Abstract
Agonist-stimulated β2-adrenergic receptor (β2AR) ubiquitination is a major factor that governs both lysosomal trafficking and degradation of internalized receptors, but the identity of the E3 ubiquitin ligase regulating this process was unknown. Among the various catalytically inactive E3 ubiquitin ligase mutants that we tested, a dominant negative Nedd4 specifically inhibited isoproterenol-induced ubiquitination and degradation of the β2AR in HEK-293 cells. Moreover, siRNA that down-regulates Nedd4 expression inhibited β2AR ubiquitination and lysosomal degradation, whereas siRNA targeting the closely related E3 ligases Nedd4-2 or AIP4 did not. Interestingly, β2AR as well as β-arrestin2, the endocytic and signaling adaptor for the β2AR, interact robustly with Nedd4 upon agonist stimulation. However, β2AR-Nedd4 interaction is ablated when β-arrestin2 expression is knocked down by siRNA transfection, implicating an essential E3 ubiquitin ligase adaptor role for β-arrestin2 in mediating β2AR ubiquitination. Notably, β-arrestin2 interacts with two different E3 ubiquitin ligases, namely, Mdm2 and Nedd4 to regulate distinct steps in β2AR trafficking. Collectively, our findings indicate that the degradative fate of the β2AR in the lysosomal compartments is dependent upon β-arrestin2-mediated recruitment of Nedd4 to the activated receptor and Nedd4-catalyzed ubiquitination.
The β2-adrenergic receptor (β2AR)3 is a prototypic member of the large and diverse seven-transmembrane receptor (7TMR, aka G protein-coupled receptor) family (1, 2). Canonical 7TMR signaling induced by receptor-G protein coupling is terminated when activated receptors are phosphorylated by G protein-coupled receptor kinases, leading to the recruitment of cytosolic β-arrestins (3). Subsequently, β-arrestins facilitate clathrin-AP-2-dependent internalization of the receptor as well as downstream mitogen-activated protein kinase signaling (3). Over the years, 7TMR trafficking and signaling have been extensively studied, and adaptor proteins in addition to β-arrestins have been shown to regulate internalization and recycling of receptors (4–7).
Continuous stimulation of cell surface receptors results in desensitization or a waning response to persistent stimuli (8, 9). While the short term or immediate desensitization results from receptor phosphorylation, β-arrestin binding, and G protein uncoupling, long term desensitization requires permanent removal of receptors from the cell surface achieved by down-regulating the total number of receptors in the cell. Receptor down-regulation is a two-step process and involves degradation of receptor protein in the lysosomes as well as a decline in receptor mRNA levels (10, 11). Recently, degradation of β2ARs induced by prolonged agonist stimulation was shown to require ubiquitination of the receptors (12). Ubiquitination is the covalent attachment of a 76-amino acid residue-containing protein, ubiquitin, to lysine residues in the substrate protein (13). Ubiquitination is the result of the sequential action of three enzymes (E1, E2, and E3). The final step of ubiquitin transfer is catalyzed by a ubiquitin protein ligase (E3), which normally links the C terminus of ubiquitin to the ε-amino group of a lysyl residue of the substrate protein. The E3 ligases in humans (totaling ∼600) are responsible for substrate specificity and interact directly with their substrates or do so through an ancillary protein that serves as an adaptor (14). Two distinct ubiquitin transfer mechanisms by E3 ligases have been described: one where the enzyme transfers ubiquitin to the substrate, as in the Homologous to E6-AP C Terminus or (HECT) domain ligases, and the other where the enzyme functions to facilitate ubiquitin transfer from E2 to the substrate as in the RING (Really Interesting New Gene) domain ligases (15–17). Regulation and modification of membrane receptors by both HECT and RING types of E3 activities have been reported (18–20).
β2AR ubiquitination occurs within 15 min of isoproterenol stimulation, and the signal decreases after hours of agonist exposure, which correlates with receptor degradation (12). A lysine-less β2AR is not ubiquitinated upon agonist treatment and is not degraded in lysosomes, although it internalizes into early endosomes as efficiently as the wild-type receptor (12). On the other hand, rapid internalization of the β2AR upon isoproterenol stimulation requires Mdm2-dependent ubiquitination of the adaptor β-arrestin2. β2AR ubiquitination and degradation occurs in the absence of Mdm2, although it requires β-arrestin2 expression, suggesting that β-arrestin2 binds an additional E3 ubiquitin ligase to facilitate receptor modification. To understand the molecular mechanisms involved in the regulation of the receptor life cycle, we sought to identify the specific E3 ubiquitin ligase(s) that modifies the β2AR.
EXPERIMENTAL PROCEDURES
Cell Lines, Plasmids, Antibodies—HEK-293 cells were obtained from ATCC and were cultured in MEM supplemented with fetal bovine serum and penicillin and streptomycin. Clonal HEK-293 cells stably expressing the Flag-β2AR or Flag-β2AR-YFP were generated and maintained under G418 selection. Clonal lines with expression levels of 0.8–1.0 and 1.8–2.0 pmol of receptor per mg of total cellular protein were utilized in this study. HA-Nedd4 and Myc-AIP4-DN (21), Mdm2-DN (12) have been reported previously. HA-Nedd4-DN used in this study is Nedd4Cys744Glu and is similar in function to the Nedd4Cys744Ser reported previously (21). Antibodies to human β2AR (H-20), LAMP2 (H4B4), AIP4, ubiquitin (P4D1) were from Santa Cruz Biotechnology. FK2 and FK2-HRP were from Biomol. Mdm2 (2A10) and β-arrestin (A1CT) antibodies were provided by Dr. Arnold Levine and Dr. Lefkowitz. Nedd4 antibodies to WW2 domain were from Millipore. Unless specified otherwise, all other reagents were from Sigma.
siRNA Sequences and Transfection Protocols—Chemically synthesized, double-stranded siRNAs, with 19-nt duplex RNA and 2-nt 3′-dTdT overhangs were purchased from Xeragon (Germantown, MD) in deprotected and desalted form. The siRNA sequences (sense, 5′ to 3′) targeting respective human mRNA are listed: Nedd4: UAGAGCCUGGCUGGGUUGUUU (22); Nedd4-2: AACCACAACACAAAGUCACAG (22); AIP4: GGUGACAAAGAGCCAACAGAG (23); Mdm2: GCCAUUGCUUUUGAAGUUA (24); β-arrestin2: GGACCGCAAAGUGUUUGUG (25); β-arrestin1: AGCCUUCUGCGCGGAGAAU (25); β-arrestin1 and 2: ACCUGCGCCUUCCGCUAUG. The control non-targeting sequence used was: UUCUCCGAACGUGUCACGU.
For experiments in Figs. 3 and 6, early passage HEK-293 cells that were 40–50% confluent on 100-mm dishes were transfected with 20 μg of siRNA, using the GeneSilencer transfection reagent (Genlantis). The cells were assayed 48–60-h post-transfection. For immunostaining experiments (Fig. 4), 70–80% confluent cells were transfected with Lipofectamine 2000 reagent (Invitrogen), and cells were assayed 48–60-h post-transfection.
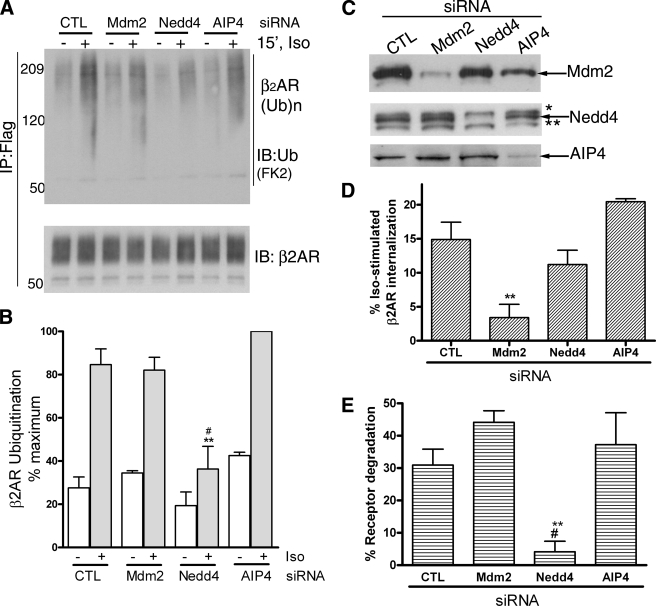
FIGURE 3.
Nedd4 RNAi inhibits agonist-stimulated β2AR ubiquitination and degradation. A, 293β2AR cells were transfected with siRNA that target nothing (CTL), Mdm2, Nedd4, or AIP4. Flag-β2AR IPs were probed with ubiquitin antibody (FK2), shown in the upper panel and reprobed with a β2AR antibody (H-20), displayed in the lower panel to detect ubiquitinated receptor and total receptor in each sample. B, ubiquitin signal in each sample is normalized to the respective receptor amount in the immunoprecipitate. All values are normalized to the maximum ubiquitin signal, which is set as 100%. The graph summarizes data from three independent experiments. **, p < 0.001, Nedd4+ versus CTL+ and Nedd4+ versus AIP4+; #, p < 0.01 Nedd4+ versus Mdm2+; repeated measures ANOVA, Bonferroni comparison. C, 20 μg of whole cell lysates from control, Mdm2, Nedd4, and AIP4 siRNA-treated cells were probed for Mdm2 (2A10 antibody) in the top panel, Nedd4 (anti-WW2 domain, Millipore) in the middle panel, and AIP4 (Santa Cruz Biotechnology) in the lowest panel. The extra cross-reactive bands in the Nedd4 blot are: *, Nedd4-2; **, unknown protein with WW domain. D, 293β2AR cells transfected with siRNA targeting no known protein (CTL), Mdm2, Nedd4, or AIP4 siRNA were stimulated with 1 μm isoproterenol for 30 min, and receptor internalization was determined by radioligand binding as described under “Experimental Procedures.” The graph summarizes results from four independent experiments. **, p < 0.01, Mdm2 versus control and AIP4. E, 293β2AR under control or depleted conditions for either Mdm2, Nedd4, or AIP4 were stimulated with vehicle or 1 μm isoproterenol for 24 h. The receptor levels under stimulated and unstimulated conditions were determined by radioligand binding. The bar graph depicts degraded receptors as a percentage of total receptors in each sample and is the summary of four independent experiments. **, p < 0.01 Nedd4 versus Mdm2; #, p < 0.05 Nedd4 versus Control and AIP4; one-way ANOVA, Bonferroni comparison.
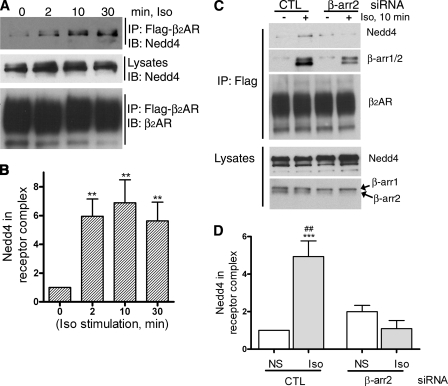
FIGURE 6.
β-Arrestin2 expression regulates Nedd4 binding to activated β2AR. A, HEK-293 cells stably expressing Flag-β2AR (293β2AR) plated on 100-mm Biocoat dishes were serum-starved for 1 h, stimulated with 1 μm isoproterenol for indicated times, subjected to chemical cross-linking (DTME), receptors solubilized in radioimmune precipitation assay buffer, and immunoprecipitated with anti-Flag affinity beads. As shown in the top panel, the immunoprecipitates (IP) were probed with an antibody specific for Nedd4 (WW2 domain, Millipore). The levels of Nedd4 in the lysates are shown in the second panel. The third panel displays the amount of receptors in the IPs as detected by a β2AR antibody (H-20, Santa Cruz Biotechnology). B, Nedd4 bands in receptor IP were quantified and displayed in the bar graph. **, p < 0.01 one-way ANOVA, Bonferroni comparisons. C, 293β2AR cells were transfected with either control or β-arrestin2-specific siRNA with GeneSilencer reagent (Genlantis). 48 h after transfection, cells were replated on Biocoat dishes, and 24 h after this were serum-starved and stimulated with isoproterenol as indicated and subjected to cross-linking as described under “Experimental Procedures.” The top three panels show the Western blotting of IP with Nedd4 antibody (top panel), anti-β-arrestin1/2 antibody (2nd panel), and anti-β2AR antibody (3rd panel). The levels of Nedd4 and β-arrestin in whole cell extracts are also shown in the lysate blots displayed in the lower panels. D, the bar graph represents quantification of Nedd4 in receptor IPs from eight independent experiments. The amount of Nedd4 in the control-unstimulated sample was assigned as 1. ***, p < 0.01 control Iso versus control NS; ##, p < 0.01; control Iso versus β-arr2 Iso.
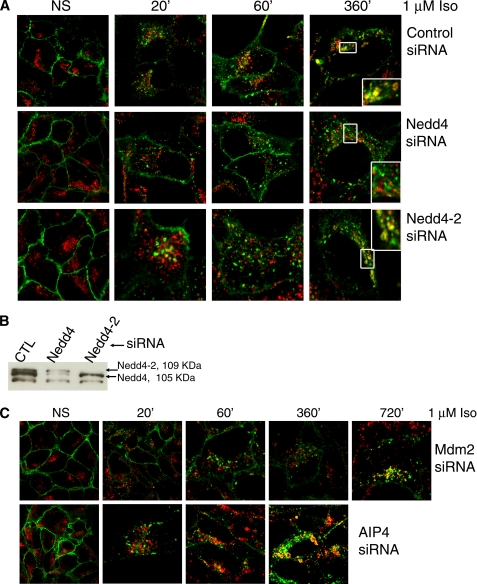
FIGURE 4.
Nedd4 is required for lysosomal targeting of the β2AR. A, 293β2AR cells were transfected with siRNA that target nothing, Nedd4, and Nedd4-2, stimulated for 6 h with 1 μm isoproterenol for the indicated times, fixed, permeabilized, and immunostained for the β2AR (green) and LAMP2 (red). Colocalization is seen in the overlay panels (yellow). The data shown are from one of four independent experiments with identical results. B, whole cell lysates (20 μg) were probed with anti-WW2 domain antibody that recognizes both Nedd4 and Nedd4-2. The predicted molecular weight of human Nedd4 is 104,086 (NP_006145) and Nedd4-2 is 109,891 (NP_056092). C, 293β2AR cells were transfected with siRNA targeting Mdm2 (top row) or AIP4 (bottom row), stimulated with 1 μm isoproterenol for indicated times and analyzed for receptor and LAMP2 distribution by immunostaining as described in A. The confocal panels displayed are representative of four independent experiments with identical results.
Immunoprecipitation and Western Blotting—Cells were solubilized in a lysis buffer (LB) containing 50 mm HEPES (pH 7.5), 0.5% Nonidet P-40, 250 mm NaCl, 2 mm EDTA, 10% (v/v) glycerol, 1 mm sodium orthovanadate, 1 mm sodium fluoride, 1 mm phenylmethylsulfonyl fluoride, leupeptin (5 μg/ml), aprotinin (5 μg/ml), pepstatin A (1 μg/ml), benzaminidine (100 μm). Soluble extracts were mixed with FLAG M2 affinity beads and rotated at 4 °C overnight. Nonspecific binding was eliminated by repeated washes with LB, and bound protein was eluted with sample buffer containing SDS. The proteins were separated on a gradient gel (4–20%, Invitrogen) and transferred to nitrocellulose membrane for Western blotting. For Western analyses with FK2-HRP, blots were blocked in 5% milk, and antibody incubations were carried out in 1% milk followed by routine methods of washes and chemiluminescence. For experiments in Fig. 6C, 10% gels were used to allow separation of endogenous β-arrestin1 and 2 isoforms. Chemiluminescence detection was performed using SuperSignal® West Pico reagent (Pierce). Mdm2 and AIP4 detection at endogenous levels required West Femto reagent (Pierce). Endogenous Nedd4 is detected as the middle band among three distinct bands that react positively against the anti-Nedd4-WW domain antibody (Upstate Biotechnology), when samples isolated with LB are separated on a 4–20% gradient gel (Figs. 3C and 4B). However, if the lysates have been subjected to chemical cross-linking, these bands either coalesce to two bands on 4–20% gels (Figs. 5A and 6A) or are further separated into 3 or 4 bands on 10% gels (Fig. 6C).
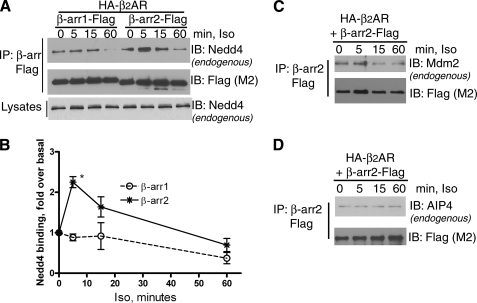
FIGURE 5.
Time course of isoproterenol-stimulated β-arrestin interaction with Nedd4, Mdm2, and AIP4. HEK-293 cells were transiently transfected with HA-β2AR and either β-arrestin1-Flag or β-arrestin2-Flag. After 1 μm isoproterenol stimulation for the indicated times, followed by DTME cross-linking, β-arrestins were immunoprecipitated and further analyzed by Western blots for Nedd4 binding (upper panel), and the amounts of β-arrestin in the immunoprecipitate (middle panel). The lower panel displays lysate levels of Nedd4 in each lane. Under these experimental conditions, we do not observe a clear separation of the three WW-positive proteins (see “Experimental Procedures”). B, Nedd4 bands detected in A were quantified, and the graph represents the mean from three independent experiments. *, p < 0.01 two-way ANOVA. C and D, HA-β2AR and β-arrestin2-Flag were transiently expressed in HEK-293 cells and Flag β-arrestin precipitates were isolated after DTME cross-linking and analyzed for bound Mdm2 in C and AIP4 in D. Data are representative blots from one of three separate experiments.
Immunostaining and Confocal Microscopy—HEK-293 cells with stable β2AR expression, plated on 10-cm dishes were transiently transfected with control, Mdm2, Nedd4, Nedd4-2, or AIP4 siRNA. 36–40-h post-transfection, cells were plated on collagen-coated 35-mm glass bottom plates. 12–15-h later, cells were starved for at least 2 h in serum-free medium prior to stimulation. After stimulation, cells were fixed with 5% formaldehyde diluted in PBS containing calcium and magnesium. Fixed cells were permeabilized with 0.01% Triton in PBS containing 2% BSA for 60 min and then incubated at 4 °C with appropriate primary antibody. The secondary antibody incubations were for 1 h at room temperature followed by repeated washes using PBS. Confocal images were obtained on a Zeiss LSM510 laser scanning microscope using multitrack sequential excitation (488, 568) and emission (515–540 nm GFP; 585–615 nm, Texas red) filter sets.
Ligand Binding—Internalization and degradation assays were done with 125I-(-)iodocyanopindolol (125I-CYP) radioligand binding on monolayers of cells on poly-d-lysine-coated 12-well dishes (Biocoat) in MEM buffered with 10 mm HEPES (pH 7.5) and 5 mm MgCl2. Binding was performed in triplicate with 400 pm 125I-CYP in the presence or absence of the hydrophobic antagonist propranolol (10 μm, to define nonspecific binding) and in the presence or absence of hydrophilic antagonist CGP12177 (0.3 μm, to assess internalized receptors). Incubation for internalization assays was at 4 °C for 3 h. After incubation, the cells were placed on ice and washed several times with PBS buffer containing calcium and magnesium. Cells were solubilized in 0.1 n NaOH and 0.1% SDS and counted for 125I. To obtain the number of internalized receptors, the percentage of total specific 125I-CYP binding sites that could not be displaced by CGP12177 was determined. The isoproterenol-stimulated internalization was determined as the difference between the percentage of total receptors internalized after stimulation and the percentage of receptors internalized in untreated cells. For degradation assays, incubation was at 37 °C for 1 h, and receptor number (total specific 125I-CYP binding sites) was determined after 24 h of isoproterenol treatment and expressed as percent of receptor number assessed in nonstimulated cells.
Cross-linking—HEK-293 cells with stable β2AR transfection (Fig. 6) or those transiently transfected with β-arrestin1-Flag or β-arrestin2-Flag and HA-β2AR (Fig. 5), plated on 10-cm dishes were stimulated at 37 °C in PBS containing 10 mm HEPES (pH 7.5), with vehicle or agonist. Stimulations were terminated by the addition of dithio-bis-maleimidoethane (DTME, Pierce) to a final concentration of 2 mm, and plates were rocked for 40 min at room temperature. Cells were washed three times with PBS/HEPES to remove unreacted DTME, lysed in radioimmune precipitation assay buffer (150 mm NaCl, 50 mm Tris, pH 8.0, 5 mm EDTA, 1% Nonidet P-40, 0.5% deoxycholate), and receptors were immunoprecipitated.
Quantification and Statistical Analyses—Protein bands were quantified by densitometry and analyzed with Genetools software (SynGene). Plotting and statistical analyses (Student's t tests or ANOVA) were done using GraphPad Prism software.
RESULTS
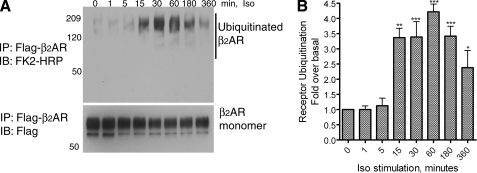
A 15-min isoproterenol treatment of HEK-293 cells with stable β2AR expression (∼2 pmol per mg of total cellular protein) leads to detectable receptor ubiquitination in receptor immunoprecipitates (Fig. 1, A and B). In general, we see a 2.5–3.5-fold increase in receptor ubiquitination at 15 min of agonist stimulation, the signal persists with prolonged agonist activation and gradually decreases after 6 h of agonist, mostly because of the degradation of ubiquitinated receptors.
FIGURE 1.
Time course of isoproterenol-stimulated β2AR ubiquitination in HEK-293 cells. A, HEK-293 cells stably expressing Flag-β2AR (293β2AR) or Flag-β2AR-mYFP were stimulated with 1 μm isoproterenol for the indicated times, and receptors were isolated with anti-Flag-agarose affinity beads. Receptor ubiquitination was detected with a ubiquitin antibody conjugated to peroxidase (FK2-HRP). The lower panel shows the amounts of receptor as detected by a Flag antibody (M2). B, ubiquitin smears in each lane from the blot shown in A were quantified and plotted as bars. The graph shown represents a summary of four independent experiments. **, p < 0.01; ***, p < 0.001 compared with non-stimulated samples; repeated measures ANOVA, Bonferroni post-test.
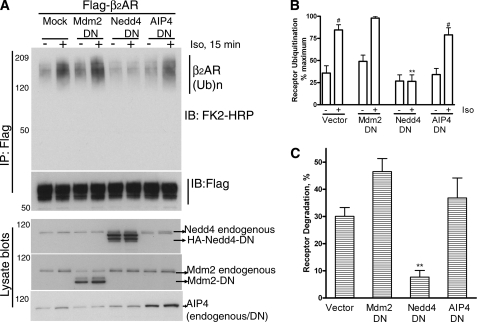
Previous studies have indicated that both HECT domain ligases such as AIP4 and RING domain ligases such as c-Cbl ubiquitinate specific GPCRs and regulate their post-endocytic sorting and degradation (23, 26). When we examined the effect of DN or catalytically inactive mutants of c-Cbl, Mdm2, (examples of RING), βTrCP (example of multisubunit RING), Nedd4, and AIP4 (examples of HECT) on isoproterenol-stimulated receptor ubiquitination at 15 min, only Nedd4-DN (Nedd4Cys744Glu) showed a dramatic inhibitory effect (Fig. 2, A and B and data not shown). We next compared the effect of dominant negative forms of Mdm2, Nedd4, and AIP4 ligases on β2AR degradation as measured after 24-h isoproterenol treatment. Receptor degradation determined by 125I-cyanopindolol binding was 30–35%, which increased to about 50 or 45% when Mdm2-DN or AIP4-DN were expressed but was dramatically decreased upon coexpression of Nedd4-DN (Fig. 2C and supplemental Table S1).
FIGURE 2.
A dominant negative Nedd4 inhibits isoproterenol-stimulated β2AR ubiquitination and degradation. A, 293β2AR were transfected with vector or catalytically inactive Nedd4, Mdm2, or AIP4, and receptors were immunoprecipitated under unstimulated or agonist-stimulated conditions and probed for ubiquitination as in Fig. 1A. Receptor amounts in each sample were determined by reprobing the blot with a M2 Flag antibody (second panel from top). The expression levels of Nedd4-DN, Mdm2-DN, and AIP4-DN are shown in the respective lysate blots. In each case, the band corresponding to endogenous Nedd4, Mdm2, and AIP4 is also indicated. B, quantification of ubiquitination signals from three independent experiments is plotted as a bar graph, where maximum signal is set as 100%. #, p < 0.001 compared with the respective nonstimulated samples; **, <0.01, Nedd4 stimulated versus all other stimulated samples, ANOVA, Bonferroni post-test. C, 293β2AR were transfected with vector, Nedd4-DN, Mdm2-DN, or AIP4-DN and stimulated with isoproterenol for 24 h or not. The receptor levels were determined by 125I-CYP binding. The percent decrease in receptor amounts compared with levels under no stimulation was calculated and plotted as a bar graph, which summarizes data from 3–5 experiments. Data were analyzed by one-way ANOVA. **, p < 0.01.
Next, we tested the effects of down-regulating endogenous levels of Nedd4, Mdm2, or AIP4 on β2AR ubiquitination as measured in HEK-293 cells (Fig. 3, A and B). AIP4 knockdown led to an increase in basal receptor ubiquitination than control transfection, whereas Nedd4 knockdown reduced the basal signals by at least 30%. Additionally, the ratio of ubiquitin to β2AR correlating with isoproterenol stimulation is dramatically reduced in Nedd4 knockdown cells. As seen in Fig. 3B, the percentage of total ubiquitinated β2ARs in the stimulated samples is significantly lower in cells with Nedd4 knockdown than control (p < 0.001), Mdm2 (p < 0.01), or AIP4 (p < 0.001) knockdown cells. On the other hand, the persistence of an absolute amount of “fold over basal” β2AR ubiquitination in the Nedd4-depleted samples (∼1.9 in Nedd4-depleted cells versus ∼3.3 in control cells), suggests that a small amount of residual enzymatic activity is prevalent despite the siRNA knockdown. As displayed in Fig. 3C, Western blot analyses of the lysates indicates >90% knockdown of Mdm2 and >95% decline in Nedd4 and AIP4 by the respective siRNA treatments. While Mdm2 siRNA specifically affected receptor internalization measured after a 30-min agonist treatment, it did not alter receptor degradation measured after chronic isoproterenol stimulation (Fig. 3, D and E). In contrast, Nedd4 siRNA, had no significant effect on receptor internalization but had a drastic inhibitory effect on receptor degradation (Fig. 3, D and E). In these assays, siRNA targeting the closely related HECT family member, AIP4 had no effect on either internalization or receptor degradation (Fig. 3, D and E). These data strongly suggest that Nedd4 expression is critical for both receptor ubiquitination and subsequent degradation in the lysosomal compartments.
To assess the intracellular trafficking and lysosomal sorting of agonist-activated β2ARs, we analyzed receptor subcellular localization in response to isoproterenol stimulation by immunostaining the receptor with an antibody that recognizes the receptor C terminus (β2AR-H20) followed by confocal microscopy. We immunostained LAMP2 (27), a protein marker of the lysosomes and late endosomes to facilitate the visualization of receptor trafficking to these compartments. As displayed in the control (top panels in Fig. 4A), β2AR is evenly distributed across the plasma membrane with no receptor staining within internal compartments under unstimulated conditions. Within 20 min of agonist treatment, a significant number of receptors are internalized into endocytic vesicles and remain in such compartments until about 6 h after isoproterenol stimulation, at which time a robust colocalization of receptor and LAMP2 is detectable. Congruently, less than 5% receptor degradation occurs at 6 h while >30% at 24 h of isoproterenol stimulation according to 125I-CYP radioligand binding (Fig. 3 and data not shown). The reason for this prolonged lag prior to receptor degradation after the initial entry of receptors into late endosomal and/or lysosomal compartments at 6 h of agonist treatment is unknown. Receptors are detected in LAMP2-positive compartments until about 16–18 h of agonist stimulation (supplemental Fig. S1) beyond which the signal for receptor in these compartments deteriorates due to degradation. As shown in supplemental Fig. S1 (right panels), at these late time points of agonist treatment, receptor-LAMP2 colocalization is more pronounced when cells are also incubated with leupeptin, which inhibits lysosomal proteases and prevents receptor degradation.
In contrast to control conditions, β2AR localization in LAMP2-positive compartments was not observed at 6 h or later when Nedd4 levels were knocked down by siRNA (Fig. 4). At 6 h of isoproterenol treatment, 80–90% of the cells contained receptor in endocytic vesicles, and 10–20% of the cells showed receptors in endosomes as well as at the plasma membrane. This is in marked contrast to what is seen under control conditions where >80% of cells showed receptors localized in the lysosomes. Interestingly, siRNA to Nedd4-2, a Nedd4 homolog expressed in humans, did not inhibit lysosomal trafficking of the β2AR (Fig. 4A, lowest row of panels). As shown in the lysate blots in Fig. 4B, both Nedd4 and Nedd4-2 were specifically and efficiently knocked down as detected by an antibody against the WW domains common to both proteins. Mdm2 siRNA delayed internalization (compare panel 20′ of different siRNA treatments in Fig. 4, A and C). Moreover, only a very few cells showed even a small number of vesicles containing the β2AR. However longer incubation with isoproterenol lead to detectable receptor internalization (see panel 60′, Fig. 4C) and lysosomal localization was detectable at 12 h of agonist activation (Fig. 4C, 1st row). Like Nedd4-2 siRNA, AIP4 siRNA did not inhibit lysosomal trafficking of the β2AR (Fig. 4C, 2nd row). These data strongly suggest that Nedd4-mediated ubiquitination is essential for the lysosomal targeting and degradation of activated β2AR.
β2AR ubiquitination is not detected in β-arrestin2-null mouse embryonic fibroblasts (MEFs) and could be recovered by repletion of β-arrestin2 in β-arrestin1/2-null MEFs, suggesting that the E3 ligase modifying the β2AR would be a β-arrestin2 binding partner (12). To assess whether β-arrestin2 does play a role in Nedd4-dependent receptor modification, we analyzed if β-arrestin2 and Nedd4 could interact and if Nedd4-β2AR interaction is bridged by β-arrestin2. When we tested the binding of endogenous Nedd4 to both β-arrestin1 and 2 in HEK-293 cells, we observed a 2–2.5-fold increase in Nedd4-β-arrestin2 interaction upon isoproterenol stimulation (Fig. 5, A and B). In contrast, β-arrestin1-binding to Nedd4 was not augmented by β2AR stimulation (Fig. 5, A and B). β-Arrestin2-Nedd4 interaction was above basal levels at 15 min of agonist, but decreased to much lower levels at 1 h of isoproterenol (Fig. 5, A and B). Additionally, in COS-7 cells a modest binding of HA-Nedd4 and β-arrestin2-Flag is seen under unstimulated conditions, but significant increase in binding is observed within 2 and 10 min of agonist treatment (supplemental Fig. S2, A and B). After this, the binding remains level until 20 min, following which it decreases to basal levels (data not shown). β-Arrestin2 displays dynamic interactions with multiple partners (28), and hence we also tested the time course of Mdm2 and AIP4 interaction with β-arrestin2. An agonist-dependent increase in β-arrestin2-Mdm2 interaction was observed at 5 min, but the two proteins dissociated upon longer periods of agonist treatment (Fig. 5C). In contrast, β-arrestin-AIP4 interaction was unaffected by β2AR stimulation (Fig. 5D). These data suggest that β2AR activation regulates the temporal nature of β-arrestin2 interaction with two distinct E3 ligases, namely, Mdm2 and Nedd4. The above binding data also indicate that β-arrestin2-Nedd4-β2AR complexes can be formed upon agonist-induced β-arrestin2 translocation, and β-arrestin2 can play a predominant role in regulating Nedd4 recruitment to activated β2ARs.
In general, Nedd4 interaction involves the binding of one or more of its WW domains to a consensus PPXY motif in its substrate proteins such as the epithelial sodium channel (ENaC) (29). Other predicted motifs for WW domain interactions include PPLP, PGM, and P(S/T)P (30). Although β-arrestin2 lacks such motifs, the sequences PPMP (amino acids 89–92) and PPRP (amino acids 94–98) within its N-domain {N and C domains of β-arrestin are indicated in Ref. 31} could serve as putative binding sites (supplemental Fig. S3A). However, in our coimmunoprecipitation assays, β-arrestin185–410 that lacks the N-domain bound HA-Nedd4 better than both the WT and β-arrestin21–185 lacking the C-domain (supplemental Fig. S3B). Additionally, we found that hNedd4 WT and hNedd4 WW domain mutants displayed similar binding to β-arrestin2-Flag (supplemental Fig. S4). Because mutations in WW domains of Nedd4 or deletions of polyproline regions in β-arrestin2 did not affect the protein-protein interaction, we believe that β-arrestin2-Nedd4 interaction is governed by yet unknown mechanisms, regulated by the conformational changes induced in β-arrestin2 upon binding the activated receptor (32, 33).
Next, we tested if Nedd4 recruitment to the β2AR complex is dependent on β-arrestin2 levels. We determined the association of endogenous Nedd4 with Flag-β2AR by coimmunoprecipitation assays (Fig. 6A). In these assays, little to no Nedd4 binding to the β2ARs was observed prior to agonist treatment. Upon isoproterenol stimulation, Nedd4 levels increased 6 ± 1.2-fold at 2 min and 8 ± 2-fold at 10 min of agonist stimulation (Fig. 6B). As shown in Fig. 6C, when β-arrestin2 levels were decreased by siRNA, endogenous Nedd4 was not recruited to the β2AR, with agonist, even though a robust agonist-dependent Nedd4 binding was observed with control siRNA treatment (Fig. 6, C and D). In these assays, we routinely observed >95% decrease in β-arrestin2 in whole cell lysates after RNAi (lysates blot, Fig. 6C), while β-arrestin protein in receptor immunoprecipitates was decreased by 80–85% (2nd panel from top, Fig. 6C). Notably, the levels of other human arrestin isoform, namely β-arrestin 1, are unaltered, but still it does not substitute for the Nedd4 recruiting role of β-arrestin2. Moreover β-arrestin1 siRNA did not result in any significant change in the levels of Nedd4 in receptor complexes, whereas knockdown of both β-arrestin1 and 2 resulted in similar effects as β-arrestin2 siRNA alone (data not shown). These findings support a model where β-arrestin2 functions as an E3 ubiquitin ligase adaptor to facilitate Nedd4-mediated β2AR ubiquitination. Additionally, these studies along with our previous findings indicate that two different E3 ligase activities, namely Mdm2 and Nedd4, function sequentially at distinct steps to regulate the trafficking of activated β2ARs.
DISCUSSION
Degradation of the β2AR upon chronic agonist stimulation occurs in the lysosomal compartments and requires post-translational ubiquitination of the receptor protein. We now demonstrate that this process is uniquely regulated by Nedd4-mediated ubiquitination and requires β-arrestin2-Nedd4 interaction. β2AR ubiquitination and postendocytic sorting is specifically mediated by Nedd4, because Nedd4-2, the second human isoform as well as closely related AIP4 are not involved in this regulation. Our studies also indicate that two distinct E3 ligases are involved in β2AR trafficking, Mdm2, which at a very early step ubiquitinates β-arrestin2 to promote its interaction with endocytic and signaling proteins (12, 34) and Nedd4 at a subsequent step to tag receptors for the degradative pathway.
Ubiquitination was originally discovered as a post-translational modification in the context of nonlysosomal protein degradation as carried out by cellular 26 S proteasomes (35). In the past decade, ubiquitin has been appreciated for its proteasome-independent functions in endocytosis and signaling (36). The endocytic role of ubiquitination was initially demonstrated for membrane transporters and pheromone receptors in yeast (37–39). Inhibition of ubiquitination of the yeast GPCR Ste2 prevents receptor internalization (38). On the other hand, mutation of ubiquitin-acceptor lysines and ablation of receptor ubiquitination of several mammalian GPCRs, such as the β2AR, vasopressin V2 receptor, chemokine receptor CXCR4, protease-activated receptor2, Neurokinin receptor, angiotensin II type1a receptor as well as the follitropin receptor does not affect receptor internalization into early endosomes (12, 26, 40–44). In contrast, several 7TMRs require ubiquitination for lysosomal trafficking and degradation (18). All these lines of investigations strongly suggest that receptor ubiquitination plays a major role in post-endocytic sorting steps. Alternate functions of ubiquitination as an inhibitory signal for constitutive (agonist-independent) internalization and ubiquitin-independent degradation of receptors have also been reported (45, 46). GPCR ubiquitination has also been demonstrated to be involved in post-synthesis ER quality control to degrade misfolded receptors (47).
β2AR internalization requires expression of β-arrestin2 (48) and moreover Mdm2-dependent β-arrestin2 ubiquitination promotes rapid clearance of cell surface receptors mostly by augmenting the rate of receptor internalization (12). Unlike β2AR ubiquitination, β-arrestin ubiquitination occurs in a transient manner, and the rate of β-arrestin2 deubiquitination regulates the formation of β-arrestin-receptor signaling complexes (signalosomes) on endocytic vesicles (34, 49). Both Mdm2-DN expression (Fig. 2) and Mdm2 knockdown (Fig. 3) do not affect β2AR ubiquitination, despite their potential inhibitory effect on β-arrestin ubiquitination. On the other hand, as demonstrated previously, the absence of β-arrestin ubiquitination does not prevent either β-arrestin recruitment or β-arrestin-receptor interaction although it renders receptor-β-arrestin complexes labile (34). Hence, even in the absence of its ubiquitination, β-arrestin can still translocate to cell surface receptors, recruit Nedd4, and mediate β2AR ubiquitination. Thus, in cells transfected with Mdm2 siRNA, receptor internalization would proceed at a much slower rate with little effect on the slower process of receptor degradation. Notably, in several experiments, transfection of Mdm2-DN led to a reduction in the levels of endogenous Mdm2 in HEK-293 cells (Fig. 2A, lysate blot). Mdm2-DN used in these experiments lacks the C-terminal RING domain, but carries an intact p53 binding domain at the N terminus. Hence, Mdm2-DN can sequester p53 protein and prevent its transcriptional activity, which in turn could result in impaired endogenous Mdm2 gene expression.
CXCR4 ubiquitination and lysosomal degradation was recently shown to be mediated by the HECT domain E3 ligase AIP4, while c-Cbl, a RING domain E3 ligase ubiquitinates PAR2 (23, 26). AIP4, Nedd4-2, c-Cbl, and Mdm2 are not involved in β2AR ubiquitination, but more surprisingly, a specific isoform, Nedd4 ubiquitinates the β2AR and regulates the lysosomal trafficking of the receptor. Nedd4 and especially Nedd4-2 are well known for their role in ubiquitinating several ion channels, mainly, the amiloride-sensitive ENaC (50). These HECT domain-containing ligases are also known to ubiquitinate membrane as well as non-membrane substrates mostly via a direct interaction with the substrate (29, 51, 52). The yeast Nedd4 homolog, Rsp5p plays a key role by modifying several membrane proteins and transporters. While a role for an adaptor protein is speculated in many cases, none has been demonstrated. In the case of the β2AR, β-arrestin2 functions as an essential adaptor and recruits Nedd4 to the receptor complex, as shown by our results demonstrating that β-arrestin2 expression is critical for Nedd4 detection in isolated receptor immunoprecipitates (Fig. 6). Recently, β-arrestin1-AIP4 binding was reported to occur via AIP4 WW domains, but this interaction was not affected by alterations of polyproline motifs in β-arrestin1 (53). On the other hand, our results (supplemental Fig. S4) suggest that β-arrestin2-Nedd4 interaction may not involve WW domain interactions. We believe that Nedd4 recruitment to the β2AR by β-arrestin2 is contingent upon specific conformational changes in β-arrestin2 when held by the activated β2AR. Future availability of high-resolution structure(s) of an active form of β-arrestin2 will help to define regions of β-arrestin, which are involved in partner interactions and will also facilitate a better characterization of β-arrestin2-Nedd4 binding.
Interestingly, Nedd4 contains a C2 domain, allowing its association with either the plasma membrane or vesicular membrane compartments. It is possible that β-arrestin translocation to the plasma membrane could lead to its interaction with Nedd4 at the membrane and activate Nedd4 to ubiquitinate β-arrestin-bound receptor. On the other hand, it is also possible that β-arrestin2 escorts other components necessary for the process such as an E2 enzyme to facilitate ubiquitin transfer via the Nedd4 HECT domain. Regardless, Nedd4-dependent β2AR ubiquitination is effective only if β-arrestin2 is present as an adaptor. Thus, β-arrestin2 binds at least two E3 ubiquitin ligases, Mdm2 and Nedd4, serving different purposes in β2AR regulation: Mdm2, which mediates β-arrestin ubiquitination (12) and regulates the initial step of receptor endocytosis, and Nedd4, which mediates receptor ubiquitination that targets receptors to lysosomal compartments. Existing studies indicate that Mdm2-β-arrestin interaction occurs efficiently before the activation of β-arrestin by receptor stimulation (54). Therefore, it is possible that Nedd4 displaces or inactivates Mdm2 (see Fig. 7) when it binds β-arrestin-receptor complexes. Nonetheless, our data suggest that Mdm2 and Nedd4 function in a sequential manner at distinct steps in the endocytic pathway to regulate the trafficking and signaling of the β2AR. Additionally, Mdm2 recruited by β-arrestins can subserve other functions as demonstrated by the roles in regulating G protein-coupled receptor kinase 2 (GRK2) and insulin-like growth factor-1 receptor turnover (55, 56).
FIGURE 7.
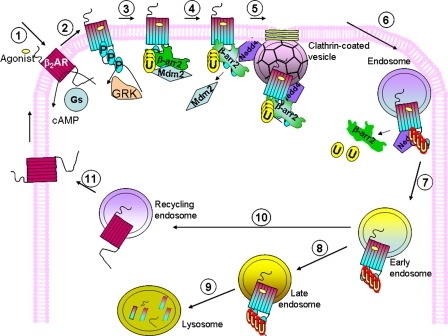
Roles of ubiquitination in the life cycle of agonist-stimulated β2AR. Step 1, within seconds of agonist exposure, β2ARs stimulate Gs and adenylyl cyclase, increasing cellular cAMP. Step 2, agonist-occupied receptors are also phosphorylated by GRKs on cytoplasmic domain seryl and/or threonyl residues, within seconds to minutes of agonist exposure. Step 3, cytosolic β-arrestin2 (βarr2) translocates to phosphorylated receptors within 1–5 min after agonist treatment. Agonist-dependent β-arrestin ubiquitination (U) occurs immediately upon β-arrestin recruitment and is mediated by Mdm2 that is bound to β-arrestin. β-Arrestin recruitment prevents further G protein coupling and β-arrestin ubiquitination allows it to form signaling and endocytic complexes, facilitating both receptor endocytosis and MAPK signaling. Step 4, β-arrestin conformational changes that occur upon receptor binding allow its interaction with Nedd4, which probably displaces Mdm2 from β-arrestin (5–15 min after agonist treatment). Step 5, by interacting simultaneously with β2AR, clathrin, and AP-2, β-arrestin2 facilitates β2AR endocytosis. Step 6, β-arrestin2 is deubiquitinated by an as-yet unidentified process, leading to its disengagement from the receptor complex (10–15 min after agonist treatment). Nedd4 mediates ubiquitination of the endosomally located β2AR. Step 7, ubiquitinated β2ARs move on into early endosomes (at >15 min after activation). Step 8, β2AR ubiquitination persists until about 6 h after agonist stimulation, when β2ARs move into late endosomal/lysosomal compartments. Step 9, level of ubiquitinated β2ARs decreases, as ubiquitinated receptors are degraded in lysosomes (6–24 h or more after agonist stimulation). Step 10, from the early endosomes, receptors may take up an alternate path and enter recycling endosomes (<15–30 min after activation), in which β2ARs become dephosphorylated and perhaps deubiquitinated, and return to the plasma membrane as “naïve receptors” (Step 11).
Based on our findings and previous studies on 7TMR trafficking by various groups (reviewed in Refs. 4, 6, 57), we propose a model in Fig. 7 to illustrate some of the rapid and slow events that occur upon β2AR activation. β2AR-mediated Gs activation and cAMP generation occur within milliseconds/seconds of receptor stimulation (58, 59). β-Arrestin recruitment to receptors phosphorylated by GRKs is detectable within a minute after agonist treatment (59, 60). Based on our studies, β-arrestin-Mdm2 complexes exist due to a constitutive interaction, and such complexes are recruited to the activated receptors, thus suggesting that Mdm2 is also recruited to the receptor rapidly (Fig. 5 and Refs. 12, 61). Although ubiquitination of β-arrestin could occur as it is being recruited, it is more likely that receptor-bound β-arrestin is the substrate that is actually ubiquitinated because β-arrestin conformational change and activation could induce its ubiquitination by exposing critical lysine residues. Such β-arrestin ubiquitination facilitates its robust binding to endocytic and signaling proteins, and initiates these processes (34). We also have reason to believe that beyond 10–15 min of agonist activation, β-arrestins are deubiquitinated (12, 62, 63) by a yet unidentified process and dissociate from the β2ARs that are now localized in endocytic vesicles. Before its dissociation, β-arrestin recruits Nedd4 activity to facilitate β2AR ubiquitination. Based on the observed rapid agonist-promoted increase in β-arrestin2-Nedd4 interaction, it appears that activated receptors that are mobilized into clathrin-coated pits within 5–10 min of agonist treatment are actually targeted for ubiquitination. Although β-arrestin-Nedd4 interaction occurs within 5 min of β2AR activation, the net effect of receptor ubiquitination is detected only beyond 15 min. Future development of real-time assays utilizing Förster resonance energy transfer (BRET or FRET) should reveal if receptor ubiquitination occurs earlier than 15 min. On the other hand, the inability to demonstrate receptor ubiquitination before 15 min could also be from the activity of deubiquitinating enzymes. Nonetheless, receptor ubiquitination is detectable as early as 15 min after agonist treatment, suggesting that it is an early event occurring at or near the membrane (Figs. 1, 2, 3, and Ref. 12).
Intriguingly, receptor ubiquitination appears to increase after 15 min with a peak signal detected at 1 h after agonist treatment. One could speculate this to be from ubiquitination of internalized receptors, or from additional rounds of ubiquitination of recycled receptors. Alternatively, this could result from recurring modifications of internalized receptors by Nedd4 at the endosomes. In this latter case, β-arrestin may mainly serve to recruit Nedd4 to the receptor at the plasma membrane, and Nedd4 could ubiquitinate the receptor after β-arrestin dissociates from the receptor complex. Although our data strongly support the role of Nedd4 in β2AR ubiquitination, regulation by additional or alternative E3 ligases in specific cell types or physiological settings cannot be ruled out.
While receptor ubiquitination can occur within minutes after agonist activation, the downstream effect of this modification on receptor sorting is obvious only after prolonged agonist stimulation. It is also likely that deubiquitination of receptors and adaptors such as β-arrestins dynamically regulate the trafficking itineraries of receptor signalosomes. Indeed, β2AR0K, a mutant that lacks lysines is not ubiquitinated, internalizes into endosomes, but is not sorted to lysosomes and recycles more efficiently than the wild-type receptors.4 This suggests that the absence of ubiquitination on β2AR0K not only prevents lysosomal degradation, but also promotes receptor recycling. It remains to be seen whether ubiquitinated and non-ubiquitinated β2ARs, both of which internalize, move via an identical chain of vesicles from the very beginning of their subcellular journey, or if their paths are sorted only at late endosomes/multivesicular bodies where only ubiquitinated receptors are taken up for destruction because of the activity of ESCRT (Endosomal Sorting Complex Required for Transport) complexes (64).
Our studies demonstrate that Nedd4-mediated ubiquitination of the β2AR is essential for lysosomal targeting and degradation of the β2AR. Our data also suggest that the absence of receptor ubiquitination upon Nedd4 knockdown does not affect receptor internalization into endocytic vesicles, but rather affects the sorting steps to reach the lysosomal compartments. We also demonstrate that β-arrestin2 functions as an E3 ubiquitin ligase adaptor to recruit Nedd4 to the activated β2AR. In conclusion, β2AR ubiquitination mediated by Nedd4 appears to be an essential mechanism for the down-regulation of receptors in lysosomes and hence is an important means of achieving long term desensitization of adrenergic signaling.
Supplementary Material
Acknowledgments
We thank Dr. Robert J. Lefkowitz, mentor extraordinaire, for his insightful comments and generous support at early stages of this work.
This work was supported, in whole or in part, by National Institutes of Health Grant HL080525. This work was also supported by the American Heart Association (0530014N) (to S. K. S.). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S4 and Table S1.
Footnotes
The abbreviations used are: β2AR, β2-adrenergic receptor; 7TMR, seven-transmembrane receptor; CYP, cyanopindolol; Nedd4, neuronal precursor cell-expressed developmentally down-regulated 4; HECT, homologous to E6-AP C terminus; Mdm2, mouse double minute2; RING, really interesting new gene; Ub, ubiquitin; PBS, phosphate-buffered saline; ANOVA, analysis of variance; WT, wild type; DTME, dithio-bis-maleimidoethane; siRNA, short interfering RNA; HA, hemagglutinin; DN, dominant negative; GPCR, G protein-coupled receptor.
S. K. Shenoy, unpublished findings.
References
- 1.Pierce, K. L., Premont, R. T., and Lefkowitz, R. J. (2002) Nat. Rev. Mol. Cell. Biol. 3 639-650 [DOI] [PubMed] [Google Scholar]
- 2.Bockaert, J., and Pin, J. P. (1999) EMBO J. 18 1723-1729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.DeWire, S. M., Ahn, S., Lefkowitz, R. J., and Shenoy, S. K. (2007) Annu. Rev. Physiol. 69 483-510 [DOI] [PubMed] [Google Scholar]
- 4.Moore, C. A., Milano, S. K., and Benovic, J. L. (2007) Annu. Rev. Physiol. 69 451-482 [DOI] [PubMed] [Google Scholar]
- 5.Tsao, P., Cao, T., and von Zastrow, M. (2001) Trends Pharmacol. Sci. 22 91-96 [DOI] [PubMed] [Google Scholar]
- 6.Drake, M. T., Shenoy, S. K., and Lefkowitz, R. J. (2006) Circ. Res. 99 570-582 [DOI] [PubMed] [Google Scholar]
- 7.Ferguson, S. S. (2001) Pharmacol. Rev. 53 1-24 [PubMed] [Google Scholar]
- 8.Dohlman, H. G. (2002) Nature 418 591. [DOI] [PubMed] [Google Scholar]
- 9.Premont, R. T., and Gainetdinov, R. R. (2007) Annu. Rev. Physiol. 69 511-534 [DOI] [PubMed] [Google Scholar]
- 10.Bouvier, M., Collins, S., O'Dowd, B. F., Campbell, P. T., de Blasi, A., Kobilka, B. K., MacGregor, C., Irons, G. P., Caron, M. G., and Lefkowitz, R. J. (1989) J. Biol. Chem. 264 16786-16792 [PubMed] [Google Scholar]
- 11.Moore, R. H., Tuffaha, A., Millman, E. E., Dai, W., Hall, H. S., Dickey, B. F., and Knoll, B. J. (1999) J. Cell Sci. 112, 329-338 [DOI] [PubMed] [Google Scholar]
- 12.Shenoy, S. K., McDonald, P. H., Kohout, T. A., and Lefkowitz, R. J. (2001) Science 294 1307-1313 [DOI] [PubMed] [Google Scholar]
- 13.Hershko, A., Ciechanover, A., and Varshavsky, A. (2000) Nat. Med. 6 1073-1081 [DOI] [PubMed] [Google Scholar]
- 14.Li, W., Bengtson, M. H., Ulbrich, A., Matsuda, A., Reddy, V. A., Orth, A., Chanda, S. K., Batalov, S., and Joazeiro, C. A. (2008) PLoS ONE 3 e1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pickart, C. M. (2001) Annu. Rev. Biochem. 70 503-533 [DOI] [PubMed] [Google Scholar]
- 16.Jackson, P. K., Eldridge, A. G., Freed, E., Furstenthal, L., Hsu, J. Y., Kaiser, B. K., and Reimann, J. D. (2000) Trends Cell Biol. 10 429-439 [DOI] [PubMed] [Google Scholar]
- 17.Joazeiro, C. A., and Weissman, A. M. (2000) Cell 102 549-552 [DOI] [PubMed] [Google Scholar]
- 18.Shenoy, S. K. (2007) Circ. Res. 100 1142-1154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miranda, M., and Sorkin, A. (2007) Mol. Interv. 7 157-167 [DOI] [PubMed] [Google Scholar]
- 20.Dikic, I. (2003) Biochem. Soc. Trans. 31 1178-1181 [DOI] [PubMed] [Google Scholar]
- 21.Magnifico, A., Ettenberg, S., Yang, C., Mariano, J., Tiwari, S., Fang, S., Lipkowitz, S., and Weissman, A. M. (2003) J. Biol. Chem. 278 43169-43177 [DOI] [PubMed] [Google Scholar]
- 22.Snyder, P. M., Steines, J. C., and Olson, D. R. (2004) J. Biol. Chem. 279 5042-5046 [DOI] [PubMed] [Google Scholar]
- 23.Marchese, A., Raiborg, C., Santini, F., Keen, J. H., Stenmark, H., and Benovic, J. L. (2003) Dev. Cell 5 709-722 [DOI] [PubMed] [Google Scholar]
- 24.Jin, Y., Lee, H., Zeng, S. X., Dai, M. S., and Lu, H. (2003) EMBO J. 22 6365-6377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ahn, S., Nelson, C. D., Garrison, T. R., Miller, W. E., and Lefkowitz, R. J. (2003) Proc. Natl. Acad. Sci. U. S. A. 100 1740-1744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jacob, C., Cottrell, G. S., Gehringer, D., Schmidlin, F., Grady, E. F., and Bunnett, N. W. (2005) J. Biol. Chem. 280 16076-16087 [DOI] [PubMed] [Google Scholar]
- 27.Cuervo, A. M., and Dice, J. F. (1996) Science 273 501-503 [DOI] [PubMed] [Google Scholar]
- 28.Xiao, K., McClatchy, D. B., Shukla, A. K., Zhao, Y., Chen, M., Shenoy, S. K., Yates, J. R., 3rd, and Lefkowitz, R. J. (2007) Proc. Natl. Acad. Sci. U. S. A. 104 12011-12016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ingham, R. J., Gish, G., and Pawson, T. (2004) Oncogene 23 1972-1984 [DOI] [PubMed] [Google Scholar]
- 30.Otte, L., Wiedemann, U., Schlegel, B., Pires, J. R., Beyermann, M., Schmieder, P., Krause, G., Volkmer-Engert, R., Schneider-Mergener, J., and Oschkinat, H. (2003) Protein Sci. 12 491-500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gurevich, V. V., Dion, S. B., Onorato, J. J., Ptasienski, J., Kim, C. M., Sterne-Marr, R., Hosey, M. M., and Benovic, J. L. (1995) J. Biol. Chem. 270 720-731 [DOI] [PubMed] [Google Scholar]
- 32.Gurevich, V. V., and Gurevich, E. V. (2004) Trends Pharmacol. Sci. 25 105-111 [DOI] [PubMed] [Google Scholar]
- 33.Xiao, K., Shenoy, S. K., Nobles, K., and Lefkowitz, R. J. (2004) J. Biol. Chem. 279 55744-55753 [DOI] [PubMed] [Google Scholar]
- 34.Shenoy, S. K., Barak, L. S., Xiao, K., Ahn, S., Berthouze, M., Shukla, A. K., Luttrell, L. M., and Lefkowitz, R. J. (2007) J. Biol. Chem. 282 29549-29562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hershko, A., and Ciechanover, A. (1998) Annu. Rev. Biochem. 67 425-479 [DOI] [PubMed] [Google Scholar]
- 36.Mukhopadhyay, D., and Riezman, H. (2007) Science 315 201-205 [DOI] [PubMed] [Google Scholar]
- 37.Kolling, R., and Hollenberg, C. P. (1994) EMBO J. 13 3261-3271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hicke, L. (1997) Faseb. J. 11 1215-1226 [DOI] [PubMed] [Google Scholar]
- 39.Galan, J. M., Moreau, V., Andre, B., Volland, C., and Haguenauer-Tsapis, R. (1996) J. Biol. Chem. 271 10946-10952 [DOI] [PubMed] [Google Scholar]
- 40.Marchese, A., and Benovic, J. L. (2001) J. Biol. Chem. 276 45509-45512 [DOI] [PubMed] [Google Scholar]
- 41.Martin, N. P., Lefkowitz, R. J., and Shenoy, S. K. (2003) J. Biol. Chem. 278 45954-45959 [DOI] [PubMed] [Google Scholar]
- 42.Cottrell, G. S., Padilla, B., Pikios, S., Roosterman, D., Steinhoff, M., Gehringer, D., Grady, E. F., and Bunnett, N. W. (2006) J. Biol. Chem. 281 27773-27783 [DOI] [PubMed] [Google Scholar]
- 43.Mihalik, B., Gaborik, Z., Varnai, P., Clark, A. J., Catt, K. J., and Hunyady, L. (2003) Int. J. Biochem. Cell Biol. 35 992-1002 [DOI] [PubMed] [Google Scholar]
- 44.Cohen, B. D., Bariteau, J. T., Magenis, L. M., and Dias, J. A. (2003) Endocrinology 144 4393-4402 [DOI] [PubMed] [Google Scholar]
- 45.Wolfe, B. L., Marchese, A., and Trejo, J. (2007) J. Cell Biol. 177 905-916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tanowitz, M., and Von Zastrow, M. (2002) J. Biol. Chem. 277 50219-50222 [DOI] [PubMed] [Google Scholar]
- 47.Petaja-Repo, U. E., Hogue, M., Laperriere, A., Bhalla, S., Walker, P., and Bouvier, M. (2001) J. Biol. Chem. 276 4416-4423 [DOI] [PubMed] [Google Scholar]
- 48.Kohout, T. A., Lin, F. S., Perry, S. J., Conner, D. A., and Lefkowitz, R. J. (2001) Proc. Natl. Acad. Sci. U. S. A. 98 1601-1606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shenoy, S. K., and Lefkowitz, R. J. (2005) J. Biol. Chem. 280 15315-15324 [DOI] [PubMed] [Google Scholar]
- 50.Snyder, P. M. (2005) Endocrinology 146 5079-5085 [DOI] [PubMed] [Google Scholar]
- 51.Harvey, K. F., and Kumar, S. (1999) Trends Cell Biol. 9 166-169 [DOI] [PubMed] [Google Scholar]
- 52.Hamilton, M. H., Tcherepanova, I., Huibregtse, J. M., and McDonnell, D. P. (2001) J. Biol. Chem. 276 26324-26331 [DOI] [PubMed] [Google Scholar]
- 53.Bhandari, D., Trejo, J., Benovic, J. L., and Marchese, A. (2007) J. Biol. Chem. 282 36971-36979 [DOI] [PubMed] [Google Scholar]
- 54.Song, X., Raman, D., Gurevich, E. V., Vishnivetskiy, S. A., and Gurevich, V. V. (2006) J. Biol. Chem. 281 21491-21499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Salcedo, A., Mayor, F., Jr., and Penela, P. (2006) EMBO J. 25 4752-4762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Girnita, L., Shenoy, S. K., Sehat, B., Vasilcanu, R., Girnita, A., Lefkowitz, R. J., and Larsson, O. (2005) J. Biol. Chem. 280 24412-24419 [DOI] [PubMed] [Google Scholar]
- 57.Tan, C. M., Brady, A. E., Nickols, H. H., Wang, Q., and Limbird, L. E. (2004) Annu. Rev. Pharmacol. Toxicol. 44 559-609 [DOI] [PubMed] [Google Scholar]
- 58.Hein, P., Rochais, F., Hoffmann, C., Dorsch, S., Nikolaev, V. O., Engelhardt, S., Berlot, C. H., Lohse, M. J., and Bunemann, M. (2006) J. Biol. Chem. 281 33345-33351 [DOI] [PubMed] [Google Scholar]
- 59.Violin, J. D., DiPilato, L. M., Yildirim, N., Elston, T. C., Zhang, J., and Lefkowitz, R. J. (2008) J. Biol. Chem. 283 2949-2961 [DOI] [PubMed] [Google Scholar]
- 60.Oakley, R. H., Laporte, S. A., Holt, J. A., Caron, M. G., and Barak, L. S. (2000) J. Biol. Chem. 275 17201-17210 [DOI] [PubMed] [Google Scholar]
- 61.Wang, P., Gao, H., Ni, Y., Wang, B., Wu, Y., Ji, L., Qin, L., Ma, L., and Pei, G. (2003) J. Biol. Chem. 278 6363-6370 [DOI] [PubMed] [Google Scholar]
- 62.Shenoy, S. K., and Lefkowitz, R. J. (2003) J. Biol. Chem. 278 14498-14506 [DOI] [PubMed] [Google Scholar]
- 63.Perroy, J., Pontier, S., Charest, P. G., Aubry, M., and Bouvier, M. (2004) Nat. Methods 1 203-208 [DOI] [PubMed] [Google Scholar]
- 64.Saksena, S., Sun, J., Chu, T., and Emr, S. D. (2007) Trends Biochem. Sci. 32 561-573 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.