Abstract
Background:
The ability to maintain muscle function decreases with age and loss of proteostatic function. Diet, drugs, and genetic interventions that restrict nutrients or nutrient signaling help preserve long-term muscle function and slow age-related decline. Previously, it was shown that attenuating protein synthesis downstream of the mechanistic target of rapamycin (mTOR) gradually increases expression of heat shock response (HSR) genes in a manner that correlates with increased resilience to protein unfolding stress. Here, we investigate the role of specific tissues in mediating the cytoprotective effects of low translation.
Methods:
This study uses genetic tools (transgenic Caenorhabditis elegans (C. elegans), RNA interference and gene expression analysis) as well as physiological assays (survival and paralysis assays) in order to better understand how specific tissues contribute to adaptive changes involving cellular cross-talk that enhance proteostasis under low translation conditions.
Results:
We use the C. elegans system to show that lowering translation in neurons or the germline increases heat shock gene expression and survival under conditions of heat stress. In addition, we find that low translation in these tissues protects motility in a body muscle-specific model of proteotoxicity that results in paralysis. Low translation in neurons or germline also results in increased expression of certain muscle regulatory and structural genes, reversing reduced expression normally observed with aging in C. elegans. Enhanced resilience to protein unfolding stress requires neuronal expression of cbp-1.
Conclusions:
Low translation in either neurons or the germline orchestrate protective adaptation in other tissues, including body muscle.
Keywords: C. elegans, lifespan, translation, ifg-1, eIF4G, healthspan, lifespan, proteostasis
1. Introduction
Loss of protein quality control plays a major role in aging and age-related diseases, including the growing pandemic of protein conformational disorders [1]. While frequently considered in the context of neurodegeneration, conformational disorders also contribute to functional decline in skeletal muscle, which exacerbates problems related to muscle wasting that diminish mobility and contribute to frailty [2,3]. Ensuring protein quality control is dependent on the ability to mount a sufficient adaptive response to stress that perturbs proteostasis. Mitigating diminution of proteostasis that occurs with normal aging is an important goal in disease prevention and treatment [4].
Ensuring proper maintenance of proteostasis requires both constitutive and induced activation of cytoplasmic and organelle-specific stress response pathways [5–7]. Prominent among these pathways is the cytoplasmic heat shock response (HSR), which governs heat shock factors (HSFs) that drive expression of genes important for restoring proteostasis [8]. A review of existing literature indicates that low heat shock gene expression is associated with an increase in cellular senescence, while overexpression is inversely correlated, across a number of tissue types [9]. Although it is clear that aging leads to loss of proteostasis [10], the reasons why proper function of this response diminishes with age are not fully understood.
Lowering translation downstream of nutrient sensing improves proteostasis [11–13]. The mechanistic target of rapamycin (mTOR) governs cellular nutrient sensing and downregulates the materially and energetically expensive process of mRNA translation when food availability is reduced [14]. Individually, restricting mTOR signaling or translation can extend lifespan and improve proteostasis, outcomes that are highly conserved across diverse species [14–17].
Translation controlled downstream of mTOR is regulated by the translation initiation cap-binding complex (CBC). It comprises the eukaryotic translation initiation factor (eIF)4G, the eIF4A RNA helicase, and the 5′ mRNA cap-binding protein eIF4E [18]. eIF4G acts as a nexus for translation by bringing in other translation factors and helping recruit the small (40S) ribosomal subunit. Although eIF4E or eIF4G can become limiting for cap-mediated translation, eIF4G is also involved in cap-independent translation and has been shown to be a limiting factor in yeast and worms under nutrient stress or when mTOR signaling is attenuated [19–21]. Depletion of eIF4G phenocopies differential translation changes from chemical or genetic inhibition of mTOR in mouse embryonic fibroblasts [22]. eIF4G also becomes sequestered in stress granules upon exposure to oxidative or thermal stress in mammalian tissue culture [23,24]. These studies show that changes in expression and availability of eIF4G form part of a conserved adaptive response to enhance survival during periods of nutrient scarcity or other environmental stress. Thus, nutrient status, mTOR, and acute stress regulate expression of the CBC factor eIF4G in diverse animal systems.
In Caenorhabditis elegans (C. elegans), changes in proteostasis occur rapidly after entry into adulthood [25] and coincide with chromatin remodeling that decreases robustness of the HSR [26]. This aspect of C. elegans biology makes it possible to rapidly assess genetic or environmental interventions that modulate age-related changes in proteostasis. Previously, we discovered that genetically attenuating eIF4G reverses proteostatic collapse that occurs early in adulthood in a manner that is partly dependent on hsf-1 [11], a gene encoding the only HSF in C. elegans. Lowering translation coincided with constitutive upregulation of HSR target genes. It also restored robustness of HSR activation to a more youthful level in response to heat stress, a phenomenon we refer to here as HSR priming.
Understanding how to maintain proteostasis for healthy aging is complicated by tissue crosstalk interactions in multicellular systems. This is due to the ability of localized stress responses to influence the activity of proteostasis machinery in distal tissues [27–32], including cell non-autonomous activation of the HSR [29]. The means and extent of intercellular signaling are not fully characterized, nor is the connection between translation and proteostasis in distal tissues.
Recently, we showed that antagonistic effects of low translation on growth and longevity were separable by tissue-type and had cell non-autonomous effects on reproduction [33]. Lowering translation downstream of mTOR in neurons, germline, or hypodermal tissue increased lifespan and negatively affected reproduction, a trade-off posited by Disposable Soma theory [34] and frequently observed in genetic or environmental interventions that increase lifespan [35]. However, lowering translation in body muscle reduced lifespan and increased the rate of development and reproductive output, a complete reversal of the typical trade-offs observed from systemic manipulation, but one in line with trade-offs between decreased motility/energy expenditure and increased reproduction predicted by foraging theory [36–38]. Taken together, results led us to wonder whether low translation in specific tissues also controls systemic responses to unfolded protein stress mediated by the HSR.
In the current study, we investigated how translation controlled by eIF4G in specific tissues influence systemic proteostasis in C. elegans. Results indicate that attenuating translation in neural or germline tissue increases resistance to stress and primes the HSR. Furthermore, we found that low translation in neurons or germline were protective in a body muscle-specific proteotoxicity model and led to increased transcription of muscle structural and regulatory genes, reversing age-related attenuation of expression and improving proteostasis in body muscle. Lastly, through an RNA interference (RNAi) screen, we found that neural expression of the transcription regulator CBP-1 is required for protective effects of low translation.
2. Materials and Methods
2.1. Nematode Culture and Strains
C. elegans strains were cultured and maintained with standard procedures as described in [39] unless otherwise specified. The N2 Bristol strain was used as the reference wild type. Genotypes of strains acquired from the Caenorhabditis Genetics Center are as follows: MAH23 rrf-1(pk1417), VP303 rde-1(ne219); kbIs7[nhx-2p::rde-1 + rol-6(su1006)], NR222 rde-1(ne219); kbIs9[lin-26p::nls::GFP, lin-26p::rde-1 + rol-6(su1006)], WM118 rde-1(ne300); neIs9[myo-3p::HA::rde-1 + pRF4(rol-6(su1006))], TU3335 lin-15B(n744); uIs57 [unc-119p::YFP + unc-119p::sid-1 + mec-6p::mec-6], PS3551 hsf-1 (syf441), CF1038 daf-16(mu86): uthIs225 [sur5p::hsf-1(CT-Delta)::unc-54 3’ UTR + myo-2p::tdTomato::unc-54 3’ UTR], and NL5901 pkIs2386 [unc-54p::α-synuclein::YFP + unc-119(+)]. Transgenic lines ANR149 rrf-1(pk1417); pkIs2386[unc-54p:: α-synuclein::YFP + unc-119(+)], ANR153 rde-1(ne300); neIs9[mvo-3p::HA::rde-1 + pRF4(rol-6(su1006))]; pkIs2386[unc-54p:: α-synuclein::YFP + unc-119(+)], and ANR168 lin-15b(n744); pkIs2386[unc-54p::alphasynuclein::YFP + unc-119(+)]; uIs57[unc-119p::YFP + unc-119p::sid-1 + mec-6p::mec-6] were created by crossing NL5901 males with MAH23, WM118 or TU3335 hermaphrodites, respectively. Polymerase Chain Reaction (PCR) combined with targeted DNA sequencing was performed to validate genotypes.
2.2. RNAi Experiments
RNAi knockdown treatments were performed as described in [40]. RNAi bacteria strains included empty vector L4440 (Addgene, Cambridge, MA, USA), ifg-1 (M110.4), cbp-1 (R10E11.1), daf-15 (C10C5.6, gifted from Han Lab), and rict-1 (F29C12.3) (Ahringer library, Source BioScience, Nottingham, UK). RNAi empty vector L4440 is referred to in text as control RNAi. RNAi was carried out from day 0 of adulthood, defined by the period immediately following L4 stage, but before animals have developed visible germline. RNAi empty vector L4440 is referred to in text (Figs. 1,2,3,4, Supplementary Figs. 1–4) as control RNAi. In Fig. 4 and RNAi screens shown in Supplementary Fig. 4, a dual RNAi strategy was used in which day 0 adults were placed on RNAi for ifg-1 or control for the first two days before being transferred to plates containing RNAi for cbp-1 or other screen target genes for 5 days prior to analysis. For a full list of RNAis used in screens shown in Supplementary Fig. 4, refer to Supplementary Table 1. For all assays except RNAi screen, animals were syncronized via 2–5 hours timed egg lays, duirng which gravid adults were allowed to lay eggs, before all animals were removed from plate, leaving behind only eggs. For the RNAi screen, animals were synchronized via bleaching gravid animals to obtain similarly staged embryos.
Fig. 1. Reduced translation in germline or neurons primes the heat shock response (HSR) and increases thermotolerance.
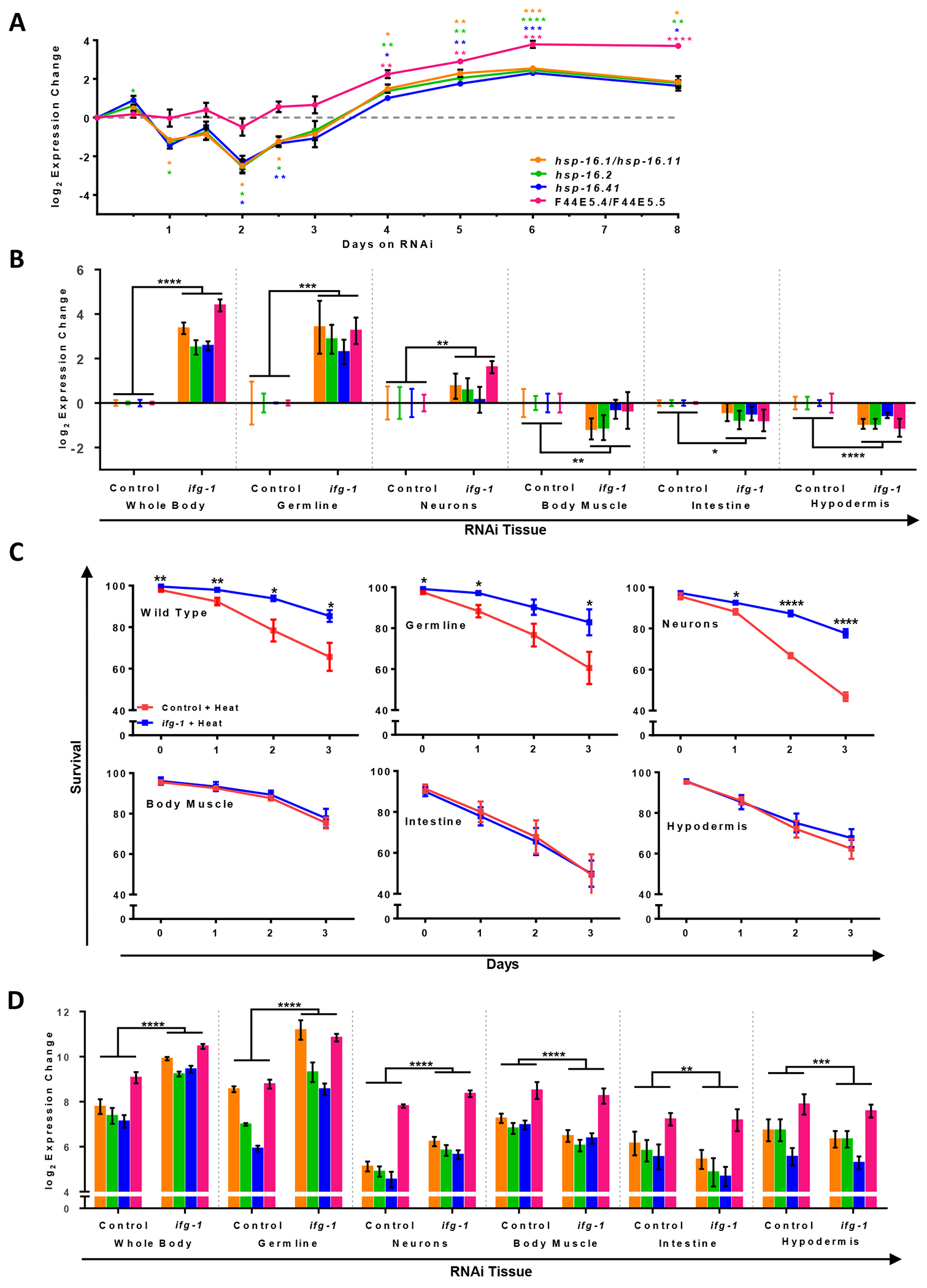
(A) Time course of heat shock gene expression in N2 animals under ifg-1 RNA interference (RNAi) normalized to control RNAi at each time point shown, beginning at the onset of adulthood. Unpaired t-tests using Welch’s correction were conducted on ΔCts for each gene at each time point (Supplementary Table 3). (B) Heat shock gene expression of tissue-specific strains on ifg-1 RNAi normalized to control RNAi at day 7 of adulthood. From left to right, panels show N2 wild type animals, MAH23 (germline-specific RNAi strain), TU3335 (neuron-specific RNAi strain), WM118 (body muscle-specific RNAi strain), VP303 (intestine-specific RNAi strain), and NR222 (hypodermis-specific RNAi strain). Two-way ANOVAs for each strain were used comparing ΔCts of all genes (Supplementary Table 4). (C) Thermotolerance of tissue-specific RNAi strains after 1 week on RNAi. Unpaired t-tests using Welch’s correction were run at each time point for each strain (Supplementary Table 5). (D) Similar to (B) except that heat shock gene expression was measured 1 hour after exposure to 35 °C for 4 hours. Two-way ANOVAs were conducted for each strain comparing heat shock gene expression (Supplementary Table 4). Error bars represent means ± SEM. * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001.
Fig. 2. Reducing translation in the germline or neurons improves motility and restores youthful transcription of muscle maintenance genes.
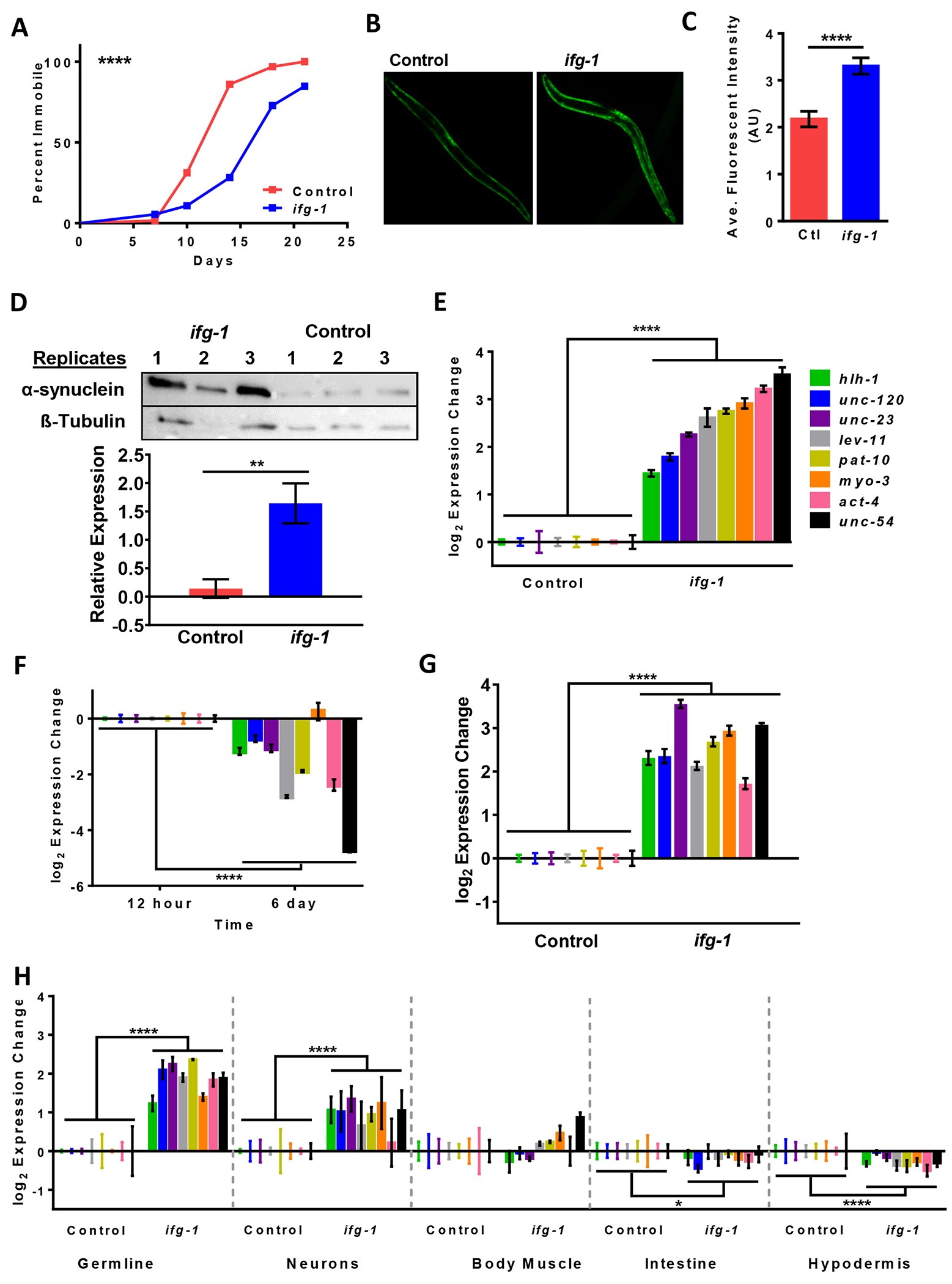
(A) Paralysis assay of proteinopathy model expressing alpha-synuclein in body muscle (strain NL5901) on control or ifg-1 RNAi from the onset of adulthood. Kaplan-Meier survival curves were plotted for paralysis assays and compared using the Mantel-Cox log rank test, **** p < 0.0001. Additional replicates show similar results (Supplementary Table 6). (B) Representative microscope images of YFP-tagged alpha-synuclein in NL5901 on control and ifg-1 RNAi for 7 days. Scale bar = 200 μM. Images were collected from at least 9 animals per replicate across 4 replicates. (C) Quantification and comparison of fluorescence in NL5901 animals from (B) using an unpaired t-test with Welch’s Correction (Supplementary Table 7). (D) Western blot probing for alpha-synuclein and beta-tubulin proteins in NL5901 animals comparing conditions from (B) using an unpaired t-test with Welch’s Correction (Supplementary Table 8). (E) Expression of muscle structural and regulatory genes in NL5901 animals on control vs. ifg-1 RNAi at day 7 of adulthood compared using two-way ANOVA (Supplementary Table 9). (F) Aging-related changes in muscle-related gene expression of N2 animals on control RNAi using two-way ANOVA, **** p < 0.0001. (G) Expression of body muscle genes in N2 animals on control or ifg-1 RNAi from day 1 to day 7 of adulthood compared using two-way ANOVA, p < 0.0001. (H) From left to right, panels show body muscle gene expression on control or ifg-1 RNAi for the first 7 days of adulthood in MAH23 (germline-specific RNAi strain), TU3335 (neuron-specific RNAi strain), WM118 (body muscle-specific RNAi strain), VP303 (intestine-specific RNAi strain), and NR222 (hypodermis-specific RNAi strain). Two-way ANOVA was used (Supplementary Table 10). Error bars represent means ± SEM. * p < 0.05, ** p < 0.01, **** p < 0.0001.
Fig. 3. Lowering translation in the germline or neurons upregulates muscle-related gene expression and improves motility in a model of body muscle proteotoxicity.
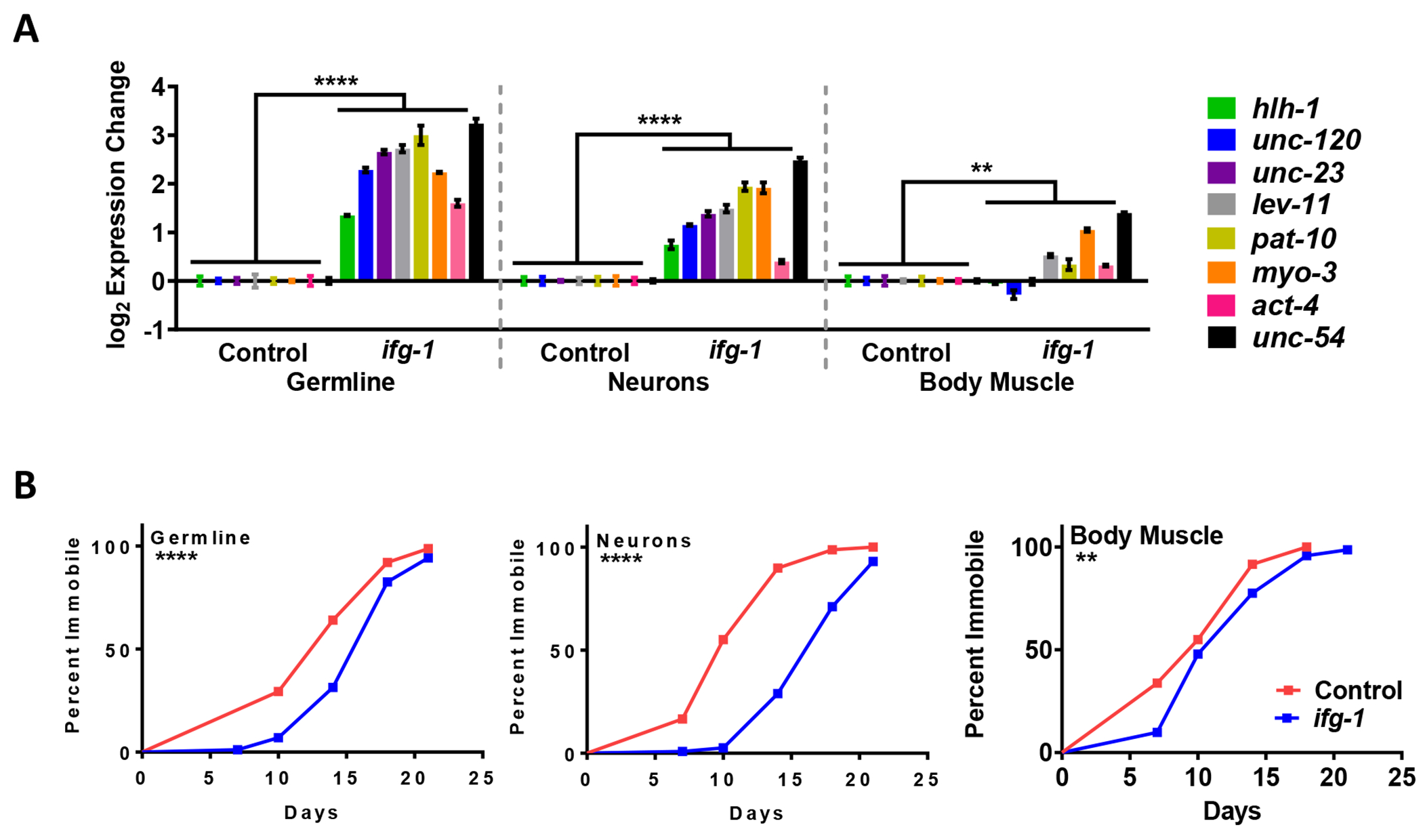
(A) Expression of muscle-related genes after 7 days of ifg-1 RNAi in the proteinopathy model strain expressing alpha-synuclein in body muscle crossed with tissue-specific RNAi strains (strains ANR149, ANR168, ANR153). Comparisons between animals treated with control or ifg-1 RNAi were carried out using two-way ANOVAs. (B) Paralysis assays carried out for strains and conditions in (A). Kaplan-Meier survival curves were plotted for paralysis assays and compared using the Mantel-Cox log rank test. Replicates for each strain were conducted with similar results (see Supplementary Table 6). Error bars represent means ± SEM. ** p < 0.01. **** p < 0.0001.
Fig. 4. cbp-1 RNAi in neurons both dampens muscle-related gene expression changes as well as enhanced survival to proteotoxicity under low translation conditions.
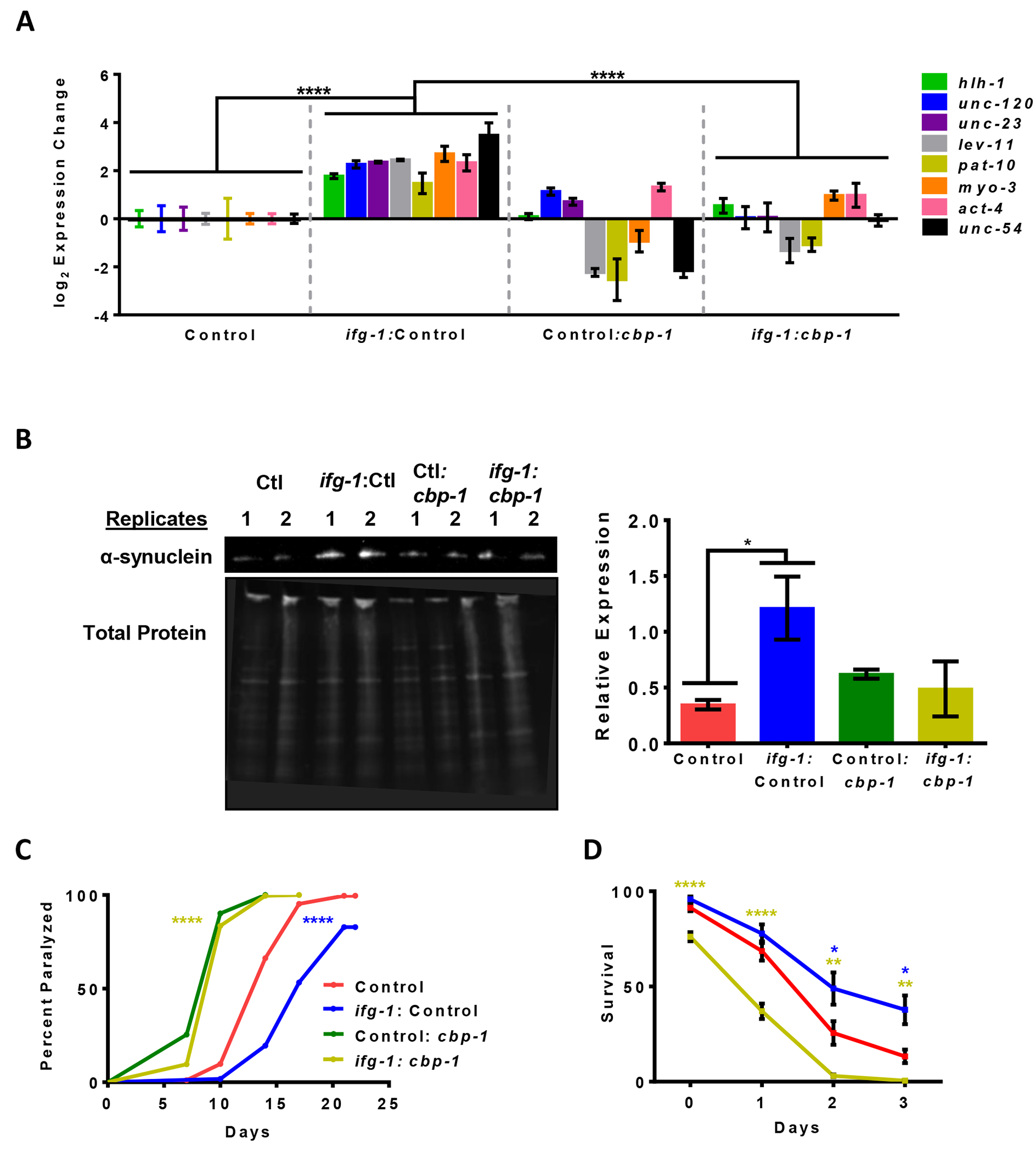
(A) Expression of genes enriched in muscle in the neuron-specific RNAi strain TU3335 crossed with NL5901 (ANR168). Day 1 adults were placed on control or ifg-1 RNAi for 2 days before transfer to control or cbp-1 RNAi for 5 days. Two-way ANOVAs were conducted comparing ΔCts of the genes shown (Supplementary Table 11). (B) Western blot of alpha-synuclein (top) and total protein measured from Ultraviolet (UV) shadowing (bottom) for conditions in (A). Quantification and comparison of alpha-synuclein was run using unpaired t-tests with Welch’s Correction (right) (Supplementary Table 12). (C) Paralysis assay of the strain and conditions from (A). Kaplan-Meier survival curves were plotted for the paralysis assay and compared using the Mantel-Cox log rank test. p < 0.0001 (blue asterisk, ctl vs. ifg1:ctl) and p < 0.0001 (yellow asterisk, ctl:cbp-1 vs. ifg-1:cbp-1) (Supplementary Table 13). (D) Thermotolerance assays (5 hours at 37 °C) using RNAi conditions from (A) in the neuron-specific RNAi strain TU3335. Comparisons at different time points were carried out with unpaired t-tests using Welch’s correction compared to control RNAi (Supplementary Tables 14,15). Error bars represent means ± SEM. * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001.
2.3. Thermotolerance
Approximately 120 synchronized animals were maintained for each condition at 20 °C until exposure to heat stress (35 °C for 4 hours or 37 °C for 5 hours). Animals were allowed to recover for 1 hour before being scored for survival. Thereafter, survival was scored at daily intervals.
2.4. RNA Processing and Qualitative Polymerase Chain Reaction (qPCR)
RNA was isolated using Trizol Reagent (Fisher Scientific, Waltham, MA, USA) following the manufacturer’s instructions using 1–200 worms per sample. RNA samples were then processed with either Sureprep RNA Cleanup and Concentration Kit (Fisher BioReagents, Fair Lawn, NJ, USA) or RNA Clean and Concentrator (Zymo Research, Irvine, CA, USA). 200 ng RNA was reverse transcribed using QuantiTect Reverse Transcription Kit (Qiagen, Valencia, CA, USA). qPCR was performed in technical duplicate or triplicate using SYBR FAST qPCR Master Mix (Kapa Biosystems, Cape Town, South Africa) on a LightCycler 480 (Roche Applied Science, Indianapolis, IN, USA). Target gene mRNA expression was normalized to the housekeeping gene cdc-42 expression. Relative expression was determined by normalizing to control samples. Primer sequences are provided in Supplementary Table 2.
2.5. Paralysis Analysis
Synchronized NL5901, ANR149, ANR153, and ANR168 strains were maintained on control or ifg-1 RNAi plates starting at adulthood and transferred fresh RNAi plates daily. On days of paralysis measurement, 100–150 adults were transferred to a new RNAi plate with their bodies aligned in a straight line. After 10 minutes, worms that had not moved from their original location were gently tapped with a sterile platinum wire 2–3 times on the head. Worms able to move only their head (from the pharynx bulb to the tip of the head) were scored as paralyzed. Worms able to move between the pharynx and tail were considered not paralyzed. Worms unable to respond to touch were scored as dead. Paralyzed or dead worms were removed from the plate on the day of paralysis measurement.
2.6. Western Blotting
Synchronized NL5901 and ANR168 strains were maintained as they were in paralysis assays until day 7 when total protein extraction occurred. To determine the levels of alpha-synuclein proteins, western blotting was performed in triplicate. Total protein extraction and preparation was performed as previously described in [ 11]. Alpha-synuclein was detected using an anti-alpha-synuclein mouse monoclonal antibody (1:500 dilution) (Santa Cruz biotechnology, Santa Cruz, CA, USA). Beta-tubulin was detected using an anti-beta-tubulin mouse monoclonal antibody (E7, 1:500 dilution) (DSHB, Iowa City, IA, USA). Peroxidase-conjugated goat anti-mouse IgG secondary antibody (1:5000 dilution) was from Pierce (Rockford, IL, USA). The density of the bands was determined using ImageJ software (1.54i, NIH LOCI, University of Wisconsin (Madison, WI, USA)) and normalized according to beta-tubulin or total protein.
2.7. Imaging
Synchronized NL5901 animals were maintained were maintained on control or ifg-1 RNAi plates starting at adulthood and transferred fresh RNAi plates daily. Visualization and quantification of alpha-synuclein:: YFP expression was conducted after 7 days of control or ifg-1 RNAi exposure. Individual worms were mounted on a 2% agarose pad and immobilized in a drop of 25 mM levamisol. Worms were imaged on a Leica M 165 FC microscope with the YFP filter (excitation 510/20 nm, emission 560/40 nm). The intensity of YFP fluorescence was measured in ImageJ by closely tracing around individual worms. In total, approximately 30–40 worms were used per condition.
2.8. Statistical Analysis
All statistics were performed using GraphPad Prism (6, GraphPad Software Inc. (Lo Jolla, CA, USA)). Kaplan-Meier survival curves were plotted for paralysis assays and compared using the Mantel-Cox log rank test. Western blots, survival assays, and fluorescent expression analyses were compared using unpaired two-tailed t-tests with Welch’s correction. Data from quantitative PCR were assessed by performing two-way ANOVA or unpaired two-tailed t-tests with Welch’s correction.
3. Results
3.1. Reduced Translation in Neurons or Germline Primes the HSR and Increases Thermotolerance
Since HSR expression is correlated with somatic protection from perturbations in proteostasis, we sought to first resolve the kinetics of HSR priming upon translation attenuation. For this, we employed RNA interference (RNAi) targeting eIF4G, which is known in C. elegans as IFG-1 and encoded by the ifg-1 gene. This treatment started on the first day of adulthood after all tissues are fully developed and continued for one week. Four HSP genes measured as a proxy for the HSR showed a decrease in the first two days after exposure to ifg-1 RNAi followed by a steady increase to peak levels by the end of the week when normalized to control RNAi for each timepoint (Fig. 1A). The delay in HSR induction corresponds with a lack of thermoprotection at two days compared to seven days of low translation observed in a previous study [11]. Thus, the time in between the rapid drop in translation and peak in HSP gene expression is considered the period of adaptation to low translation conditions.
Having established a time-course for HSR priming under ifg-1 RNAi (hereafter also referred to as a low translation condition), we sought to investigate tissue-specific effects of low ifg-1 expression. Several C. elegans strains were chosen that allow ifg-1 to be attenuated in select tissues via RNAi as previously characterized [33,41–44|. Tissues selectively targeted by RNAi include the germline, neurons, body muscle, intestine, and hypodermis (for strain details, see methods). Using the same conditions as above, we looked to see whether ifg-1 RNAi showed signs of HSR priming in these strains. Only low translation in neurons or germline elicited significantly increased HSP expression, though to varying levels, whereas other tissues exhibited a reduction (Fig. 1B, Supplementary Fig. 1). Based on these outcomes, we hypothesized that low translation in neurons or germline tissue would confer protection to thermal stress as observed for whole-body RNAi in a previous study [11].
To determine whether low translation in individual tissues could protect the entire worm from heat-derived unfolded protein stress, wild-type and tissue-specific RNAi strains were treated with ifg-1 RNAi for one week as in Fig. 1B, subjected to thermal stress for four hours, and tracked for survival. Low ifg-1 expression in N2 wild-type as well as germline or neuronal tissue increased thermotolerance, but did not increase thermotolerance through other tissues (Fig. 1C). Analysis of HSP gene expression showed that only germline- and neuron-specific RNAi strains exhibited more robust HSR gene expression following heat treatment compared to heat-treated controls (Fig. 1D). Conversely, muscle- or intestine-specific low translation significantly lowered many HSR proteins compared to heat-treated controls (Fig. 1D). Findings indicate that either low germline or neuronal translation confers enhanced protection from thermal stress.
Since eIF4G/IFG-1 is downstream of mTORC1, but not mTORC2, we tested both the pathway and tissue-specificity of the response using RNAi targeting Raptor/daf-15, a part of the mTORC1 complex and Rictor/rict-1, a part of the mTORC2 complex (Supplementary Fig. 2A). daf-15 RNAi elicited HSR priming in wild-type and germline-specific RNAi animals, but not in the neuronal RNAi strain (Supplementary Fig. 2B). However, enhanced resistance to heat was observed for the neuronal RNAi strain despite the lack of HSR priming (Supplementary Fig. 2B, lower panel). No HSR priming nor protection were conferred by rict-1 knockdown. Collectively, the data indicates the importance of mTORC1, but not mTORC2 with HSR priming and enhanced thermotolerance.
The transcription factor hsf and the C. elegans FOXO transcription factor DAF-16 regulate proteostasis and control stress resistance via the insulin-like signaling pathway [45]. To determine whether the molecular alterations and enhanced thermotolerance observed under low translation are dependent on these factors, mutant strains for daf-16 and hsf-1 were tested. HSR priming was evident in a daf-16(mu86) null mutant in response to ifg-1 RNAi. Although thermal stress survival tended to be slightly improved, results did not reach statistical significance (Supplementary Fig. 3A). Null mutants for hsf-1 are not viable and cannot be tested, however, an hsf-1(sy441) mutant lacking the carboxy-terminus DNA binding domain was available for testing and failed to induce the same level of HSR priming phenotype, demonstrating the importance of this domain for HSR induction in general (Supplementary Fig. 3B). However, despite greatly reduced HSR priming before heat challenge, following heat treatment, it resulted in a more robust HSR response and improved survival. Interestingly, a previous study showed that overexpression of hsf-1 lacking the carboxy-terminus (strain AGD794) exhibits enhanced survival [46]. Results indicate that the carboxy-terminus of HSF-1 is not necessary for enhanced thermotolerance in general. Collectively, HSR priming and thermotolerance with low translation are partially dependent on the insulin-like signaling pathway.
3.2. Reducing Translation in Neurons or the Germline Improves Motility and Restores Youthful Transcription of Muscle Maintenance Genes
The fact that selectively lowering translation in neurons or the germline improve survival from challenge with heat indicates that they are likely to improve maintenance of protein folding in other tissues. Testing this possibility requires a tissue-specific model of protein unfolding stress. For this, we employed motility assays using strain NL5901, which expresses alpha-synuclein fused with YFP in body muscle. This tissue-specific expression is due to the fact that the transgene is controlled by the major heavy myosin chain unc-54 gene promoter. Aging animals lose the ability to maintain proteostasis, which is highly exacerbated in body muscle in this strain due to expression of alpha-synuclein, resulting in paralysis in worm middle-age [47]. Here, we used paralysis to monitor the onset of proteostatic collapse in body muscle.
Prior to looking at tissue-specific effects, we tested the effect of whole body attenuation of ifg-1 during adulthood, which increased the motile period in NL5901 by an average of 44% (Fig. 2A). Unexpectedly, we observed that low translation led to increased alpha-synuclein::YFP fluorescence intensity by the end of the first week of adulthood (Fig. 2B,C). Increased protein expression was observed also in a Western blot probed with a monoclonal antibody specific for alpha-synuclein (Fig. 2D). Thus, despite increased motility under this condition, lowering systemic translation resulted in increased total protein expression for alpha-synuclein::YFP.
We wondered whether this result could be explained by increased transcriptional activity of the major heavy myosin chain gene (unc-54) promoter used to drive body muscle expression of alpha-synuclein. After seven days of ifg-1 RNAi started at the onset of adulthood, the transcript level of endogenous unc-54 was increased by more than 10-fold (Fig. 2E). In addition to unc-54, we tested expression of several other muscle structural and regulatory genes including muscle actin (act-4), minor myosin heavy chain (myo-3), troponin C (pat-10), tropomyosin (lev-11), myogenic transcription factors (hlh-1, unc-120), and the unc-23 gene encoding a negative regulator of proteosomal degradation. Expression of these genes increased in NL5901 subjected to ifg-1 RNAi. To rule out the possibility of this phenomenon being specific to this strain, we tested wild-type N2 animals with ifg-1 RNAi. A previous study showed that unc-54 decreased in the first week of adulthood in C. elegans [48]. We also observed decreased expression at the end of the first week of adulthood in wild-type animals for unc-54 and several other muscle structural and regulatory genes (Fig. 2F). Transcript expression of these muscle specific genes showed that lowering translation reversed the age-related loss of muscle specific gene expression in wild-type animals (Fig. 2G). Tissue-specific RNAi strains demonstrated that increased muscle gene expression resulted from lowering translation in the germline or neurons (Fig. 2H). Interestingly, lowering translation selectively in body muscle tissue did not induce body muscle expression significantly (Fig. 2H).
Because selectively lowering translation in neurons and germline resulted in HSR priming (Fig. 1) and an increase in muscle specific transcripts (Fig. 2), we hypothesized that selectively lowering translation in neurons or germline in the alpha-synuclein proteotoxicity model could extend their motile period and delay disease onset. We also wondered what effect lowering translation selectively in body muscle, where proteotoxicity occurs, could improve conditions in this model. Thus, we crossed the corresponding tissue-specific RNAi strains with NL5901. We confirmed that muscle structural and regulatory genes increased when translation was reduced selectively in the germline or neurons (Fig. 3A). Interestingly, slightly increased expression of muscle structural and regulatory genes was also observed when translation was selectively lowered in muscle (Fig. 3A). This result differs slightly from the results with wild-type N2 animals (Fig. 2H), which may be due to the constitutively perturbed muscle proteostasis resulting from expression of alpha-synuclein. The paralysis assay demonstrated that lowering translation in the germline or neuronal tissue resulted in the greatest increase in motility with age, whereas lowering translation in muscle resulted in a small protective effect (Fig. 3B). In summary, low translation in the neurons or germline improved body muscle proteostasis.
3.3. Neuronal CBP-1 is Required for Improved Proteostasis Due to Low Translation
Next, we sought to determine a potential mechanism behind the benefits of low translation in neurons. We chose to further examine neurons, as we previously examined other trade-offs between low translation and reproduction [33]. We performed a small screen of genes using RNAi to determine whether certain transcription factors, chromatin remodeling factors, or muscle-enriched genes were required for the increase in muscle gene expression observed under neuronal low translation conditions. Wild-type N2 animals or neuronal-specific RNAi strain TU3335 were subjected to two days of ifg-1 RNAi to lower translation before being transferred to another RNAi of interest for an additional 5 days. Translation remained low as no progeny were detected in the days following removal from ifg-1 RNAi. Because unc-54 had a robust increase in mRNA transcript abundance under low translation conditions, its expression change was used as a proxy for other muscle-specific gene expression changes. Results for a portion of the RNAi tested are shown in Supplementary Fig. 4 and Supplementary Table 1. From the RNAi screen, the transcriptional regulator gene cbp-1 was the only gene found to completely suppress the increase in unc-54 mRNA in the neuron-specific RNAi strain, but not in N2 (Supplementary Fig. 4).
Previous studies have shown that cbp-1 plays an important role in dietary restriction (DR)-mediated lifespan extension, specifically in neurons [49,50]. Based on this, and its role as a transcriptional regulator, we used the neuron-specific RNAi strain TU3335 and the dual RNAi technique used in the RNAi screen to determine whether changes in expression of other body muscle-specific genes were influenced when both ifg-1 and cbp-1 expression were reduced in neurons. Results showed that lowering neuronal cbp-1 expression significantly reduced muscle-specific gene expression changes compared with lowering ifg-1 by itself (Fig. 4A).
To determine whether CBP-1 played a role in the maintenance of muscular proteostasis with low neuronal translation, we crossed the alpha-synuclein proteotoxicity model with neuronal-specific RNAi strain to create ANR168. The combination of ifg-1 and cbp-1 RNAi prevented a significant increase alpha-synuclein protein level (Fig. 4B). In addition, knocking down expression of neuronal cbp-1 prevented a robust increase in proteostasis associated with low translation in the muscle paralysis model (Fig. 4C). Similarly, cbp-1 RNAi prevented a robust increase in proteostasis from low translation in the neuronal-specific RNAi strain TU3335 under conditions of heat stress (Fig. 4D). Together, results indicate that neuronal CBP-1 is required for the full beneficial effects of low neuronal translation on proteostasis in tissue outside the nervous system, particularly in muscle.
4. Discussion
4.1. Responses to Low Translation are Partitioned Among Tissues and Capable of Cellular Cross-talk
The basis for loss of proteostatic maintenance with age is centered on inability to properly regulate transcriptional activation of stress response pathways and maintain the protein turnover apparatus [25,26]. Results of this study address the roles of several major tissues in mediating effects of low translation on survival under perturbed proteostasis. Low translation results from downregulation of the mTOR pathway, as occurs when nutrients are scarce, leading to slowed growth but also increased lifespan and resilience to stress. Data support a model in which neurons and the germline are tissues that control physiological responses to low translation, including enhanced somatic maintenance and function of body muscle. Although we do not know how muscle maintenance factors are invoked downstream of translation, we know some of their expression changes are dependent on neuronal expression of cbp-1.
CREB-binding factor protein (CBP) acts as a histone acetyltransferase (HAT) to acetylate key transcription factors and histones, and as a recruiter for additional transcriptional machinery [49,51]. Though expressed ubiquitously due to its localization in the nuclei of most somatic cells, in mice hypothalamic expression of CBP is correlated to increases in lifespan [47]. At the same time, decreases in its expression are associated with age and diabetes [49]. In C. elegans, CBP-1 has been shown to act almost exclusively in GABAergic neurons to double lifespan in DR worms [50]. Under DR conditions, its knockdown results in loss of lifespan extension associated with this intervention [49]. The current study shows that this factor plays a critical role in adaptive changes downstream of DR and mTOR at the level of translation in neurons.
Based on results from another study, body muscle stands out because it is the only major tissue in which selectively lowering translation reverses, at least in part, the trade-offs usually observed between longevity and development [33]. The mTOR pathway is upstream of translation. When low mTOR is driven by nutrient scarcity, it increases mitochondrial respiration in skeletal muscle and mitigates normal respiratory decline observed with age in mice [52]. In addition, stem cell availability increases under dietary restriction and low mTOR signaling in injured muscles [53]. Thus, it may be that, while translation-inhibiting conditions are detrimental during growth, in adult animals, at least certain aspects of basal muscle maintenance and function are preserved better with age when translation downstream of mTOR is reduced. Our study of the effects of low translation shows an indirect association with enhanced muscle maintenance, requiring low translation in non-muscle tissue. This could help explain the otherwise paradoxical connection between catabolism-inducing dietary restriction or low mTOR signaling conditions and improved muscle maintenance with age.
4.2. The Connection between Translational Regulation and Proteostasis
At the heart of improved muscle function and enhanced resistance to unfolded cellular protein resulting from low translation is reversal of age-related diminution of proteostatic maintenance. Many factors are involved in maintaining proteostasis, which involves synthesis, folding, and turnover of cellular protein. Nascent peptide chains emerging from the ribosome are managed by folding chaperones and are subject to ubiquitination, making translation a hub for all these processes [54]. The fact that everything from protein synthesis to degradation is regulated at the same location makes translation a critical process for maintaining this balance. We show that limiting protein synthesis controlled by an essential nutrient-responsive translation factor, eIF4G/IFG-1, acts as a lever to restore robustness of stress responsiveness through the HSR. Previously, we showed that at least one other proteostasis mechanism, the ER unfolded protein response, is enhanced when translation is reduced in a manner that depends on the gene encoding the HSR transcription factor, hsf-1 [11]. Experiments with single-celled organisms and mammalian tissue culture showed that intracellular stress signaling pathways can cross-activate one another to maintain cellular proteostasis [30,55–62]. Thus, HSR priming may improve function of other proteostasis mechanisms, a possibility that future experiments will need to address.
4.3. Limitations
Like all research studies, our study does have limitations. Firstly, though C. elegans are a highly informative, simplistic model organism, further studies are needed to determine whether cell non-autonomous sigaling under conditions of reduced translation is conserved in more complex model organisms. Secondly, we conducted a limited RNAi screen that led to the importance of neuronal CBP-1 under conditions of low translation. However, CBP-1 is most likely not the only factor at play. A larger RNAi screen is needed to determine other pathways and key regulators that provide protection from proteostatic stress under low translation. Third, our studies commenced with the discovery of the importance of neuronal CBP-1 downstream of low translation, however more research is needed into the genes regulated by CBP-1 acetylation activity. Such genes would be great candidates for RNAi screens. Lastly, though CBP-1 is ubiquitously expressed, we focused our research on the importance of neuronal expression. Future studies can examine a broader role of CBP-1 in other tissues which may explain why we observed differing tissue-specific effects of low translation.
5. Conclusions
We previously found differential translation on the organismal scale when ifg-1 was inhibited in a manner consistent with antagonistic modulation between development and somatic maintenance. Results in the current study indicate that specific tissues mediate effects of this trade-off to different extents. Future studies analyzing tissue-specific translation changes may help resolve the different effects observed.
Supplementary Material
Funding
This work was supported by grants from the National Institutes of Health (R01AG062575) and by the Morris Scientific Discovery Fund. Research reported in this publication was also supported by an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of the National Institutes of Health under grant numbers P20GM0103423 and P20GM104318. Some strains were provided by the CGC, which is funded by NIH Office of Research Infrastructure Programs (P40 OD010440).
Footnotes
Availability of Data and Materials
Data tables and copies of raw western blots and gels are available in supplementary materials.
Ethics Approval and Consent to Participate
Studies using C. elegans were exempt from review and approval by the Institutional Animal Care and Use Committee at MDI Biological Laboratory in Salisbury Cove, Maine.
Conflict of Interest
The authors declare no conflict of interest.
Supplementary Material
Supplementary material associated with this article can be found, in the online version, at https://doi.org/10.31083/j.fbl2907264.
References
- [1].Dubnikov T, Ben-Gedalya T, Cohen E. Protein Quality Control in Health and Disease. Cold Spring Harbor Perspectives in Biology. 2017; 9: a023523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Fernando R, Drescher C, Nowotny K, Grune T, Castro JP. Impaired proteostasis during skeletal muscle aging. Free Radical Biology & Medicine. 2019; 132: 58–66. [DOI] [PubMed] [Google Scholar]
- [3].Ayyadevara S, Balasubramaniam M, Suri P, Mackintosh SG, Tackett AJ, Sullivan DH, et al. Proteins that accumulate with age in human skeletal-muscle aggregates contribute to declines in muscle mass and function in Caenorhabditis elegans. Aging. 2016; 8: 3486–3497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Kelly JW. Pharmacologic Approaches for Adapting Proteostasis in the Secretory Pathway to Ameliorate Protein Conformational Diseases. Cold Spring Harbor Perspectives in Biology. 2020; 12: a034108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Akerfelt M, Trouillet D, Mezger V, Sistonen L. Heat shock factors at a crossroad between stress and development. Annals of the New York Academy of Sciences. 2007; 1113: 15–27. [DOI] [PubMed] [Google Scholar]
- [6].Haynes CM, Ron D. The mitochondrial UPR - protecting organelle protein homeostasis. Journal of Cell Science. 2010; 123: 3849–3855. [DOI] [PubMed] [Google Scholar]
- [7].Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nature Reviews. Molecular Cell Biology 2007; 8: 519–529. [DOI] [PubMed] [Google Scholar]
- [8].Akerfelt M, Morimoto RI, Sistonen L. Heat shock factors: integrators of cell stress, development and lifespan. Nature Reviews. Molecular Cell Biology 2010; 11: 545–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Hebishy M, Shintouo CM, Dufait I, Debacq-Chainiaux F, Bautmans I, Njemini R. Heat shock proteins and cellular senescence in humans: A systematic review. Archives of Gerontology and Geriatrics. 2023; 113: 105057. [DOI] [PubMed] [Google Scholar]
- [10].Powers ET, Morimoto RI, Dillin A, Kelly JW, Balch WE. Biological and chemical approaches to diseases of proteostasis deficiency. Annual Review of Biochemistry. 2009; 78: 959–991. [DOI] [PubMed] [Google Scholar]
- [11].Howard AC, Rollins J, Snow S, Castor S, Rogers AN. Reducing translation through eIF4G/IFG-1 improves survival under ER stress that depends on heat shock factor HSF-1 in Caenorhabditis elegans. Aging Cell. 2016; 15: 1027–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Hansen M, Taubert S, Crawford D, Libina N, Lee SJ, Kenyon C. Lifespan extension by conditions that inhibit translation in Caenorhabditis elegans. Aging Cell. 2007; 6: 95–110. [DOI] [PubMed] [Google Scholar]
- [13].Steffen KK, MacKay VL, Kerr EO, Tsuchiya M, Hu D, Fox LA, et al. Yeast life span extension by depletion of 60s ribosomal subunits is mediated by Gcn4. Cell. 2008; 133: 292–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Kapahi P, Chen D, Rogers AN, Katewa SD, Li PWL, Thomas EL, et al. With TOR, less is more: a key role for the conserved nutrient-sensing TOR pathway in aging. Cell Metabolism. 2010; 11: 453–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Kapahi P, Zid B. TOR pathway: linking nutrient sensing to life span. Science of Aging Knowledge Environment: SAGE KE. 2004; 2004: PE34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Kapahi P, Vijg J. Aging–lost in translation? The New England Journal of Medicine. 2009; 361: 2669–2670. [DOI] [PubMed] [Google Scholar]
- [17].Kaeberlein M, Kennedy BK. Hot topics in aging research: protein translation and TOR signaling, 2010. Aging Cell. 2011; 10: 185–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Hershey JWB, Sonenberg N, Mathews MB. Principles of translational control: an overview. Cold Spring Harbor Perspectives in Biology. 2012; 4: a011528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Kaiser C, Dobrikova EY, Bradrick SS, Shveygert M, Herbert JT, Gromeier M. Activation of cap-independent translation by variant eukaryotic initiation factor 4G in vivo. RNA (New York, N.Y.). 2008; 14: 2170–2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Berset C, Trachsel H, Altmann M. The TOR (target of rapamycin) signal transduction pathway regulates the stability of translation initiation factor eIF4G in the yeast Saccharomyces cerevisiae. Proceedings of the National Academy of Sciences of the United States of America. 1998; 95: 4264–4269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Ramírez-Valle F, Braunstein S, Zavadil J, Formenti SC, Schneider RJ. eIF4GI links nutrient sensing by mTOR to cell proliferation and inhibition of autophagy. The Journal of Cell Biology. 2008; 181: 293–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Thoreen CC, Chantranupong L, Keys HR, Wang T, Gray NS, Sabatini DM. A unifying model for mTORC1-mediated regulation of mRNA translation. Nature. 2012; 485: 109–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Brown JAL, Roberts TL, Richards R, Woods R, Birrell G, Lim YC, et al. A novel role for hSMG-1 in stress granule formation. Molecular and Cellular Biology. 2011; 31: 4417–4429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Cuesta R, Laroia G, Schneider RJ. Chaperone hsp27 inhibits translation during heat shock by binding eIF4G and facilitating dissociation of cap-initiation complexes. Genes & Development. 2000; 14: 1460–1470. [PMC free article] [PubMed] [Google Scholar]
- [25].Ben-Zvi A, Miller EA, Morimoto RI. Collapse of proteostasis represents an early molecular event in Caenorhabditis elegans aging. Proceedings of the National Academy of Sciences of the United States of America. 2009; 106: 14914–14919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Labbadia J, Morimoto RI. Repression of the Heat Shock Response Is a Programmed Event at the Onset of Reproduction. Molecular Cell. 2015; 59: 639–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Durieux J, Wolff S, Dillin A. The cell-non-autonomous nature of electron transport chain-mediated longevity. Cell. 2011; 144: 79–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].O’Brien D, van Oosten-Hawle P. Regulation of cell-non-autonomous proteostasis in metazoans. Essays in Biochemistry. 2016; 60: 133–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Prahlad V, Cornelius T, Morimoto RI. Regulation of the cellular heat shock response in Caenorhabditis elegans by thermosensory neurons. Science (New York, N.Y.). 2008; 320: 811–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Senft D, Ronai ZA. UPR, autophagy, and mitochondria crosstalk underlies the ER stress response. Trends in Biochemical Sciences. 2015; 40: 141–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Takeuchi T, Suzuki M, Fujikake N, Popiel HA, Kikuchi H, Futaki S, et al. Intercellular chaperone transmission via exosomes contributes to maintenance of protein homeostasis at the organismal level. Proceedings of the National Academy of Sciences of the United States of America. 2015; 112: E2497–E2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Taylor RC, Dillin A. XBP-1 is a cell-nonautonomous regulator of stress resistance and longevity. Cell. 2013; 153: 1435–1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Howard AC, Mir D, Snow S, Horrocks J, Sayed H, Ma Z, et al. Anabolic Function Downstream of TOR Controls Trade-offs Between Longevity and Reproduction at the Level of Specific Tissues in C. elegans. Frontiers in Aging. 2021; 2: 725068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Kirkwood TB. Evolution of ageing. Nature. 1977; 270: 301–304. [DOI] [PubMed] [Google Scholar]
- [35].Maklakov AA, Chapman T. Evolution of ageing as a tangle of trade-offs: energy versus function. Proceedings. Biological Sciences 2019; 286: 20191604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Werner EE, Hall DJ. Optimal Foraging and the Size Selection of Prey by the Bluegill Sunfish (Lepomis Macrochirus). Ecology. 1974; 55: 1042–1052. [Google Scholar]
- [37].Raubenheimer D, Simpson SJ. Nutritional ecology and foraging theory. Current Opinion in Insect Science. 2018; 27: 38–45. [DOI] [PubMed] [Google Scholar]
- [38].Abrahms B, Aikens EO, Armstrong JB, Deacy WW, Kauffman MJ, Merkle JA. Emerging Perspectives on Resource Tracking and Animal Movement Ecology. Trends in Ecology & Evolution. 2021; 36: 308–320. [DOI] [PubMed] [Google Scholar]
- [39].Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974; 77: 71–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Fraser AG, Kamath RS, Zipperlen P, Martinez-Campos M, Sohrmann M, Ahringer J. Functional genomic analysis of C. elegans chromosome I by systematic RNA interference. Nature. 2000; 408: 325–330. [DOI] [PubMed] [Google Scholar]
- [41].Cai L, Phong BL, Fisher AL, Wang Z. Regulation of fertility, survival, and cuticle collagen function by the Caenorhabditis elegans eaf-1 and ell-1 genes. The Journal of Biological Chemistry. 2011; 286: 35915–35921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Espelt MV, Estevez AY, Yin X, Strange K. Oscillatory Ca2+ signaling in the isolated Caenorhabditis elegans intestine: role of the inositol-1,4,5-trisphosphate receptor and phospholipases C beta and gamma. The Journal of General Physiology. 2005; 126: 379–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Simmer F, Moorman C, van der Linden AM, Kuijk E, van den Berghe PVE, Kamath RS, et al. Genome-wide RNAi of C. elegans using the hypersensitive rrf-3 strain reveals novel gene functions. PLoS Biology. 2003; 1: E12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Calixto A, Chelur D, Topalidou I, Chen X, Chalfie M. Enhanced neuronal RNAi in C. elegans using SID-1. Nature Methods. 2010; 7: 554–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Hartl FU, Bracher A, Hayer-Hartl M. Molecular chaperones in protein folding and proteostasis. Nature. 2011; 475: 324–332. [DOI] [PubMed] [Google Scholar]
- [46].Baird NA, Douglas PM, Simic MS, Grant AR, Moresco JJ, Wolff SC, et al. HSF-1-mediated cytoskeletal integrity determines thermotolerance and life span. Science (New York, N.Y.). 2014; 346: 360–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].van Ham TJ, Thijssen KL, Breitling R, Hofstra RMW, Plasterk RHA, Nollen EAA. C. elegans model identifies genetic modifiers of alpha-synuclein inclusion formation during aging. PLoS Genetics. 2008; 4: e1000027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Adamla F, Ignatova Z. Somatic expression of unc-54 and vha-6 mRNAs declines but not pan-neuronal rgef-1 and unc-119 expression in aging Caenorhabditis elegans. Scientific Reports. 2015; 5: 10692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Zhang M, Poplawski M, Yen K, Cheng H, Bloss E, Zhu X, et al. Role of CBP and SATB-1 in aging, dietary restriction, and insulin-like signaling. PLoS Biology. 2009; 7: e1000245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Cai H, Dhondt I, Vandemeulebroucke L, Vlaeminck C, Rasulova M, Braeckman BP. CBP-1 Acts in GABAergic Neurons to Double Life Span in Axenically Cultured Caenorhabditis elegans. The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences 2019; 74: 1198–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Korzus E, Rosenfeld MG, Mayford M. CBP histone acetyltransferase activity is a critical component of memory consolidation. Neuron. 2004; 42: 961–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Hempenstall S, Page MM, Wallen KR, Selman C. Dietary restriction increases skeletal muscle mitochondrial respiration but not mitochondrial content in C57BL/6 mice. Mechanisms of Ageing and Development. 2012; 133: 37–45. [DOI] [PubMed] [Google Scholar]
- [53].Cerletti M, Jang YC, Finley LWS, Haigis MC, Wagers AJ. Short-term calorie restriction enhances skeletal muscle stem cell function. Cell Stem Cell. 2012; 10: 515–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Pechmann S, Willmund F, Frydman J. The ribosome as a hub for protein quality control. Molecular Cell. 2013; 49: 411–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Adachi M, Liu Y, Fujii K, Calderwood SK, Nakai A, Imai K, et al. Oxidative stress impairs the heat stress response and delays unfolded protein recovery. PLoS ONE. 2009; 4: e7719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Baker BM, Nargund AM, Sun T, Haynes CM. Protective coupling of mitochondrial function and protein synthesis via the eIF2α kinase GCN-2. PLoS Genetics. 2012; 8: e1002760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Hou J, Tang H, Liu Z, Österlund T, Nielsen J, Petranovic D. Management of the endoplasmic reticulum stress by activation of the heat shock response in yeast. FEMS Yeast Research. 2014; 14: 481–494. [DOI] [PubMed] [Google Scholar]
- [58].Kaushik S, Cuervo AM. Proteostasis and aging. Nature Medicine. 2015; 21: 1406–1415. [DOI] [PubMed] [Google Scholar]
- [59].Labbadia J, Morimoto RI. The biology of proteostasis in aging and disease. Annual Review of Biochemistry. 2015; 84: 435–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Liu Y, Chang A. Heat shock response relieves ER stress. The EMBO Journal. 2008; 27: 1049–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Taylor RC, Berendzen KM, Dillin A. Systemic stress signalling: understanding the cell non-autonomous control of proteostasis. Nature Reviews. Molecular Cell Biology 2014; 15: 211–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Weindling E, Bar-Nun S. Sir2 links the unfolded protein response and the heat shock response in a stress response network. Biochemical and Biophysical Research Communications. 2015; 457: 473–478. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


