ABSTRACT
Cholera caused by Vibrio cholerae O139 emerged in the early 1990s and spread rapidly to 11 Asian countries before receding for unclear reasons. Protection against cholera is serogroup-specific, which is defined by the O-specific polysaccharide (OSP) component of lipopolysaccharide (LPS). V. cholerae O139 also expresses the OSP-capsule. We, therefore, assessed antibody responses targeting V. cholerae O139 OSP, LPS, capsule, and vibriocidal responses in patients in Bangladesh with cholera caused by V. cholerae O139. We compared these responses to those of age-gender-blood group-matched recipients of the bivalent oral cholera vaccine (OCV O1/O139). We found prominent OSP, LPS, and vibriocidal responses in patients, with a high correlation between these responses. OSP responses primarily targeted the terminal tetrasaccharide of OSP. Vaccinees developed OSP, LPS, and vibriocidal antibody responses, but of significantly lower magnitude and responder frequency (RF) than matched patients. We separately analyzed responses in pediatric vaccinees born after V. cholerae O139 had receded in Bangladesh. We found that OSP responses were boosted in children who had previously received a single dose of bivalent OCV 3 yr previously but not in vaccinated immunologically naïve children. Our results suggest that OSP-specific responses occur during cholera caused by V. cholerae O139 despite the presence of capsules, that vaccination with bivalent OCV is poorly immunogenic in the short term in immunologically naïve individuals, but that OSP-specific immune responses can be primed by previous exposure, although whether such responses can protect against O139 cholera is uncertain.
IMPORTANCE
Cholera is a severe dehydrating illness in humans caused by Vibrio cholerae serogroups O1 or O139. Protection against cholera is serogroup-specific, which is defined by the O-specific polysaccharide (OSP) of V. cholerae LPS. Yet, little is known about immunity to O139 OSP. In this study, we assessed immune responses targeting OSP in patients from an endemic region with cholera caused by V. cholerae O139. We compared these responses to those of the age-gender-blood group-matched recipients of the bivalent oral cholera vaccine. Our results suggest that OSP-specific responses occur during cholera caused by V. cholerae O139 and that the OSP responses primarily target the terminal tetrasaccharide of OSP. Our results further suggest that vaccination with the bivalent vaccine is poorly immunogenic in the short term for inducing O139-specific OSP responses in immunologically naïve individuals, but OSP-specific immune responses can be primed by previous exposure or vaccination.
KEYWORDS: O-specific polysaccharide, Vibrio cholerae O139, cholera, immune response, oral cholera vaccine
INTRODUCTION
Epidemic cholera has been associated with two serogroups of Vibrio cholerae: O1 and O139 (1). Cholera caused by V. cholerae O139 emerged in the early 1990s and spread rapidly to 11 Asian countries before receding for unclear reasons (2 – 5). Although V. cholerae O139 has not been associated with large outbreaks for over a decade, it is still reported sporadically in Bangladesh (6 – 8), India (9), and China (10), indicating that it could re-emerge as a public health concern (11). Protection against cholera is serogroup-specific, and serogroup-specificity is defined by the O-specific polysaccharide (OSP) component of bacterial lipopolysaccharide (LPS) (12 – 14). Although the oligosaccharide cores of V. cholerae O1 and O139 are the same (15), the OSP of V. cholerae O139 is distinct from that of V. cholerae O1. The latter is comprised of repetitive N-(3-deoxy-L-glycero-tetronyl)-D-perosamines (16), while the former is a single hexasaccharide (17 – 20). Furthermore, V. cholerae O139 expresses a capsule comprised of a polymer of the OSP-hexasaccharide (15, 18 – 21), while V. cholerae O1 is unencapsulated. We have previously characterized the immune response to O139 OSP and its fragments using pooled human patient serum samples and found that the terminal tetrasaccharide of the OSP-hexasaccharide is particularly immunogenic (22). Despite the importance of OSP-specific responses in mediating protection against cholera caused by V. cholerae O1 (23), little is known about OSP-specific responses in individual patients infected with V. cholerae O139 and in vaccine recipients of bivalent (O1/O139) oral killed cholera vaccine (OCV) currently included in the global stockpile of cholera vaccine and used in cholera control programs. Here, we characterize O139 OSP-specific antibody responses in individual patients with cholera caused by V. cholerae O139 during an outbreak in 2002 in Bangladesh and compare these responses to those induced by OCV in the age-gender-blood group matched participants in a vaccine study in Bangladesh in 2017.
MATERIALS AND METHODS
Study design and specimen collection
This study used samples from 23 diarrheal patients admitted at the icddr,b hospital, Dhaka, Bangladesh, during a cholera epidemic in 2002 (24). All patients had V. cholerae O139 isolated from stool and had moderate to severe dehydration from diarrhea. In addition, this study included samples from 23 cholera vaccinees from an OCV clinical trial conducted in Mirpur, Dhaka in 2017, who were matched to patients by age, sex, and blood group (O versus non-O) (25). All vaccinees received two doses of bivalent (O1/O139) oral killed cholera vaccine (Shanchol; Sanofi-Shanta Biotech, India) separated by 2 weeks. Samples from 20 young children vaccinees <5 yr of age who participated in a 2014 vaccine trial, and the 2017 vaccine trial described above were also included in this analysis. Among these 20 vaccinees, 10 had previously received a single dose of bivalent (O1/O139) vaccine in 2014, while the other 10 had received a placebo in the 2014 single dose trial of oral cholera vaccine conducted in Dhaka (26, 27). These 20 children were subsequently included in the 2017 two-dose evaluation of the bivalent (O1/O139) vaccine in Bangladesh, affording us the ability to assess whether previously confirmed exposure to bivalent (O1/O139) vaccine could facilitate boosting of anti-O139 antibody responses during subsequent vaccination (25). The demographic characteristics of all the participants included in the study are provided in Tables 1 and 2.
TABLE 1.
Demographic characteristics of the cholera adult patients (2002) and vaccinees (2017)
| Characteristics | Patients | Vaccinees | P values |
|---|---|---|---|
| Sex | |||
| Male, number (%) | 11 (47.8) | 12 (52.2) | 1.00 |
| Female, number (%) | 12 (52.2) | 11 (47.8) | |
| Median age, year (25th, 75th percentiles) | 30.8 (22.8, 35.8) | 29.5 (21.5, 37) | 0.9125 |
| Blood group | |||
| O, number (%) | 10 (43.5) | 7 (30.4) | 1.00 |
| Non-O, number (%) | 13 (56.5) | 16 (69.6) |
TABLE 2.
Demographic characteristics of recipients under 5 yr of age of two doses O1/O139 vaccine in 2017, who had previously received a placebo or a single dose of O1/O139 vaccine in 2014
| Characteristics | Vaccinees receiving prior dose | Vaccinees receiving no prior dose | |
|---|---|---|---|
| Sex | |||
| Male, number (%) | 5 (50) | 5 (50) | |
| Female, number (%) | 5 (50) | 5 (50) | |
| Median age, year (25th, 75th percentile) | 4 (4, 4) | 4 (4, 4) | |
| Blood group | |||
| O, number (%) | 5 (50) | 5 (50) | |
| Non-O, number (%) | 5 (50) | 5 (50) | |
All samples were collected following written informed consent and/or assent, including permission for subsequent analysis. The 2014 vaccine study was approved by the Institutional Review Board of icddr,b, and the 2017 vaccine study was approved by the Institutional Review Boards of both icddr,b and the International Vaccine Institute (IVI). The collection and analysis of clinical samples from patients with V. cholerae O139 and use of all samples for this current analysis were approved by the Institutional Review Boards of icddr,b and the Massachusetts General Hospital. Venous blood was drawn from patients 2 days after hospitalization and clinical stabilization and then on days 7 and 21; venous blood was drawn from vaccinees on days 0, 14, and 28. Vaccinees in the 2017 cohort received bivalent (O1/O139) vaccine on days 0 and 14. All blood samples were collected and separated to obtain serum or plasma, which were subsequently stored at −80°C until further use in immunological assays.
Preparation of lipopolysaccharide, O-specific polysaccharide, its terminal tetrasaccharide, and capsule from V. cholerae O139
LPS and OSP-core from V. cholerae O139 strain CIRS245, synthetic terminal tetrasaccharide, and OSP and its terminal tetrasaccharide fragment conjugated to bovine serum albumin (BSA; for immunologic assays) were generated as previously described (17, 22). Capsule was purified from V. cholerae O139 strain CIRS245 via phenol-water extraction. Briefly, capsule was obtained by repeated (twice) extraction of wet biomass of CIRS245 with 1:1 phenol (90%)—water (v/v) at 68°C for 30 min. The extract was dialyzed against tap water for 24 h followed by deionized water for 2–4 h using a MWCO 12,000 membrane (Serva, Germany). Separation of capsule was achieved by ultracentrifugation of retentate (5 h; 136,057 × g; 4°C). Supernatant containing capsule and nucleic acids was freeze-dried, then suspended in 50 mM Tris-HCl (pH 7.5) with 1 mM CaCl2 and 2 mM MgCl2 (40 mL/10 mg of material) and incubated with 50 µg DNase from bovine pancreas (Sigma, Germany) along with 50 µg RNase A from bovine pancreas (Sigma, Germany) overnight at 37°C, followed by 100 µg Proteinase K from Tritirachium album (Sigma, Germany) for 4 h at 37°C. Enzymes were inactivated by heating at 80°C for 1 h, and the material was dialyzed through a MWCO 3500 membrane (Serva, Germany). The capsule was further purified by repeated (three times) ultrafiltration using 100,000 MWCO Amicon Ultra 4 mL filters (Merck, Germany) followed by size exclusion chromatography on a Bio-Gel P30 column (Bio-Rad Laboratories, USA) eluted with water. The first eluting double peak detected in the refractive index chromatogram corresponding to the capsule was concentrated and freeze-dried. Absence of nucleic acids and proteins was verified spectrophotometrically using Shimadzu UVmini-1240 UV-VIS spectrophotometer by measuring absorbance at 256 nm and 280 nm, respectively. In order to remove residual traces of LPS detected on SDS-PAGE using Bio-Rad Mini-PROTEAN Cell system, an extra step of ultracentrifugation was performed as described above.
Antibody responses to O-specific polysaccharide, terminal tetrasaccharide, lipopolysaccharide, and capsule of V. cholerae O139
Antibody responses in plasma or serum against O-specific polysaccharide, terminal tetrasaccharide, lipopolysaccharide, and capsule of V. cholerae O139 were assessed using ELISA as previously described (28, 29). Briefly, 96-well polystyrene plates (NuncF, Denmark) were coated per well with 100 µL of the specific antigen (OSP:BSA, LPS, tetrasaccharide:BSA, capsule) and then blocked in 1% BSA (Sigma, St. Louis, MO) in phosphate-buffered saline (PBS). All antigens were coated in 50 mM carbonate buffer (pH 9.6) at a concentration of 1 µg/mL, except for LPS, which was coated at 2.5 µg/mL. Each serum or plasma sample was diluted 1:50 in 0.1% BSA in PBS-0.05% Tween and added in triplicate (100 µL per well) to the coated plate, followed by incubation for 90 min at 37°C. Antigen-specific IgA, IgG, and IgM antibodies in samples were detected using horseradish peroxidase-conjugated rabbit anti-human antibodies of the relevant isotype (1:1,000 dilution; Jackson ImmunoResearch, West Grove, PA) as secondary antibodies. Following further incubation for 90 min, bound secondary antibodies were detected using o-phenylene diamine (Sigma, St. Louis, MO) in 0.1 M sodium citrate buffer (pH 4.5) and 0.012% hydrogen peroxide. Optical density was measured at 450 nm for 5 min at 14 s intervals, and the rate of change in optical density was expressed as milli-absorbance units per minute. The mean absorbance value for each sample was normalized to ELISA units by dividing the value for the sample against that of a pooled convalescent-phase sera standard. A responder was defined as any patient or vaccinee who had a ≥1.5-fold increase in ELISA units at the assessed time compared to day 2 or day 0, respectively.
Vibriocidal antibody assay
Vibriocidal antibody titers were measured using serum or plasma from patients and vaccinees as previously described with a less encapsulated strain of V. cholerae O139 CIRS134B as the target organism (24). The vibriocidal titer was defined as the reciprocal of the highest dilution of the serum or plasma, resulting in a greater than 50% reduction in optical density compared to that of control wells containing no serum or plasma. A responder was defined as any patient or vaccinee who had a ≥fourfold increase in vibriocidal titer at the assessed time point compared to day 2 or day 0, depending on the cohort.
Statistical analyses
Differences in demographic characteristics between the two groups were tested using the Wilcoxon signed-rank test or the Fischer’s exact test, as appropriate. Differences in immune responses within groups were assessed using the Wilcoxon signed-rank test, while differences between groups were assessed using the Mann–Whitney U test. Linear relationships between immune responses were evaluated using the Spearman rank correlation coefficient. All reported P values were two-tailed, with a cutoff of P ≤ 0.05 considered a threshold for statistical significance. All statistical analyses were performed using GraphPad Prism 7 (GraphPad Software, Inc.).
RESULTS
Plasma antibody responses to OSP, tetrasaccharide, LPS, and capsule
We assessed antibody responses in plasma or serum against OSP, the terminal tetrasaccharide of OSP, LPS, and capsule of V. cholerae O139 in patients and vaccinees across all isotypes and across all three time points. In patients, plasma IgA, IgG, and IgM antibodies targeting OSP (Fig. 1), its terminal tetrasaccharide (Fig. 2), and LPS (Fig. 3) increased significantly at day 7 (early convalescent stage) compared to day 2 (acute stage) and remained significantly elevated at day 21 (late convalescent stage) compared to baseline. Similarly, in vaccinees, plasma IgA, IgG, and IgM antibodies against OSP, tetrasaccharide, and LPS increased significantly at day 14 (14 days after the first dose of vaccine) compared to day 0 and remained significantly elevated at day 28 (14 days after the second vaccination) compared to baseline, except for anti-OSP IgM responses that had returned to baseline on day 28 (Fig. 1 to 3). No significant increases in IgA, IgG, and IgM antibodies against the capsule of V. cholerae O139 were observed in patients or vaccinees (Fig. S1).
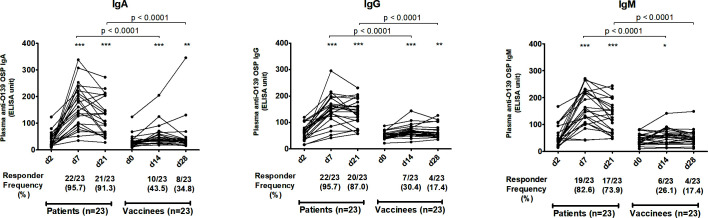
Fig 1.
Plasma antibody responses against V. cholerae O139 O-specific polysaccharide in naturally infected patients and bivalent O1/O139 vaccinees. P values represent statistical differences in the mean between patients and vaccinees. Asterisks represent statistically significant differences in immune responses within patients or vaccinees compared to baseline (***P ≤ 0.001, **P ≤ 0.01, and *P ≤ 0.05). Responder frequencies are shown in parentheses below the X-axes (see text).
Fig 2.
Plasma antibody responses against V. cholerae O139 terminal tetrasaccharide of O-specific polysaccharide in naturally infected patients and bivalent O1/O139 vaccinees. P values represent statistical differences in the mean between patients and vaccinees. Asterisks represent statistically significant differences in immune responses within patients or vaccinees compared to baseline (***P ≤ 0.001, **P ≤ 0.01, and *P ≤ 0.05). Responder frequencies are shown in parentheses below the X-axes (see text).
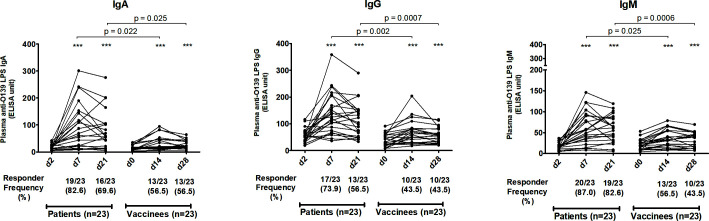
Fig 3.
Plasma antibody responses against V. cholerae O139 lipopolysaccharide in naturally infected patients and bivalent O1/O139 vaccinees. P values represent statistical differences of the mean between patients and vaccinees. Asterisks represent statistically significant differences in immune responses within patients or vaccinees compared to baseline (***P ≤ 0.001, **P ≤ 0.01, and *P ≤ 0.05). Responder frequencies are shown in parentheses below the X-axes (see text).
The magnitude of antibody responses against OSP, the OSP terminal tetrasaccharide, and LPS was significantly higher in patients compared to those in vaccinees in all antibody isotypes and at all follow-up time points (Fig. 1 to 3). Responder frequencies were also significantly higher in patients compared to vaccinees (Fig. 1 to 3).
Significant correlations were observed between anti-OSP and anti-terminal tetrasaccharide antibodies, anti-LPS and anti-OSP antibodies, and between anti-LPS and anti-tetrasaccharide antibodies in both patients and vaccinees (Fig. S2).
Vibriocidal antibody responses
We assessed vibriocidal antibody titers in plasma against V. cholerae O139 in both patients and vaccinees at all time points (Fig. 4). Vibriocidal responses in patients peaked on day 7 compared to day 2 and remained significantly elevated over baseline on day 21. Similarly, vibriocidal responses in vaccinees also increased significantly after the first dose of vaccine compared to baseline. There was no significant boosting of vibriocidal responses after the second dose of vaccine but values remained higher than baseline. Vibriocidal responses were significantly higher in patients than in vaccinees across all follow-up time points, with no differences in responses between groups at baseline. Vibriocidal responder frequency (RF) was also significantly higher in patients compared to vaccinees (Fig. 4).
Fig 4.
Vibriocidal antibody responses against V. cholerae O139 strain CIRS134B (thinly encapsulated) in naturally infected patients and bivalent O1/O139 vaccinees. P values represent statistical differences of the mean between patients and vaccinees. Asterisks represent statistically significant differences in vibriocidal titer within patients or vaccinees compared to baseline (***P ≤ 0.001, **P ≤ 0.01, and *P ≤ 0.05). Responder frequency) is defined as the percentage of subjects with a ≥fourfold increase in vibriocidal titer over the baseline.
Comparison of plasma antibody responses to OSP, terminal tetrasaccharide, and LPS with vibriocidal antibody responses
We compared vibriocidal antibody responses against responses to OSP, terminal tetrasaccharide of OSP, and LPS in both patients and vaccinees (Fig. S2). IgM responses to these antigens are best correlated with vibriocidal responses among isotype responses. IgA and IgG responses to these antigens also significantly correlated with vibriocidal responses in both patients and vaccinees, except for IgA antibody responses against terminal tetrasaccharide in vaccinees (Fig. S2).
Comparison of plasma antibody responses to OSP related to baseline vibriocidal antibody responses
In order to assess whether previous exposure to V. cholerae O139 had an effect on subsequent immune responses, we compared anti-OSP responses within patients (Fig. S3) and within vaccinees (Fig. S4) separately based on baseline vibriocidal titer. No differences in IgA, IgG, and IgM anti-OSP responses were observed at any later time points between study participants with baseline vibriocidal titers ≥80 versus <80.
Plasma antibody responses to OSP, LPS, and vibriocidal antibody responses of vaccine recipients in young children
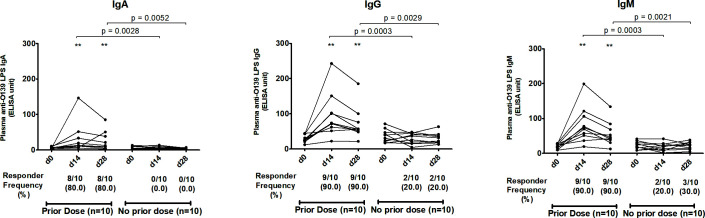
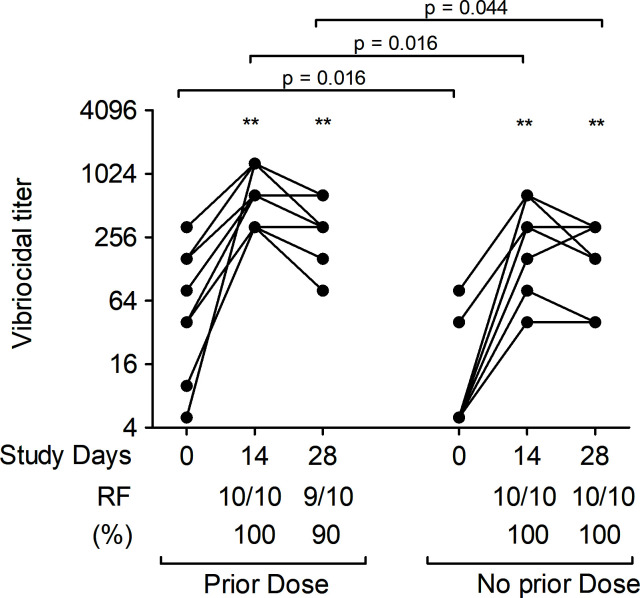
In order to further address the potential impact of previous exposure to V. cholerae O139, we assessed anti-OSP, anti-LPS, and vibriocidal antibody responses in 20 child vaccinees who were under 5 yr of age in 2017. These individuals were chosen since they had been born after V. cholerae O139 was widely circulating in Bangladesh, in order to lessen the possibility of any previous unknown exposure to V. cholerae O139. Among these 20 vaccinees, we identified 10 who had received a single dose of OCV in a previous single dose vaccine study performed 3 yr previously in 2014 and 10 who had received only a placebo in that earlier study. Among these young children, only vaccinees who had received a prior single dose of OCV in 2014 mounted significant IgA, IgG, and IgM anti-OSP (Fig. 5) and anti-LPS (Fig. 6) responses against O139 at day 14 compared to day 0, with the responses remaining significantly higher than baseline on day 28. Interestingly, unlike anti-OSP and anti-LPS responses that were only seen in previously vaccinated children, significant vibriocidal responses were observed at day 14 compared to day 0 among both groups of vaccinees (Fig. 7).
Fig 5.
Boosting effect in children vaccinees (<5 yr of age) against V. cholerae O139 O-specific polysaccharide. P values represent statistical differences of the mean between the two groups of recipients of 2-dose O1/O139 vaccine in 2017: those who received a single dose of O1/O139 vaccine 3 yr previously and those who received a single dose of placebo then. Asterisks represent statistically significant differences in immune responses within the vaccinee group compared to baseline (***P ≤ 0.001, **P ≤ 0.01, and *P ≤ 0.05). Responder frequencies are shown in parentheses below the X-axes (see text).
Fig 6.
Boosting effect in children (<5 yr of age) receiving a bivalent O1/O139 vaccine against V. cholerae O139 lipopolysaccharide). P values represent statistical differences of the mean between the two groups of recipients of two-dose O1/O139 vaccine in 2017: those who received a single dose of O1/O139 vaccine 3 yr previously and those who received a single dose of placebo then. Asterisks represent statistically significant differences in immune responses within the vaccinee group compared to baseline (***P ≤ 0.001, **P ≤ 0.01, and *P ≤ 0.05). Responder frequencies are shown in parentheses below the X-axes (see text).
Fig 7.
Vibriocidal antibody responses against V. cholerae O139 strain CIRS134B (thinly encapsulated) in children (<5 yr of age) receiving a bivalent O1/O139 vaccine. P values represent statistical differences of the mean between the two groups of recipients of two-dose O1/O139 vaccine in 2017: those who had received a single dose of O1/O139 vaccine 3 yr previously and those who had received a single dose of placebo then. Asterisks represent statistically significant differences in vibriocidal titer within the group compared to baseline (***P ≤ 0.001, **P ≤ 0.01, and *P ≤ 0.05). Responder frequency is defined as the percentage of participants with a ≥ fourfold increase in titer over the baseline.
DISCUSSION
Protection against cholera is serogroup-specific, and serogroup-specificity is determined by the O-specific polysaccharide component of bacterial LPS (13). Previous infection with V. cholerae O1 does not provide protection against O139 and vice versa (12), despite the fact that V. cholerae O139 evolved from V. cholerae O1 and expresses identical proteins including cholera toxin (11). The major difference between V. cholerae O139 and O1 is in the rfb genes involved in OSP synthesis (21, 30, 31) and the presence of a capsule in O139, with the O139 capsule comprised of a polymer of O139 OSP (15, 18 – 21). Specifically, the OSP of V. cholerae O1 is comprised of 10–20 repetitive (1→2)-α-linked (4-N-3-deoxy-l-glycero-tetronyl)-perosamines, with or without a 2-O-methyl group on the terminal saccharide determining the Ogawa or Inaba serogroup, respectively (32, 33). In comparison, the OSP of V. cholerae O139 is a single hexasaccharide containing N-acetyl-d-quinovosamine (QuiNAc), d-galacturonic acid (GalA), N-acetyl-d-glucosamine (GlcNAc), d-galactose (Gal), and two colitose (Col) residues. It contains two negatively charged groups: a carboxyl group of d-galacturonic acid and a cyclic phosphate bound to O-4 and O-6 of d-galactose (18, 19, 34). The capsule of V. cholerae O139 contains a flexible, complex, and branched polymer of this hexasaccharide (18, 19, 34).
In our analysis, we found that patients with cholera caused by V. cholerae O139 develop prominent OSP-specific immune responses, despite the presence of a capsule. These immune responses mirrored immune responses against LPS and were largely directed against the terminal tetrasaccharide component of the O139 non-repeating OSP hexasaccharide. We have previously shown that the tetrasaccharide is the most immunogenic component of the V. cholerae O139 OSP (22), suggesting pocket-like interaction between antibody and antigen, as observed for V. cholerae O1 OSP (33) and other bacterial surface polysaccharides (35 – 38).
In our previous analysis profiling immune responses against a range of O139-related saccharides, we performed an analysis using pooled plasma samples (n = 10) collected on day 2 and day 7 from patients with cholera caused by V. cholerae O139 in Bangladesh and pooled samples (n = 10; enrollment and 7 d after receipt of vaccine dose one and 7 d after vaccine dose two) from Haitian recipients of bivalent OCV (22). Here, we report a detailed analysis at the individual patient/participant level of patients and vaccinees in Bangladesh, an area in which V. cholerae O139 had previously emerged. In our prior analysis of immune responses in Haitian vaccinees, we found only low-level IgM OSP-specific responses and no induction of IgA or IgG responses (22). In contrast, in our current analysis of immune responses in the age-gender-blood group matched vaccinees compared to naturally infected patients in Bangladesh, we found significant IgA, IgG, and IgM OSP-specific responses following vaccination, although these responses were less prominent than those induced following natural infection. It is possible that these higher immune responses following natural disease may reflect in part the impact of cholera toxin, a potent immunoadjuvant, during natural disease; the bivalent vaccine does not contain this immunoadjuvant. We considered that these different levels of immunogenicity in Bangladeshi vaccinees versus Haitian vaccinees may reflect different previous exposure to V. cholerae O139 in the two populations. V. cholerae O139 has never been reported in Haiti. In comparison, V. cholerae O139 caused large outbreaks for over a decade in the 1990s (2) and then smaller outbreaks in the early 2000s (39) in Bangladesh and is still occasionally identified in stool cultures (6, 8) and in the environment in Bangladesh (40, 41).
We were constrained in our current analysis in that available samples of serum of our naturally infected patients from 2002 were largely from adults (20 of 23 samples). Since we matched to this cohort, our vaccinee samples were, therefore, also largely from adults. The naturally infected samples had been collected in 2002, years before the vaccine study in 2017, since O139 had disappeared as a significant cause of cholera when the bivalent OCV was developed and deployed. As such, the adults included in our vaccine cohort were children when Bangladesh was afflicted by large outbreaks of cholera caused by O139, and our detection of O139-specific immune responses in our vaccinees could possibly represent a boosting response from previous undocumented exposure. To address this possibility, we first analyzed O139-specific OSP responses in Bangladeshi vaccinees based on a baseline vibriocidal titer of 80 or greater (possibly consistent with previous exposure). However, we were unable to discern significant differences in subsequent OSP responses by this cohort analysis, perhaps because of the poor predictive value of the vibriocidal parameter in O139 (42, 43) and its unknown predictive value to identify a possible exposure 10–20 yr prior.
We, therefore, performed an additional analysis using vaccinees who were children born after V. cholerae O139 had receded in Bangladesh. In this sub-analysis, we had samples from children who received the standard two doses of bivalent vaccine (day 0 and 14) in 2017 and who had been randomized to receive either a single dose of bivalent OCV or a placebo in 2014. We found no significant induction of immune responses against OSP in immunologically naïve Bangladeshi children (vaccine recipients in 2017 who were born after O139 cholera had receded in Bangladesh and who had received a placebo in the previous single-dose vaccine study in 2014). However, we detected significant responses in children who had received a prior single dose of bivalent (O1/O139) vaccine 3 yr previously. These results are consistent with the poor O139 OSP-specific immunogenicity of initial vaccination with bivalent OCV that we observed in immunologically naïve Haitian vaccinees (22) but also suggest that such vaccination can prime OSP and related O139-specific responses that can subsequently be boosted. As such, previous exposure or vaccination would suggest induction of memory responses.
We found a high correlation of OSP-hexasaccharide, OSP-terminal tetrasaccharide, LPS, and vibriocidal responses following natural infection across antibody isotypes (IgA, IgM, and IgG). Vibriocidal responses during V. cholerae O139 can vary depending on the strain, capsule thickness, growth media, inoculum size, and concentration of complement (44). To maximize the detection of vibriocidal responses, we used a thinly encapsulated V. cholerae O139 strain (22, 24). Although we found a correlation between OSP responses and vibriocidal responses in vaccinees, the degree of these correlations was less prominent than those observed following natural disease. Specifically, a number of vaccinees had moderate vibriocidal responses in the setting of low OSP-specific responses. We have previously shown that the affinity of V. cholerae O1 OSP-specific antibodies does not predict functionality, including vibriocidal activity (45); specifically, low-affinity OSP-specific antibodies can have significant vibriocidal activity, and our current observations may reflect this effect. Another possibility is that these responses could reflect the presence of antibodies to antigens other than OSP. We also found that IgA antibodies that targeted terminal tetrasaccharide in vaccinees did not correlate with vibriocidal responses. This could be attributed to the fact that the vibriocidal response is a surrogate marker of protection against cholera and assesses complement-mediated bacterial lysis in vitro (43), while IgA does not bind complement via the classical pathway.
V. cholerae O139 is encapsulated, and the capsule is comprised of a polymer of the V. cholerae O139 OSP hexasaccharide (15, 18 – 21). Despite our ability to detect antibody responses to the O139 OSP hexasaccharide, we were unable to detect significant increases in antibody responses to the capsule in both patients and vaccinees, perhaps suggesting altered immunologic display of the polysaccharide in its polymerized form, and perhaps underscoring that the ability of anti-OSP and anti-LPS antibodies to provide mechanistic protection against O139 cholera is yet to be determined.
In summary, naturally acquired cholera caused by V. cholerae O139 in an endemic area induces prominent OSP-specific responses despite capsule. These responses correlate with LPS and vibriocidal responses and are primarily directed against the terminal tetrasaccharide of OSP. In comparison, the bivalent OCV does not induce detectable OSP responses in immunologically naïve individuals but boosts OSP-specific responses in individuals previously exposed (or primed) to V. cholerae O139 antigens via community exposure or vaccination. The degree of protection afforded by such priming is unknown. Indeed, the ability of bivalent OCV to protect against cholera caused by V. cholerae O139 has never been demonstrated, and efficacy can no longer be easily assessed since the incidence of V. cholerae O139 infection is now rare; assessing protection afforded by vaccination would now require use of a human challenge model. Moreover, since O139 has not been of public health importance for many years, new simplified versions of OCV are being developed that do not contain V. cholerae O139 (46). It is unknown why V. cholerae O139 has receded but should it re-emerge, our data suggest that oral ingestion of killed O139 can prime immune responses. However, it remains unclear whether such priming would protect against virulent V. cholerae O139.
ACKNOWLEDGMENTS
This research was supported through programs funded by the National Institutes of Health, including the National Institute of Allergy and Infectious Diseases (AI106878 [E.T.R. and F.Q.], AI099243 [J.B.H.], and AI137164 [R.C.C. and J.B H.]), the Fogarty International Center, Training Grant in Vaccine Development and Public Health (TW005572 [M.K.]), and Emerging Global Leader Award TW010362 (T.R.B.), the Intramural Research Program of the NIH and NIDDK (P.X. and P.K.), and the Operational Program Integrated Infrastructure for the project ITMS: 313021Y920, co-financed by the European Regional Development Fund (A.C.). The 2017 vaccine study was supported by grants from the Bill and Melinda Gates Foundation (OPP52797) and the government of South Korea to the International Vaccine Institute (IVI). icddr,b is also grateful to the governments of Bangladesh, Canada, Sweden, and the United Kingdom for providing core/unrestricted support. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
There were no conflicts of interest for any of the authors.
Contributor Information
Edward T. Ryan, Email: etryan@mgh.harvard.edu.
David W. Pascual, University of Florida, Gainesville, Florida, USA
DATA AVAILABILITY
The raw data supporting the findings of this article will be made fully available by the authors, without undue reservation.
SUPPLEMENTAL MATERIAL
The following material is available online at https://doi.org/10.1128/msphere.00255-23.
Plasma antibody responses against Vibrio cholerae O139 capsule in naturally infected patients, and bivalent O1/O139 vaccinees.
Correlation heatmap showing pairwise spearman correlation matrices of V. cholerae O139 antigen-specific antibody responses for patients (left) and bivalent vaccinees (right).
Differences in plasma antibody responses against Vibrio cholerae O139 O-specific polysaccharide (OSP) in naturally infected patients based on vibriocidal titer at "baseline" (day 2 post-infection).
Differences in plasma antibody responses against Vibrio cholerae O139 O-specific polysaccharide (OSP) in vaccinees based on vibriocidal titer on the day of first dose of vaccination.
ASM does not own the copyrights to Supplemental Material that may be linked to, or accessed through, an article. The authors have granted ASM a non-exclusive, world-wide license to publish the Supplemental Material files. Please contact the corresponding author directly for reuse.
REFERENCES
- 1. Harris JB, LaRocque RC, Qadri F, Ryan ET, Calderwood SB. 2012. Cholera. Lancet 379:2466–2476. doi: 10.1016/S0140-6736(12)60436-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Albert MJ, Ansaruzzaman M, Bardhan PK, Faruque ASG, Faruque SM, Islam MS, Mahalanabis D, Sack RB, Salam MA, Siddique AK, Yunus MD, Zaman K, Cholera Working Group, International Centre for Diarrhoeal Diseases Research, Bangladesh . 1993. Large epidemic of cholera-like disease in Bangladesh caused by Vibrio cholerae O139 synonym Bengal. The Lancet 342:387–390. doi: 10.1016/0140-6736(93)92811-7 [DOI] [PubMed] [Google Scholar]
- 3. Ramamurthy T, Garg S, Sharma R, Bhattacharya SK, Nair GB, Shimada T, Takeda T, Karasawa T, Kurazano H, Pal A. 1993. Emergence of novel strain of Vibrio cholerae with epidemic potential in southern and eastern India. Lancet 341:703–704. doi: 10.1016/0140-6736(93)90480-5 [DOI] [PubMed] [Google Scholar]
- 4. Nair GB, Ramamurthy T, Bhattacharya SK, Mukhopadhyay AK, Garg S, Bhattacharya MK, Takeda T, Shimada T, Takeda Y, Deb BC. 1994. Spread of Vibrio cholerae O139 Bengal in India. J Infect Dis 169:1029–1034. doi: 10.1093/infdis/169.5.1029 [DOI] [PubMed] [Google Scholar]
- 5. Bodhidatta L, Echeverria P, Hoge CW, Pitarangsi C, Serichantalergs O, Henprasert-Tae N, Harikul S, Kitpoka P. 1995. Vibrio cholerae O139 in Thailand in 1994. Epidemiol Infect 114:71–73. doi: 10.1017/s095026880005192x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chowdhury F, Mather AE, Begum YA, Asaduzzaman M, Baby N, Sharmin S, Biswas R, Ikhtear Uddin M, LaRocque RC, Harris JB, Calderwood SB, Ryan ET, Clemens JD, Thomson NR, Qadri F, Yang R. 2015. Vibrio cholerae serogroup O139: isolation from cholera patients and asymptomatic household family members in Bangladesh between 2013 and 2014. PLoS Negl Trop Dis 9:e0004183. doi: 10.1371/journal.pntd.0004183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Alam M, Hasan NA, Sadique A, Bhuiyan NA, Ahmed KU, Nusrin S, Nair GB, Siddique AK, Sack RB, Sack DA, Huq A, Colwell RR. 2006. Seasonal cholera caused by Vibrio cholerae serogroups O1 and O139 in the coastal aquatic environment of Bangladesh. Appl Environ Microbiol 72:4096–4104. doi: 10.1128/AEM.00066-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Parvin I, Shahid ASMSB, Das S, Shahrin L, Ackhter MM, Alam T, Khan SH, Chisti MJ, Clemens JD, Ahmed T, Sack DA, Faruque ASG. 2021. Vibrio cholerae O139 persists in Dhaka, Bangladesh since 1993. PLoS Negl Trop Dis 15:e0009721. doi: 10.1371/journal.pntd.0009721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pal BB, Mohanty A, Biswal B, Nayak SR. 2019. New variant of Vibrio cholerae O139 in Odisha, India. J Clin Microbiol 57:e01877-18. doi: 10.1128/JCM.01877-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhang P, Zhou H, Diao B, Li F, Du P, Li J, Kan B, Morris JG, Wang D. 2014. A molecular surveillance reveals the prevalence of Vibrio cholerae O139 isolates in China from 1993 to 2012. J Clin Microbiol 52:1146–1152. doi: 10.1128/JCM.03354-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ramamurthy T, Pragasam AK, Taylor-Brown A, Will RC, Vasudevan K, Das B, Srivastava SK, Chowdhury G, Mukhopadhyay AK, Dutta S, Veeraraghavan B, Thomson NR, Sharma NC, Nair GB, Takeda Y, Ghosh A, Dougan G, Mutreja A. 2022. Vibrio cholerae O139 genomes provide a clue to why it may have failed to usher in the eighth cholera pandemic. Nat Commun 13:3864. doi: 10.1038/s41467-022-31391-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Qadri F, Wennerås C, Albert MJ, Hossain J, Mannoor K, Begum YA, Mohi G, Salam MA, Sack RB, Svennerholm AM. 1997. Comparison of immune responses in patients infected with Vibrio cholerae O139 and O1. Infect Immun 65:3571–3576. doi: 10.1128/iai.65.9.3571-3576.1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ali M, Emch M, Park JK, Yunus M, Clemens J. 2011. Natural cholera infection-derived immunity in an endemic setting. J Infect Dis 204:912–918. doi: 10.1093/infdis/jir416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Johnson RA, Uddin T, Aktar A, Mohasin M, Alam MM, Chowdhury F, Harris JB, LaRocque RC, Bufano MK, Yu Y, Wu-Freeman Y, Leung DT, Sarracino D, Krastins B, Charles RC, Xu P, Kovác P, Calderwood SB, Qadri F, Ryan ET. 2012. Comparison of immune responses to the O-specific polysaccharide and lipopolysaccharide of Vibrio cholerae O1 in Bangladeshi adult patients with cholera. Clin Vaccine Immunol 19:1712–1721. doi: 10.1128/CVI.00321-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cox AD, Perry MB. 1996. Structural analysis of the lipopolysaccharide from Vibrio cholerae O139. Carbohydr Res 290:59–65. doi: 10.1016/0008-6215(96)00131-0 [DOI] [PubMed] [Google Scholar]
- 16. Hisatsune K, Kondo S, Isshiki Y, Iguchi T, Haishima Y. 1993. Occurrence of 2-O-methyl-N-(3-deoxy-L-glycero-tetronyl)-D-perosamine (4-amino-4,6-dideoxy-D-manno-pyranose) in lipopolysaccharide from Ogawa but not from Inaba O forms of O1 Vibrio cholerae. Biochem Biophys Res Commun 190:302–307. doi: 10.1006/bbrc.1993.1046 [DOI] [PubMed] [Google Scholar]
- 17. Xu P, Korcová J, Baráth P, Čížová A, Valáriková J, Qadri F, Kelly M, O’Connor RD, Ryan ET, Bystrický S, Kováč P. 2019. Isolation, purification, characterization and direct conjugation of the lipid A-free lipopolysaccharide of Vibrio cholerae O139. Chemistry 25:12946–12956. doi: 10.1002/chem.201902263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Knirel YA, Paredes L, Jansson PE, Weintraub A, Widmalm G, Albert MJ. 1995. Structure of the capsular polysaccharide of Vibrio cholerae O139 synonym Bengal containing D-galactose 4,6-cyclophosphate. Eur J Biochem 232:391–396. doi: 10.1111/j.1432-1033.1995.391zz.x [DOI] [PubMed] [Google Scholar]
- 19. Preston LM, Xu Q, Johnson JA, Joseph A, Maneval DR, Husain K, Reddy GP, Bush CA, Morris JG. 1995. Preliminary structure determination of the capsular polysaccharide of Vibrio cholerae O139 Bengal Al1837. J Bacteriol 177:835–838. doi: 10.1128/jb.177.3.835-838.1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cox AD, Perry MB. 1996. Structural analysis of the O-antigen-core region of the lipopolysaccharide from Vibrio cholerae O139. Carbohydr Res 290:59–65. doi: 10.1016/0008-6215(96)00131-0 [DOI] [PubMed] [Google Scholar]
- 21. Waldor MK, Colwell R, Mekalanos JJ. 1994. The Vibrio cholerae O139 serogroup antigen includes an O-antigen capsule and lipopolysaccharide virulence determinants. Proc Natl Acad Sci U S A 91:11388–11392. doi: 10.1073/pnas.91.24.11388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kamruzzaman M, Kelly M, Charles RC, Harris JB, Calderwood SB, Akter A, Biswas R, Kaisar MH, Bhuiyan TR, Ivers LC, Ternier R, Jerome J-G, Pfister HB, Lu X, Soliman SE, Ruttens B, Saksena R, Mečárová J, Čížová A, Qadri F, Bystrický S, Kováč P, Xu P, Ryan ET, Pascual DW. 2021. Defining polysaccharide-specific antibody targets against Vibrio cholerae O139 in humans following O139 cholera and following vaccination with a commercial bivalent oral cholera vaccine, and evaluation of conjugate vaccines targeting O139. mSphere 6. doi: 10.1128/mSphere.00114-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ryan ET, Leung DT, Jensen O, Weil AA, Bhuiyan TR, Khan AI, Chowdhury F, LaRocque RC, Harris JB, Calderwood SB, Qadri F, Charles RC. 2021. Systemic, mucosal, and memory immune responses following cholera. Trop Med Infect Dis 6:192. doi: 10.3390/tropicalmed6040192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Qadri F, Svennerholm AM, Shamsuzzaman S, Bhuiyan TR, Harris JB, Ghosh AN, Nair GB, Weintraub A, Faruque SM, Ryan ET, Sack DA, Calderwood SB. 2005. Reduction in capsular content and enhanced bacterial susceptibility to serum killing of Vibrio cholerae O139 associated with the 2002 cholera epidemic in Bangladesh. Infect Immun 73:6577–6583. doi: 10.1128/IAI.73.10.6577-6583.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chowdhury F, Bhuiyan TR, Akter A, Bhuiyan MS, Khan AI, Tauheed I, Ahmed T, Ferdous J, Dash P, Basher SR, Hakim A, Lynch J, Kim JH, Excler J-L, Kim DR, Clemens JD, Qadri F. 2020. Augmented immune responses to a booster dose of oral cholera vaccine in Bangladeshi children less than 5 years of age: revaccination after an interval of over three years of primary vaccination with a single dose of vaccine. Vaccine 38:1753–1761. doi: 10.1016/j.vaccine.2019.12.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Qadri F, Wierzba TF, Ali M, Chowdhury F, Khan AI, Saha A, Khan IA, Asaduzzaman M, Akter A, Khan A, Begum YA, Bhuiyan TR, Khanam F, Chowdhury MI, Islam T, Chowdhury AI, Rahman A, Siddique SA, You YA, Kim DR, Siddik AU, Saha NC, Kabir A, Cravioto A, Desai SN, Singh AP, Clemens JD. 2016. Efficacy of a single-dose, inactivated oral cholera vaccine in Bangladesh. N Engl J Med 374:1723–1732. doi: 10.1056/NEJMoa1510330 [DOI] [PubMed] [Google Scholar]
- 27. Qadri F, Ali M, Lynch J, Chowdhury F, Khan AI, Wierzba TF, Excler J-L, Saha A, Islam MT, Begum YA, Bhuiyan TR, Khanam F, Chowdhury MI, Khan IA, Kabir A, Riaz BK, Akter A, Khan A, Asaduzzaman M, Kim DR, Siddik AU, Saha NC, Cravioto A, Singh AP, Clemens JD. 2018. Efficacy of a single-dose regimen of inactivated whole-cell oral cholera vaccine: results from 2 years of follow-up of a randomised trial. Lancet Infect Dis 18:666–674. doi: 10.1016/S1473-3099(18)30108-7 [DOI] [PubMed] [Google Scholar]
- 28. Uddin T, Aktar A, Xu P, Johnson RA, Rahman MA, Leung DT, Afrin S, Akter A, Alam MM, Rahman A, Chowdhury F, Khan AI, Bhuiyan TR, Bufano MK, Rashu R, Yu Y, Wu-Freeman Y, Harris JB, LaRocque RC, Charles RC, Kováč P, Calderwood SB, Ryan ET, Qadri F. 2014. Immune responses to O-specific polysaccharide and lipopolysaccharide of Vibrio cholerae O1 Ogawa in adult Bangladeshi recipients of an oral killed cholera vaccine and comparison to responses in patients with cholera. Am J Trop Med Hyg 90:873–881. doi: 10.4269/ajtmh.13-0498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Akter A, Dash P, Aktar A, Jahan SR, Afrin S, Basher SR, Hakim A, Lisa AK, Chowdhury F, Khan AI, Xu P, Charles RC, Kelly M, Kováč P, Harris JB, Bhuiyan TR, Calderwood SB, Ryan ET, Qadri F. 2019. Induction of systemic, mucosal and memory antibody responses targeting Vibrio cholerae O1 O-specific polysaccharide (OSP) in adults following oral vaccination with an oral killed whole cell cholera vaccine in Bangladesh. PLoS Negl Trop Dis 13:e0007634. doi: 10.1371/journal.pntd.0007634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Berche P, Poyart C, Abachin E, Lelievre H, Vandepitte J, Dodin A, Fournier JM. 1994. The novel epidemic strain O139 is closely related to the pandemic strain O1 of Vibrio cholerae. J Infect Dis 170:701–704. doi: 10.1093/infdis/170.3.701 [DOI] [PubMed] [Google Scholar]
- 31. Comstock LE, Johnson JA, Michalski JM, Morris JG, Kaper JB. 1996. Cloning and sequence of a region encoding a surface polysaccharide of Vibrio cholerae O139 and characterization of the insertion site in the chromosome of Vibrio cholerae O1. Mol Microbiol 19:815–826. doi: 10.1046/j.1365-2958.1996.407928.x [DOI] [PubMed] [Google Scholar]
- 32. Wang J, Villeneuve S, Zhang J, Lei P, Miller CE, Lafaye P, Nato F, Szu SC, Karpas A, Bystricky S, Robbins JB, Kovác P, Fournier JM, Glaudemans CP. 1998. On the antigenic determinants of the lipopolysaccharides of Vibrio cholerae O:1, serotypes Ogawa and Inaba. J Biol Chem 273:2777–2783. doi: 10.1074/jbc.273.5.2777 [DOI] [PubMed] [Google Scholar]
- 33. Villeneuve S, Souchon H, Riottot MM, Mazie JC, Lei P, Glaudemans CP, Kovác P, Fournier JM, Alzari PM. 2000. Crystal structure of an anti-carbohydrate antibody directed against Vibrio cholerae O1 in complex with antigen: molecular basis for serotype specificity. Proc Natl Acad Sci U S A 97:8433–8438. doi: 10.1073/pnas.060022997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kossaczka Z, Szu SC. 2000. Evaluation of synthetic schemes to prepare immunogenic conjugates of Vibrio cholerae O139 capsular polysaccharide with chicken serum albumin. Glycoconj J 17:425–433. doi: 10.1023/a:1007164216202 [DOI] [PubMed] [Google Scholar]
- 35. Roche MI, Lu Z, Hui JH, Sharon J. 2011. Characterization of monoclonal antibodies to terminal and internal O-antigen epitopes of Francisella tularensis lipopolysaccharide. Hybridoma (Larchmt) 30:19–28. doi: 10.1089/hyb.2010.0083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Vyas NK, Vyas MN, Chervenak MC, Johnson MA, Pinto BM, Bundle DR, Quiocho FA. 2002. Molecular recognition of oligosaccharide epitopes by a monoclonal Fab specific for Shigella flexneri Y lipopolysaccharide: X-ray structures and thermodynamics. Biochemistry 41:13575–13586. doi: 10.1021/bi0261387 [DOI] [PubMed] [Google Scholar]
- 37. Carlin NI, Bundle DR, Lindberg AA. 1987. Characterization of five Shigella flexneri variant Y-specific monoclonal antibodies using defined saccharides and glycoconjugate antigens. J Immunol 138:4419–4427. [PubMed] [Google Scholar]
- 38. Rose DR, Przybylska M, To RJ, Kayden CS, Oomen RP, Vorberg E, Young NM, Bundle DR. 1993. Crystal structure to 2.45 A resolution of a monoclonal Fab specific for the Brucella A cell wall polysaccharide antigen. Protein Sci 2:1106–1113. doi: 10.1002/pro.5560020705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Faruque SM, Chowdhury N, Kamruzzaman M, Ahmad QS, Faruque ASG, Salam MA, Ramamurthy T, Nair GB, Weintraub A, Sack DA. 2003. Reemergence of epidemic Vibrio cholerae O139, Bangladesh. Emerg Infect Dis 9:1116–1122. doi: 10.3201/eid0909.020443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Righetto L, Zaman RU, Mahmud ZH, Bertuzzo E, Mari L, Casagrandi R, Gatto M, Islam S, Rinaldo A. 2015. Detection of Vibrio cholerae O1 and O139 in environmental waters of rural Bangladesh: a flow-cytometry-based field trial. Epidemiol Infect 143:2330–2342. doi: 10.1017/S0950268814003252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Rashed SM, Iqbal A, Mannan SB, Islam T, Rashid MU, Johura FT, Watanabe H, Hasan NA, Huq A, Stine OC, Sack RB, Colwell RR, Alam M. 2013. Vibrio cholerae O1 El Tor and O139 Bengal strains carrying ctxB(ET). Emerg Infect Dis 19:1713–1715. doi: 10.3201/eid1910.130626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Losonsky GA, Lim Y, Motamedi P, Comstock LE, Johnson JA, Morris JG, Tacket CO, Kaper JB, Levine MM. 1997. Vibriocidal antibody responses in North American volunteers exposed to wild-type or vaccine Vibrio cholerae O139: specificity and relevance to immunity. Clin Diagn Lab Immunol 4:264–269. doi: 10.1128/cdli.4.3.264-269.1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Saha D, LaRocque RC, Khan AI, Harris JB, Begum YA, Akramuzzaman SM, Faruque ASG, Ryan ET, Qadri F, Calderwood SB. 2004. Incomplete correlation of serum vibriocidal antibody titer with protection from Vibrio cholerae infection in urban Bangladesh. J Infect Dis 189:2318–2322. doi: 10.1086/421275 [DOI] [PubMed] [Google Scholar]
- 44. Attridge SR, Qadri F, Albert MJ, Manning PA. 2000. Susceptibility of Vibrio Cholerae O139 to antibody-dependent, complement-mediated bacteriolysis. Clin Diagn Lab Immunol 7:444–450. doi: 10.1128/CDLI.7.3.444-450.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kauffman RC, Adekunle O, Yu H, Cho A, Nyhoff LE, Kelly M, Harris JB, Bhuiyan TR, Qadri F, Calderwood SB, Charles RC, Ryan ET, Kong J, Wrammert J, Mantis NJ, McDaniel LS. 2021. Impact of immunoglobulin isotype and epitope on the functional properties of Vibrio Cholerae O-specific polysaccharide-specific monoclonal antibodies. mBio 12:e03679-20. doi: 10.1128/mBio.03679-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sit B, Fakoya B, Waldor MK. 2022. Emerging concepts in cholera vaccine design. Annu Rev Microbiol 76:681–702. doi: 10.1146/annurev-micro-041320-033201 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Plasma antibody responses against Vibrio cholerae O139 capsule in naturally infected patients, and bivalent O1/O139 vaccinees.
Correlation heatmap showing pairwise spearman correlation matrices of V. cholerae O139 antigen-specific antibody responses for patients (left) and bivalent vaccinees (right).
Differences in plasma antibody responses against Vibrio cholerae O139 O-specific polysaccharide (OSP) in naturally infected patients based on vibriocidal titer at "baseline" (day 2 post-infection).
Differences in plasma antibody responses against Vibrio cholerae O139 O-specific polysaccharide (OSP) in vaccinees based on vibriocidal titer on the day of first dose of vaccination.
Data Availability Statement
The raw data supporting the findings of this article will be made fully available by the authors, without undue reservation.