Abstract
Background
The ANP32 family of proteins have been implicated in neuronal function through biochemical and cellular biology studies in neurons, as well as by recent behavioural studies of a gene-trapped loss-of-function mutation of Anp32e in mice, particularly with respect to fine motor function. A second targeted allele of the Anp32e, however, did not appear to demonstrate neurological phenotypes.
Methodology/Principal Findings
Using a stringently controlled cohort of ten-generation backcrossed, co-caged, sex-matched, littermate pairs, we assayed for potential motor defects in the targeted ANP32E-deficient mice. We found no phenotypic difference in any assays.
Conclusion
Since it is unlikely that the gene-trap is a more complete loss-of-function, our results suggest that ANP32E has no appreciable effect on motor functions and that genetic background differences most likely account for the gene-trap phenomena.
Introduction
The acidic (leucine-rich) nuclear phosphoprotein 32 kD (ANP32) family of proteins have been implicated in regulating neuronal function from several lines of inquiry. Firstly, the founding member of the family ANP32A (a.k.a. PHAPI, LANP, I1PP2A, pp32) was found to bind and colocalize with the spinocellular ataxia protein, SCA1 [1]. Secondly, the same protein was shown to affect phosphorylation of the microtubule-associated factor tau, which is abundant in neurons and notable in the pathogenesis of Alzheimer's disease [2], [3]. Finally, a separate family member, ANP32E (a.k.a. CPD1, LANP-L), was originally cloned from postnatal rat cerebellums [4] and is proposed to regulate synaptogenesis and Purkinje cell function [5].
Three of the annotated ANP32 genes, ANP32A, ANP32B and ANP32E, are expressed and are distinguished by the presence of two functional regions; an N-terminal leucine-rich domain and a C-terminal tail comprised predominantly of acidic amino-acid residues [6], [7]. These proteins have been ascribed a surprisingly diverse number of biochemical activities including inhibition of PP2A [8], [9], association with microtubules [3], [10], [11], apoptotic caspase inhibition [12]–[15], regulation of mRNA transport and stability [16]–[18], and control of gene transcription [19]–[24]. Whereas PP2A inhibition is most frequently reported as critical in ANP32-mediated neuronal regulation [2], [5], the regulation of transcriptional activity, potentially through E4F1, has also been suggested to mediate ANP32 neuronal effects [25], [26].
Recently, two loss-of-function mutant alleles of Anp32e were characterized in mice. Mice homozygous for the targeted allele demonstrated no motor deficiency in qualitative analysis or in an accelerating rotorod analysis [27]. The “gene-trapped” allele carrying a “β-Geo” promoter trap insertion in intron 3, on the other hand, was analysed more thoroughly for movement disorders and was likewise normal in accelerating rotorod behaviour but had subtle phenotypes in balance beam and limb-clasp testing [28].
In an attempt to resolve the discrepancy between the results for the two lines of mice, we re-examined the targeted Anp32e allele that has been backcrossed ten generations into the C57BL/6 strain for a panel of movement assessments. Here we present the lack of evidence for any movement defects in the ANP32E-deficient mouse in balance beam performance or limb clasping. Similarly, we did not detect any gait, accelerating rotorod, and grip strength deficiencies in quantitative analyses. Since it is highly unlikely that this targeted allele retains more gene function than the trapped allele, we propose that previous findings are more likely due to genetic background of the mice used for the gene-trapped allele. Importantly, this study finds no detectable motor defects associated with ANP32E deficiency.
Materials and Methods
Mice
Targeted Anp32e heterozygous mice (Anp32e +/−) were backcrossed an addition four generations into C57BL/6 background to give a final 10-generation C57BL/6 congenic. After final backcrossing, Anp32e +/− mice were interbred to give sex-matched littermate pairs of Anp32e nullizygous (Anp32e −/−) and wild-type (Anp32e +/+) mice. Genotyping was performed as previously reported [29]. All littermates were co-caged and provided 5% irradiated food and water ad libitum.
Rotorod test
Mice were placed on the Rotor-rod apparatus (San Diego Instruments) that linearly accelerates from 4 to 40 rpm at a rate of 0.1 rpm/sec. Mice were tested in four trials over four consecutive days, with a 15 min rest period between trials. The latency to fall and distance travelled by a mouse were recorded.
Balance beam test
Beams 50 mm in length, with two different widths, 6 and 12 mm, were used in the balance beam test for motor coordination [30]. A bright light was used as an aversive stimulus at the start platform and an escape box (20×20×20 cm) was placed at the end of the beam. The mice were trained for three trials on each beam for three consecutive days, and then tested on five consecutive days. The time taken to traverse the beam was recorded for each trial. The maximum time cutoff was 20 s. On the test days 1 to 5, the number of slips and grips were also recorded.
Hindlimb clasping test
Mice were held by their tails and recorded for 30 s to measure for limb clasping behavior. Clasping behaviour was scored by an observer blinded to the individual mouse genotype.
Runway test
Mice were allowed to free walk across a runway (Cleversys Inc.) from a white acrylic start box to a darkened escape box. The walls of the runway are 12 cm apart and its length is 100 cm, with a 0.8 cm thick glass floor. Adjustable light panels distribute light uniformly over the entire glass floor to enable the detection of the paws. Low intensity green background light allows for the separation of the body of the mouse from its brightly lit paws. Images are captured by a high-speed digital video camera mounted to record the ventral view of the runway (Basler Cam Inc.).
Treadmill test
The treadmill (Columbus instruments, OH) was used for a forced walking gait analysis. The apparatus consisted of a motor-driven transparent belt with a 17 cm×5 cm compartment over it, where the mouse is placed. A high-speed digital video camera mounted to record the ventral view of the treadmill (Basler Cam Inc.).
Automated gait analysis
Data acquisition and analysis of the gait in the runway and treadmill were done using Gaitscan (Cleversys Inc.). The training and analysis parameters have been described in detail elsewhere [31]. In brief, Gaitscan could reliably identify each paw and the measures taken include the stance, swing and stride times.
Grip Strength test
Grip strength tests were performed using a grip strength meter from Columbus Instruments (Columbus, OH). The forelimb and full body grips of each mouse were measure in three successive trials and recorded. Hindlimb measures were calculated using the difference between the grams-force (gF) recorded for the full body and the forelimb. The results of the three tests were averaged for each mouse.
Statistical analysis
Data were analyzed using R statistical package (R Foundation for Statistical Computing). Data are presented as means±SEM. Rotorod and balance beam data were analyzed using a mixed factorial design ANOVA. Between subjects factor for all tests was genotype, and within subjects factor for the rotorod and balance beam is test days. Measures scored in the hind limb clasping, runway and treadmill tests were analyzed using the two-tailed, paired t test. Bonferroni corrected pair-wise comparisons were used as the post-hoc tests. A p<0.05 was considered as significant.
Ethics Statement
Mice were maintained under protocols 2009/SHS/447 and 2012/SHS/725, which were approved under the legal authority of the Singhealth Institutional Animal Care and Use Committee as per the Singapore N.A.C.L.A.R. guidelines. To alleviate undue suffering, all animals were socially housed in enriched environments with food and water available ad libitum. Animals were sacrificed either by carbon dioxide asphyxiation followed by cervical dislocation or by ketamine/medetomidine overdose followed by exsanguination.
Results
In order to resolve the discrepancy between earlier publications regarding the phenotype of the ANP32E-deficient mouse, we generated seven co-caged, sex-matched, littermate pairs of Anp32e +/+ and Anp32e −/− mice (Figure S1). Consistent with previous results in mixed-bred populations [27], [28], the C57BL/6-congenic Anp32e deficiency segregated at normal Mendelian ratios (Table S1). At ages between 16–20 weeks old, we performed an array of established motor tests.
We first examined whether the results of rotorod analysis from earlier cohorts in both publications consistently showed no effect in these mice. The seven pairs of mice were tested on an accelerating rotorod for four consecutive days, and the latency to fall was recorded. A mixed-design ANOVA revealed significant main effect of day (F3,48 = 24.3, p<0.0001), with no significant main effect of genotype and no significant interaction (Figure S2). Bonferroni corrected pair-wise comparisons for each test day showed no significant differences between genotypes, with both Anp32e +/+ and Anp32e −/− mice showing improvement in performance with each increasing test day.
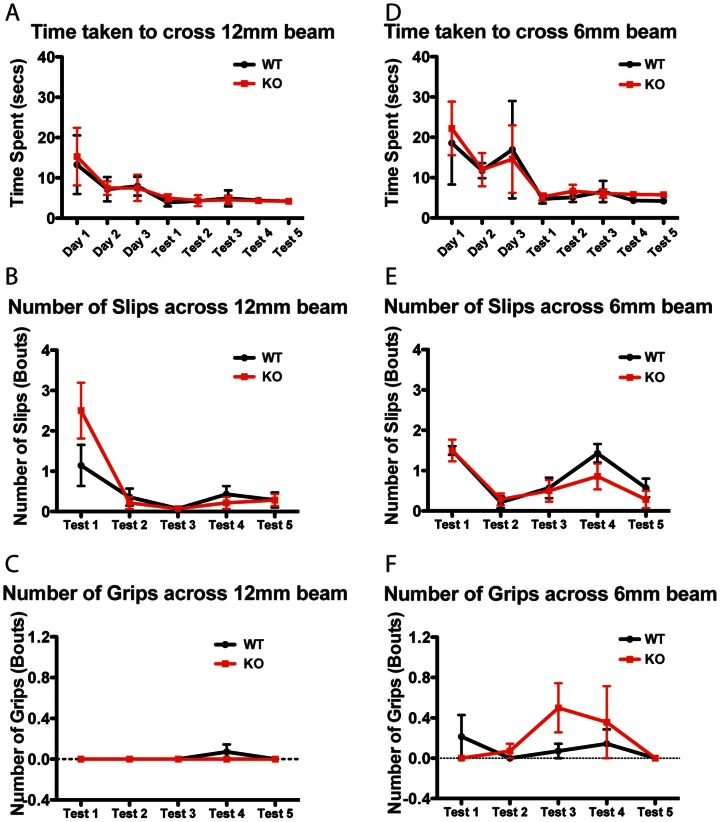
Because the gene-trapped ANP32E-deficient mice have been reported to have poorer performance in balance beam testing, we assessed the motor balance and coordination abilities of the mice using the balance beam test. The time taken to cross were recorded for the 12 mm and 6 mm beams for the 3 training days and 5 test days. The mixed-design ANOVA only showed significant effects of test day for either beam (F12,84 = 21.3, p<0.0001 and F = 19.012,84, p<0.0001 respectively), with no significant main effect of genotype and no significant interaction. For a more detailed analysis, the number of slips and grips were scored on the test days. The number of slips on the 12 or 6 mm beams showed significant main effects of only test day (F = 11.412,48, p<0.0001 and F = 10.912,48, p<0.0001 respectively). There were no significant main effects for the number of grips on the 12 or 6 mm beams. These results suggest that both Anp32e +/+ and Anp32e −/− mice show improvement in the balance beam task across days, taking progressively shorter amounts of time to transverse the beams with fewer slips. Importantly, we saw no significant differences between genotypes (Figure 1).
Figure 1. ANP32E-deficient mice perform normally on balance beam assay.
Mice were assayed, on light aversion balance beam across a 12 mm diameter dowel (panels A–C) and a 6 mm diameter dowel (panels D–F), for crossing time (panel A and D), number of slips (panels B and E) and number of grips (panels C and F). No statistically significant differences were detected between Anp32e −/− (KO, red) and Anp32e +/+ (WT, black).
The gene-trapped ANP32E-deficient mice have also been reported to have limb-clasping defects that are commonly associated with neuronal dysfunction. We, therefore, examined clasping response for the Anp32e +/+ and Anp32e −/− littermates while being suspended from their tails for a total of 30 s. Using a variety of published quantification strategies [28], [32]–[34], we found no statistically significant differences between genotypes using any of the quantification methods. Indeed, a clasping response was only detected in one mutant mouse for a total duration of 1 sec in this first analysis (Table 1). Even using the most liberal clasping quantification measure we could find in the literature [35], where limbs are assessed independently and retraction was scored in the absence of clasping, we found only three mice generated any clasping score (1 point each of a possible 3) and each of those mice were wild-type, not the mutant (Table 1).
Table 1. Limb clasping assay quantification for targeted ANP32E-deficiency.
| Genotype | Kular et al. Scoring [28] (average ± SEM) | Tanaka et al. Scoring [34] (average ± SEM) | Homma et al. Scoring [32] (average clasping time in sec ± SEM) | SHIRPA scoring [33], [36] (binary response) | Guyenet et al. Scoring [35] (average ± SEM) |
| Trial 1 | |||||
| Anp32e +/+ | 0±0 | 0±0 | 0 s±0 s | 0 of 7 | 0.428±0.202 |
| Anp32e −/− | 0.142±0.142 | 0.142±0.142 | 0.142 s±0.142 s | 1 of 7 | 0±0 |
| Trial 2 | |||||
| Anp32e +/+ | 0±0 | 0±0 | 0 s±0 s | 0 of 7 | 0±0 |
| Anp32e −/− | 0±0 | 0±0 | 0 s±0 s | 0 of 7 | 0±0 |
Data shown are post-hoc analysis of hindlimb clasping tests for genotype-paired mice done in two trials on sequential days.
To confirm this result, we performed the assay again on the following day. In this analysis, we found no clasping response in the mice by any standard (Video SV1). Thus, we conclude that there is no apparent limb-clasping defect in the targeted ANP32E-deficient mouse.
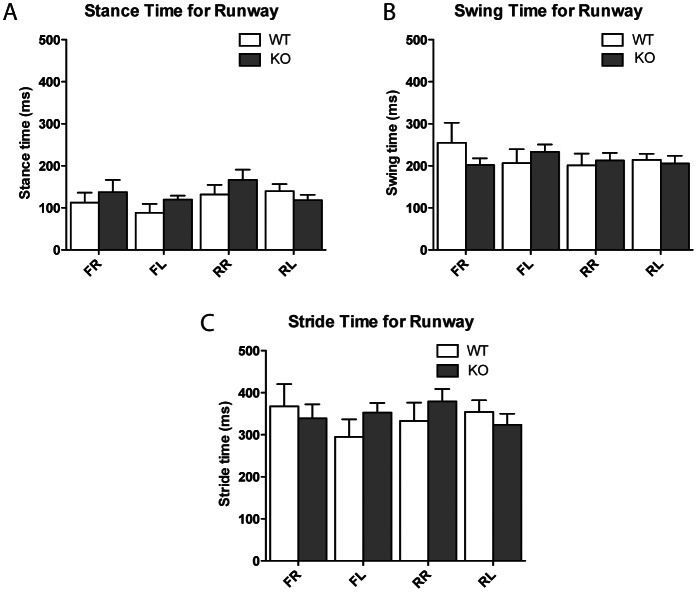
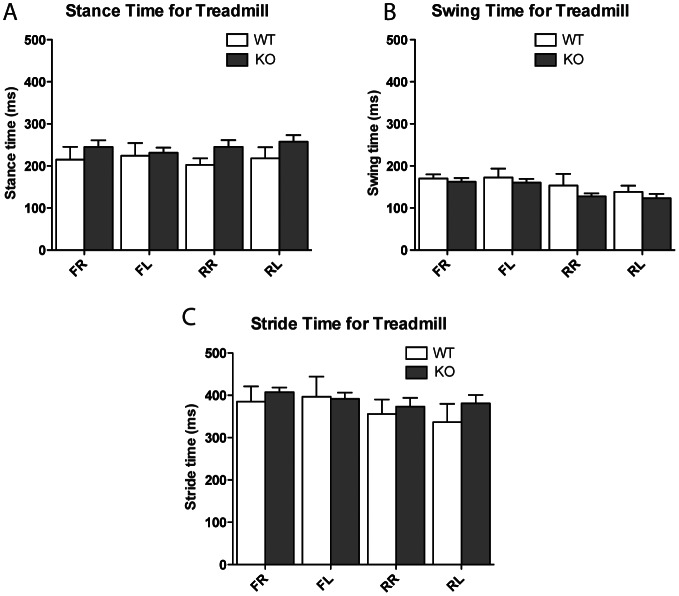
In order to further examine any potential motor defects in the targeted ANP32E-deficient mice, we performed gait analysis on these mice. Gait parameters such as swing, stance and stride time were measured in the runway (free-walk, Figure 2) and treadmill (forced-walk, Figure 3) gait tests. In neither of these gait tests did the gait measures for the front left, front right, rear left and rear right paws show any significant genotype differences, with the exception of the swing time for the rear left foot in the treadmill (p = 0.021). These results suggest that there were no major differences between Anp32e +/+ and Anp32e −/− mice in both the runway and treadmill tests.
Figure 2. No phenotypic difference of ANP32E-deficient mice in free stride gait.
Mouse gait analysis was performed on stationary runway and analysed for stance time (panel A), swing time (panel B) and stride time (panel C). Values for separate legs are presented separately. No statistically significant differences were detected by paired t-test analysis. FR, front right; FL, front left; RR, rear right; RL, rear right.
Figure 3. No phenotypic difference of ANP32E-deficient mice in forced stride gait.
Mouse gait analysis was performed on a treadmill and analysed for stance time (panel A), swing time (panel B) and stride time (panel C). Values for separate legs are presented separately. FR, front right; FL, front left; RR, rear right; RL, rear right.
Finally, in order to assess the neuromuscular function of the forelimbs, hindlimbs and whole body, we performed grip strength testing on these littermate pairs of Anp32e +/+ and Anp32e −/− mice (Figure 4). The maximum force of pull for each test was recorded and normalized to the weight of each mouse. We could detect no statistically significant differences between the genotypes in these three tests.
Figure 4. ANP32E-deficient mice do not show defects in grip strength.
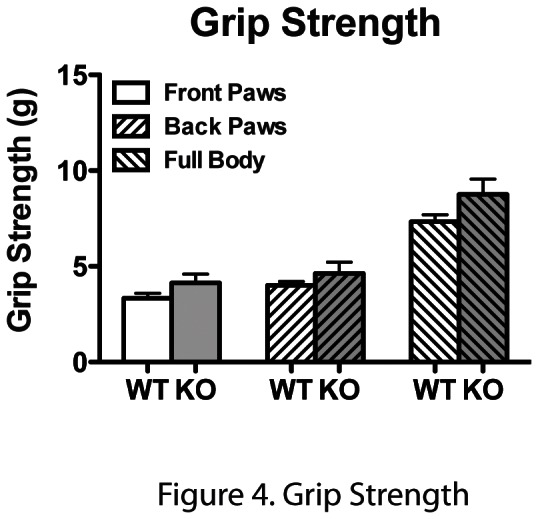
Mice were analysed for grip strength of front paws, hind paws, and total grip strength. No statistically significant differences were noted by paired t-test analysis.
Discussion
ANP32E has been implicated in Purkinje cell function [5] and a previous report suggested subtle neurological defects with respect to motor function in a gene-trapped, Anp32e-deficient mutant [28]. In contrast, our previous limited analysis of motor function in the targeted Anp32e mutant mice did not show any phenotype [27]. To readdress in a more complete and quantitative manner, we performed a panel of motor-function assays on a stringently controlled cohort of mice. We found in no case, including balance beam performance and limb clasping, was a phenotype evident as was described for the gene-trapped Anp32e mutant.
There is little consistency for quantification of limb-clasping phenomena in the literature [28], [32], [34]–[36]. To ensure that we did not miss any phenotype due to scoring, we applied five different scoring strategies, including the same one used for the gene-trapped allele. Even the most liberal scoring strategy, whereby hindlimbs are treated individually and retraction without clasping is scored, did not give any statistically significant difference between the genotypes. Intriguingly, when we applied these scoring systems for the representative mutant video provided as an example in the gene-trapped analysis, we also did not score any limb-clasping positivity by these techniques. Indeed, we perceive the animal in this video attempting escape behaviour by including flexing of their trunks, which is generally not standard limb-clasping behaviour as described in the literature [35], [37].
Different alleles of the same gene can have different phenotypes and gene traps are known to previously give rise to hypomorphic or gain-of-function alleles. In the case of Anp32e however, both alleles purport to be complete loss-of-function alleles. Since gene-trap alleles depend on engineered splicing events to generate loss of function, which may not happen to completion, the targeted allele generated by removal of exons (four of six coding exons for Anp32e), therefore, is more likely to be a complete loss of function. Hence the difference in apparent phenotypes is unlikely to be due to more complete loss-of-function of the gene-trapped allele.
We have performed our experiments on a cohort of co-caged, sex-matched, littermate pairs from parents that were ten-times backcrossed into C57BL/6. We feel that this is critical for assessing subtle phenotypes, particularly where a single ES isolate is used. No mention is made of such refinements in analysis of the gene-trapped allele. In particular, the background differences could explain the differences in results. Significant strain-dependent differences have been noted in the Mouse Phenome Database for both balance beam performance and limb clasping response [34], [37]. The C57BL/6 performed much better than other strains although the 129P2/OlaHsd strain, the background of the gene-trapped allele, was not directly compared. Thus, to conclude that a mutation is responsible for a subtle effect, we propose it is critical to use a minimal of six-generations congenic.
It has been shown that rodent environment is critical for proper experimental control [38]–[40]. Our standard of using co-caged, littermate pairs provides maximum control of environmental factors that can influence behavioural tasks. The unequal numbers of different genotypes analysed for the gene-trap allele suggest that this cocaging of compared animals was likely not performed.
ANP32E is a highly conserved factor in mammals with expression in a broad array of tissues including neurons. In contrast to the conclusions of a previous report, we do not find any neurological phenotypes associated with the complete mutation of this gene. We propose that difference previously reported for the gene-trapped allele are likely due to linked genes on mouse chromosome 3 that will vary significantly in a mixed-bred background.
Supporting Information
PCR-based genotyping of the experimental cohort. Ethidium bromide stained agarose gels of PCR reactions for sex-matched littermate pairs used in this analysis. The upper band of 850 bp detects Anp32e + (WT) allele whereas the lower band detects the Anp32e − (KO) band. +/+, mice ascertained to be Anp32e +/+; −/−, mice ascertained to be Anp32e −/−; +/−, mice ascertained to be Anp32e +/−.
(EPS)
No rotorod defect seen in the targeted ANP32E-deficient mouse. Mice were analysed using the accelerating rotorod to determine latency time to fall (panel A) and total distance travelled (Panel B). No statistically significant differences were detected between Anp32e −/− (KO, red) and Anp32e +/+ (WT, black).
(EPS)
Expected and observed Mendelian ratios of progeny derived from the intercrossing of C57BL/6 backcrossed Anp32e+/− mice. Data shown are the number of mice of a given genotype (percentage of total progeny). Chi square analysis determined that there was no significant difference from expected ratios.
(DOCX)
No difference in limb-clasping behaviour in the mice. Video presents recording of a representative littermate pair of mice second trial for limb-clasping assay. No limb-clasping or retraction is evident in either the Anp32e −/− (first animal shown) or Anp32e +/+ (second animal shown).
(ZIP)
Acknowledgments
We would like to thank Tan Lihua, Cecilia Chang Chin Roei, Teo Weiling, and Arlaine Amoyo-Brion for technical assistance, as well as Dr. Christopher Lee Asplund and Lim Lin Xindi for valuable discussions. We would also recognize the Behavioral Phenotyping Core Facility at Duke-NUS for provision of equipment.
Funding Statement
This work was supported by funding from the Singhealth Research Foundation and the National Cancer Centre Singapore. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Matilla A, Koshy BT, Cummings CJ, Isobe T, Orr HT, et al. (1997) The cerebellar leucine-rich acidic nuclear protein interacts with ataxin-1. Nature 389: 974–978. [DOI] [PubMed] [Google Scholar]
- 2. Chen S, Li B, Grundke-Iqbal I, Iqbal K (2008) I1PP2A affects tau phosphorylation via association with the catalytic subunit of protein phosphatase 2A. J Biol Chem 283: 10513–10521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ulitzur N, Humbert M, Pfeffer SR (1997) Mapmodulin: a possible modulator of the interaction of microtubule-associated proteins with microtubules. Proc Natl Acad Sci U S A 94: 5084–5089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Radrizzani M, Vila-Ortiz G, Cafferata EG, Di Tella MC, Gonzalez-Guerrico A, et al. (2001) Differential expression of CPD1 during postnatal development in the mouse cerebellum. Brain Res 907: 162–174. [DOI] [PubMed] [Google Scholar]
- 5. Costanzo RV, Vila-Ortiz GJ, Perandones C, Carminatti H, Matilla A, et al. (2006) Anp32e/Cpd1 regulates protein phosphatase 2A activity at synapses during synaptogenesis. Eur J Neurosci 23: 309–324. [DOI] [PubMed] [Google Scholar]
- 6. Matilla A, Radrizzani M (2005) The Anp32 family of proteins containing leucine-rich repeats. Cerebellum 4: 7–18. [DOI] [PubMed] [Google Scholar]
- 7. Santa-Coloma TA (2003) Anp32e (Cpd1) and related protein phosphatase 2 inhibitors. Cerebellum 2: 310–320. [DOI] [PubMed] [Google Scholar]
- 8. Li M, Guo H, Damuni Z (1995) Purification and characterization of two potent heat-stable protein inhibitors of protein phosphatase 2A from bovine kidney. Biochemistry 34: 1988–1996. [DOI] [PubMed] [Google Scholar]
- 9. Li M, Makkinje A, Damuni Z (1996) Molecular identification of I1PP2A, a novel potent heat-stable inhibitor protein of protein phosphatase 2A. Biochemistry 35: 6998–7002. [DOI] [PubMed] [Google Scholar]
- 10. Itin C, Ulitzur N, Muhlbauer B, Pfeffer SR (1999) Mapmodulin, cytoplasmic dynein, and microtubules enhance the transport of mannose 6-phosphate receptors from endosomes to the trans-golgi network. Mol Biol Cell 10: 2191–2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Opal P, Garcia JJ, Propst F, Matilla A, Orr HT, et al. (2003) Mapmodulin/leucine-rich acidic nuclear protein binds the light chain of microtubule-associated protein 1B and modulates neuritogenesis. J Biol Chem 278: 34691–34699. [DOI] [PubMed] [Google Scholar]
- 12. Hill MM, Adrain C, Duriez PJ, Creagh EM, Martin SJ (2004) Analysis of the composition, assembly kinetics and activity of native Apaf-1 apoptosomes. EMBO J 23: 2134–2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jiang X, Kim HE, Shu H, Zhao Y, Zhang H, et al. (2003) Distinctive roles of PHAP proteins and prothymosin-alpha in a death regulatory pathway. Science 299: 223–226. [DOI] [PubMed] [Google Scholar]
- 14. Shen SM, Yu Y, Wu YL, Cheng JK, Wang LS, et al. (2010) Downregulation of ANP32B, a novel substrate of caspase-3, enhances caspase-3 activation and apoptosis induction in myeloid leukemic cells. Carcinogenesis 31: 419–426. [DOI] [PubMed] [Google Scholar]
- 15. Sun W, Kimura H, Hattori N, Tanaka S, Matsuyama S, et al. (2006) Proliferation related acidic leucine-rich protein PAL31 functions as a caspase-3 inhibitor. Biochem Biophys Res Commun 342: 817–823. [DOI] [PubMed] [Google Scholar]
- 16. Fries B, Heukeshoven J, Hauber I, Gruttner C, Stocking C, et al. (2007) Analysis of nucleocytoplasmic trafficking of the HuR ligand APRIL and its influence on CD83 expression. J Biol Chem 282: 4504–4515. [DOI] [PubMed] [Google Scholar]
- 17. Gallouzi IE, Brennan CM, Steitz JA (2001) Protein ligands mediate the CRM1-dependent export of HuR in response to heat shock. RNA 7: 1348–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Higashino F, Aoyagi M, Takahashi A, Ishino M, Taoka M, et al. (2005) Adenovirus E4orf6 targets pp32/LANP to control the fate of ARE-containing mRNAs by perturbing the CRM1-dependent mechanism. J Cell Biol 170: 15–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fan Z, Zhang H, Zhang Q (2006) Tumor suppressor pp32 represses cell growth through inhibition of transcription by blocking acetylation and phosphorylation of histone H3 and initiating its proapoptotic activity. Cell Death Differ 13: 1485–1494. [DOI] [PubMed] [Google Scholar]
- 20. Kutney SN, Hong R, Macfarlan T, Chakravarti D (2004) A signaling role of histone-binding proteins and INHAT subunits pp32 and Set/TAF-Ibeta in integrating chromatin hypoacetylation and transcriptional repression. J Biol Chem 279: 30850–30855. [DOI] [PubMed] [Google Scholar]
- 21. Schneider R, Bannister AJ, Weise C, Kouzarides T (2004) Direct binding of INHAT to H3 tails disrupted by modifications. J Biol Chem 279: 23859–23862. [DOI] [PubMed] [Google Scholar]
- 22. Seo SB, Macfarlan T, McNamara P, Hong R, Mukai Y, et al. (2002) Regulation of histone acetylation and transcription by nuclear protein pp32, a subunit of the INHAT complex. J Biol Chem 277: 14005–14010. [DOI] [PubMed] [Google Scholar]
- 23. Seo SB, McNamara P, Heo S, Turner A, Lane WS, et al. (2001) Regulation of histone acetylation and transcription by INHAT, a human cellular complex containing the set oncoprotein. Cell 104: 119–130. [DOI] [PubMed] [Google Scholar]
- 24. Tochio N, Umehara T, Munemasa Y, Suzuki T, Sato S, et al. (2010) Solution structure of histone chaperone ANP32B: interaction with core histones H3–H4 through its acidic concave domain. J Mol Biol 401: 97–114. [DOI] [PubMed] [Google Scholar]
- 25. Cvetanovic M, Kular RK, Opal P (2012) LANP mediates neuritic pathology in Spinocerebellar ataxia type 1. Neurobiol Dis 48: 526–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cvetanovic M, Rooney RJ, Garcia JJ, Toporovskaya N, Zoghbi HY, et al. (2007) The role of LANP and ataxin 1 in E4F-mediated transcriptional repression. EMBO Rep 8: 671–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Reilly PT, Afzal S, Wakeham A, Haight J, You-Ten A, et al. (2010) Generation and characterization of the Anp32e-deficient mouse. PLoS One 5: e13597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kular RK, Gogliotti RG, Opal P (2010) Cpd-1 null mice display a subtle neurological phenotype. PLoS One 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Reilly PT, Afzal S, Gorrini C, Lui K, Bukhman YV, et al. (2011) Acidic nuclear phosphoprotein 32 kDa (ANP32)B-deficient mouse reveals a hierarchy of ANP32 importance in mammalian development. Proc Natl Acad Sci U S A 108: 10243–10248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Luong TN, Carlisle HJ, Southwell A, Patterson PH (2011) Assessment of motor balance and coordination in mice using the balance beam. J Vis Exp [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Beare JE, Morehouse JR, DeVries WH, Enzmann GU, Burke DA, et al. (2009) Gait analysis in normal and spinal contused mice using the TreadScan system. J Neurotrauma 26: 2045–2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Homma D, Sumi-Ichinose C, Tokuoka H, Ikemoto K, Nomura T, et al. (2011) Partial biopterin deficiency disturbs postnatal development of the dopaminergic system in the brain. J Biol Chem 286: 1445–1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Maddatu TP, Grubb SC, Bult CJ, Bogue MA (2012) Mouse Phenome Database (MPD). Nucleic Acids Res 40: D887–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tanaka M, Machida Y, Niu S, Ikeda T, Jana NR, et al. (2004) Trehalose alleviates polyglutamine-mediated pathology in a mouse model of Huntington disease. Nat Med 10: 148–154. [DOI] [PubMed] [Google Scholar]
- 35. Guyenet SJ, Furrer SA, Damian VM, Baughan TD, La Spada AR, et al. (2010) A simple composite phenotype scoring system for evaluating mouse models of cerebellar ataxia. J Vis Exp [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rogers DC, Peters J, Martin JE, Ball S, Nicholson SJ, et al. (2001) SHIRPA, a protocol for behavioral assessment: validation for longitudinal study of neurological dysfunction in mice. Neurosci Lett 306: 89–92. [DOI] [PubMed] [Google Scholar]
- 37. Komatsu M, Waguri S, Chiba T, Murata S, Iwata J, et al. (2006) Loss of autophagy in the central nervous system causes neurodegeneration in mice. Nature 441: 880–884. [DOI] [PubMed] [Google Scholar]
- 38. Cheng L, Wang SH, Jia N, Xie M, Liao XM (2012) Environmental stimulation influence the cognition of developing mice by inducing changes in oxidative and apoptosis status. Brain Dev [DOI] [PubMed] [Google Scholar]
- 39. Crabbe JC, Wahlsten D, Dudek BC (1999) Genetics of mouse behavior: interactions with laboratory environment. Science 284: 1670–1672. [DOI] [PubMed] [Google Scholar]
- 40. Diaz Heijtz R, Wang S, Anuar F, Qian Y, Bjorkholm B, et al. (2011) Normal gut microbiota modulates brain development and behavior. Proc Natl Acad Sci U S A 108: 3047–3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PCR-based genotyping of the experimental cohort. Ethidium bromide stained agarose gels of PCR reactions for sex-matched littermate pairs used in this analysis. The upper band of 850 bp detects Anp32e + (WT) allele whereas the lower band detects the Anp32e − (KO) band. +/+, mice ascertained to be Anp32e +/+; −/−, mice ascertained to be Anp32e −/−; +/−, mice ascertained to be Anp32e +/−.
(EPS)
No rotorod defect seen in the targeted ANP32E-deficient mouse. Mice were analysed using the accelerating rotorod to determine latency time to fall (panel A) and total distance travelled (Panel B). No statistically significant differences were detected between Anp32e −/− (KO, red) and Anp32e +/+ (WT, black).
(EPS)
Expected and observed Mendelian ratios of progeny derived from the intercrossing of C57BL/6 backcrossed Anp32e+/− mice. Data shown are the number of mice of a given genotype (percentage of total progeny). Chi square analysis determined that there was no significant difference from expected ratios.
(DOCX)
No difference in limb-clasping behaviour in the mice. Video presents recording of a representative littermate pair of mice second trial for limb-clasping assay. No limb-clasping or retraction is evident in either the Anp32e −/− (first animal shown) or Anp32e +/+ (second animal shown).
(ZIP)