mg, once a day, 15 d a course, 2–4 courses), with effective rate at 94%, 90%, and 80%, respectively50. To search for highly potent, low toxic immunosuppressive agents, more artemisinin derivatives were synthesized and screened in this Institute since 200151, 52, 53, 54. Compounds with various structures were tested in respect to their cytotoxicity to lymphocyte, inhibition activity on ConA-induced T cell proliferation and LPS-induced B cell proliferation, in comparison with QHS, artesunate, artemether and cyclosporin A (CsA). At last, SM 735, SM 905, SM 933, and SM 934 (Figure 2) were selected and tested in the animal models for 2,4-dinitrofluorobenzene (DNFB)-induced delayed-type hypersensitivity (DTH) reaction, sheep red blood cell (SRBC)-induced antibody production, and experimental autoimmune encephalomyelitis (EAE). Up to date, a number of papers related their immuno-suppressive activity and possible mechanisms have been published mainly from this Institute55, 56, 57, 58, 59, 60, 61, 62, 63, 64. The preclinical research of SM 934 is in progress.
During the latter period of the program of the relationship between chemical structure and activity of artemisinins, SM 1044 was isolated as a by-product during the preparation of SM 934, and interestingly showed excellent antileukemia activity in vitro and in vivo in the Ruijin Hospital, Shanghai Jiao Tong University School of Medicine65, 66, 67, 68, 69. These alkaline artemisinins may be a new kind of promising candidates. I sincerely hope they will be successfully developed for treatment of cancer, auto-immune or other diseases, like artemether as antimalarial drug 30 years ago.
References
- Zhang JF.A detailed chronological record of project 523 and the discovery and development of Qinghaosu (Artemisinin)Guangzhou: Yangcheng Evening News Publisher; 2006. Chinese.
- Li Y, Wu YL. A golden phoenix arising from the herbal net — A review and reflection on the study of antimalarial drug qinghaosu. Front Chem China. 2010;5:357–422. [Google Scholar]
- Liu JM, Ni MY, Fan JF, Tu YY, Wu ZH, Wu YL, et al. Structure and reactions of arteannuin Acta Chim Sin 197937129–43.Chinese. [Google Scholar]
- Li Y, Yu PL, Chen YX, Li LQ, Gai YZ, Wang DS, et al. Synthesis of some derivatives of artemisinin Ke Xue Tong Bao 197924667–9.Chinese. [Google Scholar]
- Li Y, Yu PL, Chen YX, Li LQ, Gai YZ, Wang DS, et al. Studies on analogs of artemisinin I. The synthesis of ethers, carboxylic esters and carbonates of dihydroartemisinine Yao Xue Xue Bao 198116429–39.Chinese. [PubMed] [Google Scholar]
- Gu HM, Lu BF, Qu ZX.Antimalarial activities of 25 derivatives of artemisinine against chloroquine-resistant Plasmodium berghei Acta Pharmacol Sin 1980148–50.Chinese. [PubMed] [Google Scholar]
- Liu X.Study on artemisinin derivatives Yao Xue Tong Bao 198015183Chinese. [Google Scholar]
- Meshnick SR, Tsang TW, Lin FB, Pan HZ, Chang CN, Kuypers F, et al. Activated oxygen mediates the antimalarial activity of qinghaosu. Prog Clin Biol Res. 1989;313:95–104. [PubMed] [Google Scholar]
- Meshnick SR, Tomas A, Ranz A, Xu CM, Pan HZ. Artemisinin (qinghaosu): the role of intracellular hemin in its mechanism of antimalarial action. Mol Biochem Parasitol. 1991;49:181–9. doi: 10.1016/0166-6851(91)90062-b. [DOI] [PubMed] [Google Scholar]
- Posner GH, Oh CH. A regiospecifically oxygen-18 labeled 1,2,4-trioxane: a simple chemical mode system to probe the mechanism(s) for the antimalarial activity of artemisinin (qinghaosu) J Am Chem Soc. 1992;114:8328–9. [Google Scholar]
- Jefford CW. Why artemisinin and certain synthetic peroxides are potent antimalarials. Implications for the mode of action. Curr Med Chem. 2001;8:1803–26. doi: 10.2174/0929867013371608. [DOI] [PubMed] [Google Scholar]
- Olliaro PL, Haynes RK, Meunier B, Yuthavong Y. Possible modes of action of the artemisinin-type compounds. Trends Parasitol. 2001;17:122–6. doi: 10.1016/s1471-4922(00)01838-9. [DOI] [PubMed] [Google Scholar]
- Posner GH, O'Neill PM. Knowledge of the proposed chemical mechanism of action and cytochrome P 450 metabolism of antimalarial trioxanes like artemisinin allows rational design of new antimalarial peroxides. Acc Chem Res. 2004;37:394–404. doi: 10.1021/ar020227u. [DOI] [PubMed] [Google Scholar]
- O'Neill PM, Barton VE, Ward SA. The molecular mechanism of action of artemisinin — the debate continues. Molecules. 2010;15:1705–21. doi: 10.3390/molecules15031705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qinghaosu antimaraia coordinating research group: Antimararia studies on qinghaosu. Chin Med J. 1979;92:811–6. [PubMed] [Google Scholar]
- China cooperative research group on qinghaosu and its derivatives as antimalarials: Antimararial efficacy and mode of action of qinghaosu and its derivatives in experimental models. China Cooperative Research Group on qinghaosu and its derivatives as antimalarials. J Tradit Chin Med. 1982;2:17–24. [PubMed] [Google Scholar]
- Jin YG, Teng XH, Sun CP.Free radicals and mechanism of toxicity of sodium artesunate Chin J Pharmacol Toxicol 1989260–5.Chinese. [Google Scholar]
- Wang JY, Yuan LZ, Wang MD.Inhibition of sodium artesunate on rat erythrocyte membrane Na+-K+-exchanging ATPase in vitro Yao Xue Xue Bao 199516524–6.Chinese. [PubMed] [Google Scholar]
- Shi XC, Zhang QL, Wu K, Wang S, Sun CP, Wang ZQ.Hemopoietotoxicity in pregnant mice and their embryos induced by free radicals derived from sodium artesunate Chin J Pharmacol Toxicol 199711226–8.Chinese. [Google Scholar]
- Chen Y, Zhu SM, Chen HY, Li Y. Artesunate interaction with heme. Bioelectrochem Bioenerg. 1988;44:295–300. [Google Scholar]
- Cheng F, Shen JH, Luo XM, Zhu WL, Gu JD JI RY, et al. Molecular docking and 3-D-QSAR studies on the possible antimalarial mechanism of artemisinin analogues. Bioorg Med Chem. 2002;10:2883–91. doi: 10.1016/s0968-0896(02)00161-x. [DOI] [PubMed] [Google Scholar]
- Wu WM, Yao ZJ, Wu YL, Jiang K, Wang YF, Chen HB, et al. Ferrous ion induced cleavage of the peroxy bond in qinghaosu and its derivatives and the DNA damage associated with this process. Chem Commun. 1996;18:2213–4. [Google Scholar]
- Wu WM, Wu YK, Wu YL, Yao ZJ, Zhou CM, Li Y, et al. A unified mechanism framework for the Fe(II)-induced cleavage of qinghaosu and derivatives/analogs. The first spin-trapping evidence for the earlier postulated secondary C-4 radical. J Am Chem Soc. 1998;120:3316–25. [Google Scholar]
- Butler AR, Gilbert BC, Hulme P, Irvine LR, Renton L, Whitwood AC. EPR evidence for the involvement of free radicals in the iron-catalysed decomposition of qinghaosu (artemisinin) and some derivatives: Antimalarial action of some polycyclic endoperoxides. Free Rad Res. 1998;28:471–6. doi: 10.3109/10715769809066884. [DOI] [PubMed] [Google Scholar]
- Zhu DY, Huang BS, Chen ZL, Yin ML, Yang YM, Dai ML, et al. Isolation and identification of the metabolite of artemisinin in human Yao Xue Xue Bao 19834194–7.Chinese. [PubMed] [Google Scholar]
- Lee IS, Hufford CD. Metabolism of antimalarial sesquiterpene lactones. Pharmacol Ther. 1990;48:345–55. doi: 10.1016/0163-7258(90)90053-5. [DOI] [PubMed] [Google Scholar]
- Wang DY, Wu YL, Wu YK, Liang J, Li Y. Further evidence for the participation of primary carbon-centered free-radicals in the antimalarial action of the the qinghaosu (artemisinin) series of compounds. ChemInform. 2001;32:605, 9. [Google Scholar]
- Wang DY, Wu YK, Wu YL, Li Y, Shan F. Synthesis, iron(II)-induced cleavage and in vivo antimalarial efficacy of 10-(2-hydroxy-1-naphthyl)-dexoxqinghaosu (-deoxoartemisinin) J Chem Soc Perkin Trans 1. 1999. pp. 1827–31.
- Wang J, Huang L, Li J, Fan Q, Long Y, Li Y, et al. Artemisinin directly targets malaria mitochondria through its specific mitochondrial activation. PLoS One. 2010;5:e9582. doi: 10.1371/journal.pone.0009582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu YL, Chen HB, Jiang K, Li Y, Shan F, Wang DY, et al. Interaction of biomolecules with qinghaosu (artemisinin) and its derivatives in the presence of ferrous ion -— an exploration of antimalarial mechanism. Pure Appl Chem. 1999;71:1139–42. [Google Scholar]
- Wang DY, Wu YL. A possible antimalarial action mode of qinghaosu (artemisinin) series compounds. Alkylation of reduced glutathione by C-centered primary radicals produced from antimalarial compound qinghaosu and 12-(2,4-dimethoxyphenyl)-12-deoxoqinghaosu. Chem Commun. 2000. pp. 2193–4.
- Wu YK, Yue ZY, Wu YL. Interaction of qinghaosu (artemisinin) with cysteine sulfhydryl mediated by traces of non-heme iron. Angew Chem Int Ed. 1999;38:2580–2. doi: 10.1002/(sici)1521-3773(19990903)38:17<2580::aid-anie2580>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Wu WM, Chen YL, Zhai ZL, Xiao SH, Wu YL. Study on the mechanism of action of artemether against schistosomes — The identification of cysteine adducts of both carbon-centered free-radicals derived from artemether. Bioorg Med Chem Lett. 2003;13:1645–7. doi: 10.1016/s0960-894x(03)00293-2. [DOI] [PubMed] [Google Scholar]
- Chen DJ, Fu LF, Shao PP, Wu FZ, Fan CZ, Shu Y, et al. The experimental studies of artemisinin against Schistosoma japonicum in animals Zhonghua Yi Xue Za Zhi 198060422–5.Chinese. [Google Scholar]
- Le WJ, Wang GF, You JQ, Xie RR, Mei JY.The experimental studies of artemisinin derivatives against Schistosoma japonicum in animals Yao Xue Tong Bao 198015182Chinese. [Google Scholar]
- Xiao SH, Tanner M, N'Goran EK, Utzinger J, Chollet J, Bergquist R, et al. Recent investigations of artemether, a novel agent for the prevention of Schistosomiasis japonica, mansoni and haematobia. Acta Trop. 2002;82:175–81. doi: 10.1016/s0001-706x(02)00009-8. [DOI] [PubMed] [Google Scholar]
- Sun WC, Han JX, Yang WY, Deng DA, Yue XF. Antitumor activities of 4 derivatives of artemisinic acid and artemisinin B, in vitro. Acta Pharmacol Sin. 1992;13:541–3. [PubMed] [Google Scholar]
- Deng DA, Xu CH, Cai JC.Derivatives of arteannuin B with antileukemia activity Yao Xue Xue Bao 199227317–20.Chinese. [PubMed] [Google Scholar]
- Efferth T, Dunstan H, Sauerbrey A, Miyachi H, Chitambar CR. The anti-malarial artesunate is also active ahainst cancer. Int J Oncol. 2001;18:767–73. doi: 10.3892/ijo.18.4.767. [DOI] [PubMed] [Google Scholar]
- Crespo-Ortiz MP, Wei MQ. Antitumor activity of artemisinin and its derivatives: from a well-known antimalarial agent to a potential anticancer drug. J Biomed Biotech. 2012;2012:247597. doi: 10.1155/2012/247597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Shan F, Wu JM, Wu GS, Ding J, Xiao D, et al. Novel antitumor artemisinin derivatives targeting G1 phase of the cell cycle. Bioorg Med Chem Lett. 2001;11:5–8. doi: 10.1016/s0960-894x(00)00578-3. [DOI] [PubMed] [Google Scholar]
- Wu JM, Shan F, Wu GS, Li Y, Ding J, Xiao D, et al. Synthesis and cytotoxicity of artemisinin derivatives containing cyanoarylmethyl group. Eur J Med Chem. 2001;36:240–5. doi: 10.1016/s0223-5234(01)01240-5. [DOI] [PubMed] [Google Scholar]
- Efforth T. Willmar Schwabe Award 2006: Antiplasmodial and antitumor activity of artemisinin from bench to bedside. Planta Med. 2007;73:299–309. doi: 10.1055/s-2007-967138. [DOI] [PubMed] [Google Scholar]
- Liu Y, Lok CN, Ko BCB, Shum TYT, Wong MK, Che CM. Subcellular localization of a fluorescent artemisinin derivative to endoplasmic reticulum. Org Lett. 2010;12:1420–3. doi: 10.1021/ol902890j. [DOI] [PubMed] [Google Scholar]
- Morrissey C, Gallis B, Solazzi JW, Kim BJ, Gulati R, Vakar-Lopez F, et al. Effect of artemisinin derivatives on apoptosis and cell cycle in prostate cancer cells. Anticancer Drug. 2010;21:423–32. doi: 10.1097/CAD.0b013e328336f57b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu JJ, Meng LH, Cai YJ, Chen Q, Tong LJ, Lin LP, et al. Dihydroartemisinin induces apoptosis in HL-60 leukemia cells dependent of iron and p38 mitogen-activated protein kinase activation but independent of reactive oxygen species. Cancer Biol Ther. 2008;7:1017–23. doi: 10.4161/cbt.7.7.6035. [DOI] [PubMed] [Google Scholar]
- Lu JJ, Meng LH, Shankavaram UT, Zhua CH, Tong LJ, Chen G, et al. Dihydroartemisinin accelerates c-MYC oncoprotein degradation and induces apoptosis in c-MYC-overexpressing tumor cells. Biochem Pharmacol. 2010;80:22–30. doi: 10.1016/j.bcp.2010.02.016. [DOI] [PubMed] [Google Scholar]
- Lu JJ, Chen SM, Zhang XW, Ding J, Meng LH. The anti-cancer activity of dihydroartemisinin is associated with induction of iron-dependent endoplasmic reticulum stress in colorectal carcinoma HCT116 cells. Invest New Drugs. 2011;29:1276–83. doi: 10.1007/s10637-010-9481-8. [DOI] [PubMed] [Google Scholar]
- Lu JJ, Chen SM, Ding J, Meng LH. Characterization of dihydroartemisinin-resistant colon carcinoma HCT116/R cell line. Mol Cell Biochem. 2012;360:329–37. doi: 10.1007/s11010-011-1072-2. [DOI] [PubMed] [Google Scholar]
- Yu QB, Gao YX.56 cases of lupus erythematosus treated with artesunate Zhonghua Pi Fu Ke Za Zhi 19973051–2.Chinese. [Google Scholar]
- Yang ZS, Zhou WL, Sui Y, Wang JX, Wu JM, Zhou Y, et al. Synthesis and immunosuppressive activity of new artemisinin derivatives. Part I. [12 (β or α)-Dihydroartemisininoxy] phen(ox)yl aliphatic acids/esters. J Med Chem. 2005;48:4608–17. doi: 10.1021/jm048979c. [DOI] [PubMed] [Google Scholar]
- Yang ZS, Wang JX, Zhou Y, Zuo JP, Li Y. Synthesis and immunosuppressive activity of new artemisinin derivatives. Part 2: 2-[12(β or α)-Dihydro-artemisininoxymethyl- (or 1′-ethyl) phenoxyl propionic acids and esters. Bioorg Med Chem. 2006;14:8043–9. doi: 10.1016/j.bmc.2006.07.038. [DOI] [PubMed] [Google Scholar]
- Zhang JX, Wang JX, Zhang Y, Zuo JP, Wu JM, Sui Y, et al. Synthesis and immunosuppressive activity of new artemisinin derivatives containing polyethylene glycol group Yao Xue Xue Bao 20064165–70.Chinese. [PubMed] [Google Scholar]
- Li Y, Zhu YM, Jiang HJ, Pan JP, Wu GS, Wu JM, et al. Synthesis and antimalarial activity of artemisinin derivatives containing an amino group. J Med Chem. 2000;43:1635–40. doi: 10.1021/jm990552w. [DOI] [PubMed] [Google Scholar]
- Zhou WL, Wu JM, Wu QL, Wang JX, Zhou Y, Zhou R, et al. A novel artemisinin derivative, 3-(12-beta-artemisininoxy) phenoxyl succinic acid (SM735), mediates immunosuppressive effects in vitro and in vivo. Acta Pharmacol Sin. 2005;26:1352–8. doi: 10.1111/j.1745-7254.2005.00232.x. [DOI] [PubMed] [Google Scholar]
- Wang JX, Tang W, Shi LP, Wan J, Zhou R, Ni J, et al. Investigation of the immunosuppressive activity of artemether on T cell activation and proliferation. Br J Pharmacol. 2007;150:652–61. doi: 10.1038/sj.bjp.0707137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Qiu J, Guo TB, Liu A, Wang Y, Li Y, et al. Anti-inflammatory properties and regulatory mechanism of a novel derivative of artemisinin in experimental autoimmune encephalomyelitis. J Immunol. 2007;179:5958–65. doi: 10.4049/jimmunol.179.9.5958. [DOI] [PubMed] [Google Scholar]
- Wang JX, Tang W, Shi LP, Wan J, Zhou R, Ni J, et al. Investigation of the immunosuppressive activity of artemether on T-cell activation and proliferation. Br J Pharmacol. 2007;150:652–61. doi: 10.1038/sj.bjp.0707137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JX, Tang W, Yang ZS, Wan J, Shi LP, Zhang Y, et al. Suppressive effect of a novel water-soluble artemisinin derivative SM 905 on T cell activation and proliferation in vitro and in vivo. Eur J Pharmacol. 2007;564:211–8. doi: 10.1016/j.ejphar.2007.01.092. [DOI] [PubMed] [Google Scholar]
- Wang JX, Tang W, Zhou R, Wan J, Shi LP, Zhang Y, et al. The new water-soluble artemisinin derivative SM 905 ameliorates collagen-induced arthritis by suppression of inflammatory and Th17 responses. Br J Pharmacol. 2008;153:1303–10. doi: 10.1038/bjp.2008.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JX, Hou LF, Yang Y, Tang W, Li Y, Zuo JP. SM 905, a novel artemisinin derivative, inhibition of NO and pro-inflammatory cytokine production by suppressing MAPK and NF-kappa B pathways in RAW 264.7 macrophages. Acta Pharmacol Sin. 2009;30:1428–35. doi: 10.1038/aps.2009.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou LF, He SJ, Wang JX, Yang Y, Zhu FH, Zhou Y, et al. SM 934, a water-soluble derivative of artemisinin, exerts immunosuppressive functions in vitro and in vivo. Int Immunopharmacol. 2009;9:1509–17. doi: 10.1016/j.intimp.2009.09.003. [DOI] [PubMed] [Google Scholar]
- Hou LF, He SJ, Li X, Yang Y, He PL, Zhou Y, et al. Oral administration of artemisinin analog SM934 ameliorates lupus syndromes in MRL/lpr mice by inhibiting Th1 and Th17 cell responses. Arthritis Rheum. 2011;63:2445–55. doi: 10.1002/art.30392. [DOI] [PubMed] [Google Scholar]
- Hou LF, He SJ, Li X, Wan CP, Yang Y, Zhang XH, et al. SM934 treated lupus-prone NZB×NZW F1 mice by enhancing macrophage interleukin-10 production and suppressing pathogenic T cell development. PLoS One. 2012;7:e32424. doi: 10.1371/journal.pone.0032424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Zhu Y, Zhang Y, Zhou JY, Cai L, Xie SW.inventors; Qinghaosu dimer containing nitrogen atom(s), its preparation and use. Shanghai Institute of Materia Medica, Chinese Academy of Sciences and Shanghai Institute of Planned Parenthood Research Chinese Patent201110034154.9.2011. Jan 31.
- Mi JQ, Li Y, Liu JJ, Zhang Y, Cai X, Wang ZY.inventors; Use of a qinghaosu dimer. Ruijin Hospital, Shanghai Jiao Tong University School of Medicine and Shanghai Institute of Materia Medica, Chinese Academy of Sciences Chinese Patent201110034201.X, 2011. Jan 31.
- Mi JQ, Li Y, Ni RM, Zhang Y, Wang J, Cai X, Wang ZY.inventors; Qinghaosu derivative and its salt, a new type of agent for treatment of acute leukemia. Ruijin Hospital, Shanghai Jiao Tong University School of Medicine and Shanghai Institute of Materia Medica, Chinese Academy of Sciences Chinese Patent201210011774.5, 2012. Jan 16.
- Mi JQ, Li Y, Ni RM, Zhang Y, Wang J, Cai X, Wang ZY.inventors; Qinghaosu derivative and its salt, a new type of agent for treatment of leukemia. Ruijin Hospital, Shanghai Jiao Tong University School of Medicine and Shanghai Institute of Materia Medica, Chinese Academy of Sciences Chinese Patent201210012386.9, 2012. Jan 16.
- Mi JQ, Li Y, Peng Y, Zhang Y, Wang J, Cai X, Wang ZY.inventors; Qinghaosu derivative and its salt, a new type of agent for treatment of acute myelocytie leukemia. Ruijin Hospital, Shanghai Jiao Tong University School of Medicine and Shanghai Institute of Materia Medica, Chinese Academy of Sciences Chinese Patent201210012204.8, 2012. Jan 16.