Abstract
PURPOSE
Merkel cell carcinoma (MCC) is a rare, aggressive skin cancer commonly driven by the Merkel cell polyomavirus (MCPyV). The programmed death-1 (PD-1)/programmed death-ligand 1 (PD-L1) immunosuppressive pathway is often upregulated in MCC, and advanced metastatic MCC frequently responds to PD-1 blockade. We report what we believe to be the first trial of anti–PD-1 in the neoadjuvant setting for resectable MCC.
METHODS
In the phase I/II CheckMate 358 study of virus-associated cancer types, patients with resectable MCC received nivolumab 240 mg intravenously on days 1 and 15. Surgery was planned on day 29. Tumor regression was assessed radiographically and microscopically. Tumor MCPyV status, PD-L1 expression, and tumor mutational burden (TMB) were assessed in pretreatment tumor biopsies.
RESULTS
Thirty-nine patients with American Joint Committee on Cancer stage IIA-IV resectable MCC received ≥ 1 nivolumab dose. Three patients (7.7%) did not undergo surgery because of tumor progression (n = 1) or adverse events (n = 2). Any-grade treatment-related adverse events occurred in 18 patients (46.2%), and grade 3-4 events in 3 patients (7.7%), with no unexpected toxicities. Among 36 patients who underwent surgery, 17 (47.2%) achieved a pathologic complete response (pCR). Among 33 radiographically evaluable patients who underwent surgery, 18 (54.5%) had tumor reductions ≥ 30%. Responses were observed regardless of tumor MCPyV, PD-L1, or TMB status. At a median follow-up of 20.3 months, median recurrence-free survival (RFS) and overall survival were not reached. RFS significantly correlated with pCR and radiographic response at the time of surgery. No patient with a pCR had tumor relapse during observation.
CONCLUSION
Nivolumab administered approximately 4 weeks before surgery in MCC was generally tolerable and induced pCRs and radiographic tumor regressions in approximately one half of treated patients. These early markers of response significantly predicted improved RFS. Additional investigation of these promising findings is warranted.
INTRODUCTION
Merkel cell carcinoma (MCC) is a rare, aggressive skin cancer that tends to affect older people, with a median age at diagnosis of 75-79 years. Although 65% of patients present with localized disease, regional and distant metastases are frequent.1,2 Approximately 80% of MCCs result from clonal integration of the Merkel cell polyomavirus (MCPyV), but MCC can also arise from ultraviolet (UV) light–induced mutations. These 2 etiologies are strikingly different in their mutational characteristics, with nearly 100-fold higher tumor mutational burden (TMB) in UV-induced (virus-negative) versus virus-positive MCC.3-5 In principle, either etiology could generate immunogenic neoantigens recognizable by T and/or B cells.
CONTEXT
Key Objectives
To evaluate the safety and potential efficacy of nivolumab (anti–programmed death-1) administered in the neoadjuvant setting to patients with high-risk resectable Merkel cell carcinoma (MCC) in, to our knowledge, the first study of its kind (CheckMate 358).
Knowledge Generated
Among 39 adults with American Joint Committee on Cancer stage IIA-IV MCC who received nivolumab for approximately 4 weeks before planned surgical resection, 7.7% experienced treatment-related grade 3-4 toxicities and 36 underwent surgery. Thereafter, patients underwent surveillance or additional treatment according to institutional standards of care. High rates of pathologic complete response (pCR [47.2%]) and radiographic response (54.5%) were observed, and responders had prolonged recurrence-free survival; no patient with a pCR experienced tumor relapse, with 19.3 months of median follow-up postoperatively.
Relevance
Neoadjuvant nivolumab therapy in high-risk resectable MCC mediated major pathologic and radiographic responses in approximately one half of patients, with significantly improved recurrence-free survival among the responders. This treatment regimen seemed to be generally tolerable and should be further explored as a potentially beneficial adjunct to surgery in patients with MCC.
Until recently, cytotoxic chemotherapy was the primary systemic therapy for advanced MCC. Although initial responses to chemotherapy are frequent (approximately 60%), durability is disappointing, with 95% of patients experiencing progression within 1 year.6 The programmed death-1 (PD-1)/programmed death-ligand 1 (PD-L1) immunosuppressive pathway is often upregulated in MCC,7 and several trials of PD-(L)1 inhibitors have demonstrated high response rates (approximately 40%-60%) with excellent durability.8-11 In aggregate, these findings rapidly changed the treatment landscape for advanced MCC, with immunotherapy now preferred over chemotherapy for eligible patients.12
Given the rapid and durable responses seen in advanced MCC, there is great interest in exploring immunotherapy for earlier disease stages. Neoadjuvant treatment targeting the PD-1/PD-L1 pathway has the potential to eradicate microscopic disease that may escape regional therapy with surgery and radiation, a common occurrence in MCC. Neoadjuvant anti–PD-1–based therapies have been explored in several malignancies, including non–small-cell lung cancer (NSCLC),13 bladder cancer,14 and glioblastoma.15,16 In melanoma, studies of neoadjuvant anti–PD-1 plus anti–CTLA-4 suggest that pathologic response in surgical specimens might be an early indicator of long-term benefit.17,18 However, for nonresponders, neoadjuvant therapy may delay regional treatment, potentially losing a window of opportunity for local control. We conducted the current trial to assess the safety and potential efficacy of neoadjuvant nivolumab in resectable MCC.
METHODS
Study Design, Patients, and Treatment
CheckMate 358 is a multicenter, open-label, multicohort phase I/II trial investigating nivolumab monotherapy or nivolumab-based combination therapies in patients with virus-associated solid tumors in the recurrent/metastatic or neoadjuvant setting. In the neoadjuvant MCC cohort, eligible patients were ≥ 18 years of age with an Eastern Cooperative Oncology Group performance status of 0 or 1 and histopathologic confirmation of MCC. Patients had tumors amenable to pretreatment biopsy that were deemed surgically resectable, including (1) stage IIA-IIIB disease with primary tumor ≥ 2 cm, or primary tumor of any size with palpable regional lymph node metastases or resectable in-transit metastases; (2) oligometastatic stage IV disease; or (3) locoregional recurrence with total tumor burden ≥ 1 cm.19 Patients with brain metastases, another invasive malignancy within ≤ 3 years, a history of autoimmunity, or a requirement for systemic immunosuppressive medications, and those who had had prior treatment with T-cell–modulating drugs were ineligible.
Patients received nivolumab 240 mg intravenously on days 1 and 15, and underwent surgery on day 29 (± 7 days). Delayed administration of the second dose was acceptable up to day 22. Conventional postoperative therapy was allowed at the investigator’s discretion, provided that any nivolumab-related toxicity had resolved to grade ≤ 1.
Study End Points
The primary end point for this MCC cohort was the safety and tolerability of neoadjuvant nivolumab measured by treatment-related adverse events (TRAEs) and surgical delays, defined as the proportion of patients experiencing TRAE-related delays > 4 weeks from planned surgery date. Exploratory end points included pathologic complete response (pCR) rate (assessed by investigators [site review] and by an independent pathologist [central review]), radiographic response (modified RECIST v1.1),20 recurrence-free survival (RFS, time from surgery to date of recurrence per investigator or death from any cause, whichever occurs first), overall survival (OS, time from first nivolumab dose to date last known alive or death date), immunologic changes in blood and tumor, and association of tumor MCPyV status and PD-L1 expression with efficacy.
Tumor assessments by computed tomography and/or magnetic resonance imaging were conducted ≤ 35 days before the first nivolumab dose, at day 29 (or ≤ 7 days preoperatively), and at approximately 4, 8, and 12 months. Safety was monitored throughout the study and until 100 days after study drug discontinuation; TRAEs were monitored until they resolved, returned to baseline, or were deemed irreversible, or until the patient was lost to follow-up, withdrew consent, or started another anticancer therapy. Adverse events (AEs) were assessed using worst grade per National Cancer Institute Common Terminology Criteria for Adverse Events v4 by system organ class and MedDRA preferred terms.21 Survival was monitored at the first follow-up assessment approximately 35 days after the last nivolumab dose, approximately 80 days after the first follow-up assessment, and every 3 months thereafter.
Pathologic Response Assessment, PD-L1 Immunohistochemistry, and Multispectral Immunofluorescence Tumor Imaging and Analysis
Site pathology reviews categorized patients who underwent surgery as achieving pCR or non-pCR; central pathology review categorized patients using immune-related pathologic response criteria22,23 as achieving pCR, major pathologic response (MPR), or non-pCR/MPR. pCR was defined as the absence of residual viable invasive cancer on hematoxylin and eosin evaluation of completely resected tumor specimens including all sampled lymph nodes; MPR was defined as ≤ 10% residual viable tumor. Tumor cell PD-L1 expression was assessed in recent archival or fresh pretreatment tumor biopsy specimens using an automated immunohistochemistry (IHC) assay (PD-L1 IHC 28-8 pharmDx; Dako, an Agilent Technologies, Inc. Company, Santa Clara, CA). PD-L1–positive tumors had ≥ 1% of tumor cells with cell surface expression at any intensity. In a subset of patients, pre-nivolumab tumor biopsy specimens paired with post-nivolumab surgical resection specimens from the same patients were subjected to multispectral immunofluorescence staining at the Johns Hopkins Bloomberg∼Kimmel Institute for Cancer Immunotherapy using multiplex panels to assess immune cell subsets and their activation state (PD-1, CD4, CD8, CD20, Ki67, neuron-specific enolase [NSE], DAPI), and features of anti–PD-1–induced tumor regression (PD-1, CD3, CD79a, CD163, ERG, NSE, DAPI)22,23; see the Data Supplement (online only) for details.
Tumor Viral Status and Whole-Genome Sequencing
Tumors were characterized as MCPyV positive if patients had small T-antigen–specific immunoglobulin G antibodies detected in their serum via a customized Luminex immunoassay (Thermo-Fisher Scientific, Waltham, MA)24,25 performed at the University of Washington or demonstrated large T-antigen expression in tumor biopsy specimens via IHC using the CM2B4 antibody.26,27 To call a case virus-negative, both tests needed to be negative. If only 1 test could be performed and was negative, viral status was classified as uninterpretable, owing to the incomplete sensitivity of either test alone.
TMB was assessed using whole-genome sequencing (Illumina, San Diego, CA) to determine the number of mutations per exome found within a tumor sample relative to normal host tissue. In silico filtering was used to derive TMB for patients without germline sequence data, as described.28 The UV mutational signature score was derived as described.29
Study Oversight
The protocol was approved by an institutional review board or independent ethics committee at each site before study activation. The study was conducted in accordance with Good Clinical Practice guidelines as defined by the International Conference on Harmonisation, and in accordance with the ethical principles of the European Union Directive and United States Code of Federal Regulations. All patients provided written informed consent in accordance with the Declaration of Helsinki.
Statistical Analysis
Enrollment of ≥ 21 evaluable patients was planned for this cohort, on the basis of estimation precision of safety event and pathologic response rates (details in Data Supplement). Safety was summarized in all treated patients using descriptive statistics. Pathologic and radiographic responses were evaluated only in patients receiving ≥ 1 nivolumab dose and who had relevant baseline and/or postbaseline assessments. RFS and OS were estimated using the Kaplan-Meier techniques (Data Supplement).30 Median RFS and OS are reported together with corresponding 95% CIs, using the Brookmeyer and Crowley method31 (applying log-log transformation for CI construction32). Survival rates at fixed time points were derived from the Kaplan-Meier estimate, and corresponding CIs were derived per the Greenwood formula for variance derivation33; log-log transformation was applied on the survivor function. Comparisons of RFS or OS between patient subgroups were exploratory, and all reported P values are nominal. TMB was analyzed according to MCPyV status or site pathologic response using the Wilcoxon signed-rank test.
RESULTS
Patient, Tumor, and Treatment Characteristics
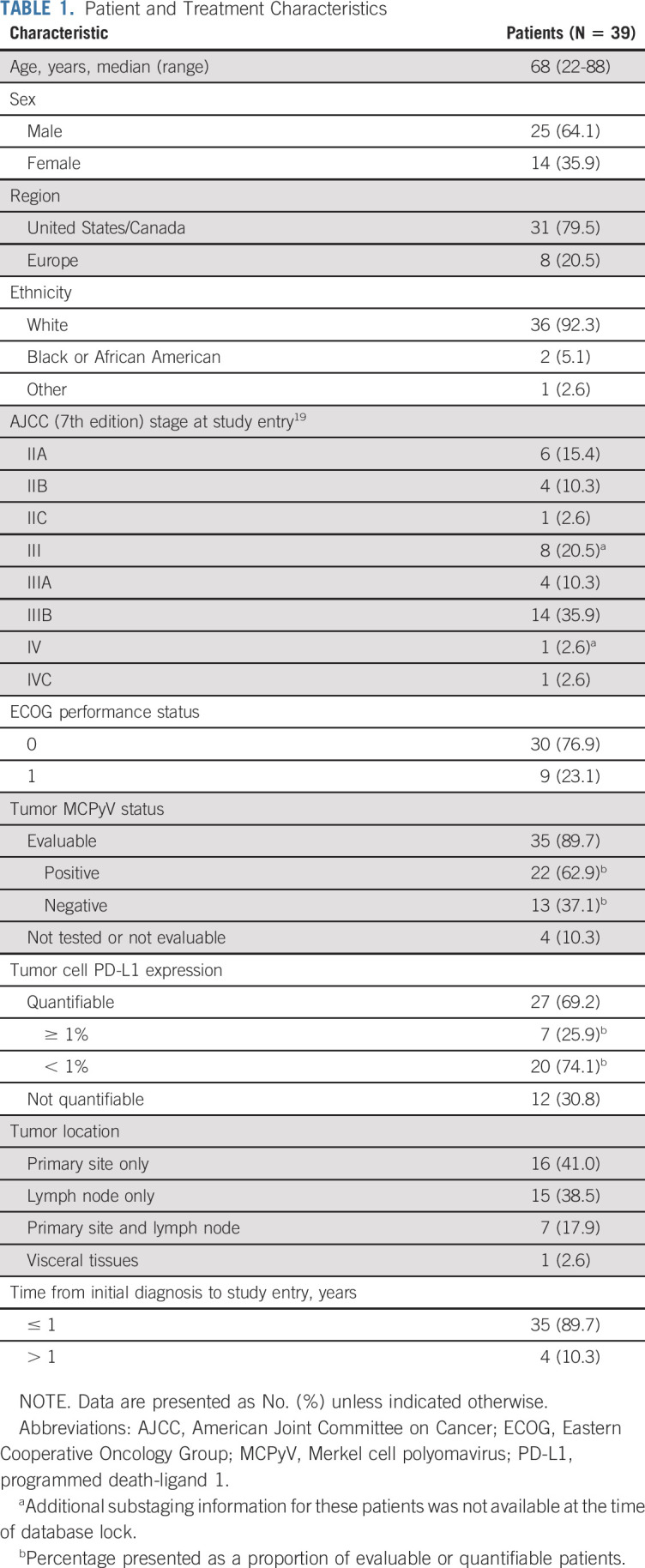
Between January 2016 and March 2019, 39 patients were treated in the neoadjuvant MCC cohort of CheckMate 358. The database lock for this analysis was June 26, 2019, and the median follow-up was 20.3 months (range, 0.5-39.7 months). The median age was 68 years (range, 22-88 years; Table 1). Most patients had American Joint Committee on Cancer stage III disease at enrollment (66.7%). Among 35 patients with tumors evaluable for MCPyV status, 22 (62.9%) were positive. Among 27 patients with quantifiable tumor cell PD-L1 expression, 7 (25.9%) had expression ≥ 1%.
TABLE 1.
Patient and Treatment Characteristics
Thirty-six of 39 treated patients received both planned doses of nivolumab. Three patients received only 1 dose: one with grade 2 binocular diplopia proceeded to surgery and later resolved; one with non–treatment-related grade 2 nausea withdrew consent; and one discontinued because of grade 3 treatment-related rash (Data Supplement).
Safety
Any-grade TRAEs were reported in 18 of 39 treated patients (46.2%; Data Supplement). Three patients (7.7%) experienced grade 3-4 TRAEs; no treatment-related deaths occurred. Select TRAEs (AEs with potential immunologic cause), including 2 grade 3-4 events (5.1%; 1 skin, 1 GI), occurred in 6 patients (15.4%). The most common any-grade select TRAEs were skin reactions (10.3%).
Among the 39 treated patients, 3 did not undergo surgery: one discontinued because of tumor progression, one withdrew consent because of non–treatment-related grade 2 nausea, and one discontinued because of grade 3 treatment-related rash (Data Supplement). Among 36 patients who underwent surgery, the median interval between first nivolumab dose and surgery was 4.3 weeks (range, 2.9-15.0 weeks). One patient (2.6%) had surgery delayed per protocol definition (> 4 weeks) because of treatment-related autoimmune colitis (surgery performed on day 105), and 3 (7.7%) had surgery delayed > 7 days but ≤ 4 weeks for administrative reasons (surgery performed on days 37, 39, and 44). There were 6 deaths on-study: 4 because of disease progression and 2 resulting from non–treatment-related AEs (Data Supplement).
Pathologic and Radiographic Tumor Response
All 36 patients undergoing surgery were evaluated for pathologic response by study investigators (site review), and 17 (47.2%) achieved a pCR. Definitive resection specimens from 26 patients were also evaluable by central pathologic review: 12 (46.2%) achieved a pCR, and 4 (15.4%) achieved an MPR, yielding a pCR plus MPR rate of 61.5%. MPR was not evaluated by site review. There was good concordance between site and central reviews, with 12 patients achieving pCR by both; the remaining 5 specimens scored as pCR by site review were not available or not evaluable on central review (Data Supplement).
Thirty-three of 36 patients who underwent surgery were evaluable for radiographic response (ie, change in sum of diameters of target lesions). Figure 1 presents the change from baseline in these patients according to tumor MCPyV and PD-L1 status and pathologic response. Twenty-nine of 33 patients (87.9%) showed any radiographic tumor reduction and 18 (54.5%) had tumor reduction ≥ 30%; the median change in tumor burden from baseline was −32.8% (range, –100% to +73%). Radiographic reduction ≥ 30% was observed in tumors that were MCPyV positive or negative, and PD-L1 positive or negative, with no apparent trends. Radiographic response generally underestimated the degree of pathologic response: among 11 tumors with radiographic reduction insufficient for meeting partial response criteria (ie, < 30%), 5 had pCR by site review; furthermore, only 2 tumors showed complete radiographic resolution, although there were 15 pCRs among radiographically evaluable tumors. Figure 2 presents 2 patients whose MCC responded to neoadjuvant nivolumab therapy. The patient depicted in Fig 2A provided consent to the use of photographs and medical information.
FIG 1.
Characteristics of treatment response. Change from baseline in the sum of target lesion diameters according to modified RECIST v1.1 in 33 evaluable patients who underwent surgery, by (A) tumor Merkel cell polyomavirus (MCPyV) status and pathologic response and (B) tumor cell programmed death-ligand 1 (PD-L1) expression and pathologic response. Pathologic response according to both site investigator review and central review is shown. Dashed horizontal lines indicate 30% target lesion reduction (consistent with a partial response in the absence of new lesions) and 20% increase (consistent with progressive disease). Note that radiographic responses per modified RECIST v1.1 were based on a single imaging scan before surgery, with no confirmatory scan performed. MPR, major pathologic response; pCR, pathologic complete response.
FIG 2.
Treatment response to neoadjuvant nivolumab in 2 patients with Merkel cell carcinoma (MCC). (A) Complete response in a 53-year-old woman with an advanced facial primary MCC, T3N0 (patient No.13 per Data Supplement). The tumor was Merkel cell polyomavirus (MCPyV) positive and programmed death-ligand 1 (PD-L1) negative. This patient received 2 doses of nivolumab preoperatively, with evidence of rapid tumor regression on physical examination (top row) and CT scans (bottom row, red arrows) at day 17. On day 20, she underwent surgery as originally planned (radical cheek resection, parotidectomy, and cervical lymph node dissection), revealing a pathologic complete response by site and central pathology reviews. Adjuvant radiotherapy totaling 50 Gy was administered to the primary tumor site as standard of care at the investigator’s discretion. At 3.5 years of follow-up, this patient remains tumor-free per the investigator. (B) Near-complete response of stage III MCC in a 67-year-old man with bulky left axillary lymph node metastases and an unknown primary tumor site (patient No. 21 per Data Supplement). The axillary tumor was MCPyV negative and PD-L1 negative. This patient received 2 doses of nivolumab on days 1 and 15, with a CT scan at day 18 showing partial tumor regression (top row, red circles). He underwent a complete left axillary lymph node dissection on day 23, with 2 of 47 lymph nodes showing residual microscopic tumor deposits (major pathologic response by central review, non-pCR by site review). No postoperative therapy was administered. This patient remains tumor-free 3 years later per the investigator. Features of immune-mediated pathologic response in the surgical specimen were evident with hematoxylin and eosin staining (bottom left panel: yellow arrow, infiltrating lymphocytes; red arrows, prominent plasma cell infiltrates; green arrow, proliferative fibrosis) and with multispectral immunofluorescence staining (bottom right panel: red stain, ERG+ neovasculature; green, CD79a+ B-lineage cells; yellow, CD3+ T cells; purple, programmed death-1 (PD-1)+ cells; blue, CD163+ myeloid cells). Note individual cells with both purple and yellow staining, indicating PD-1+ T cells (white arrow).
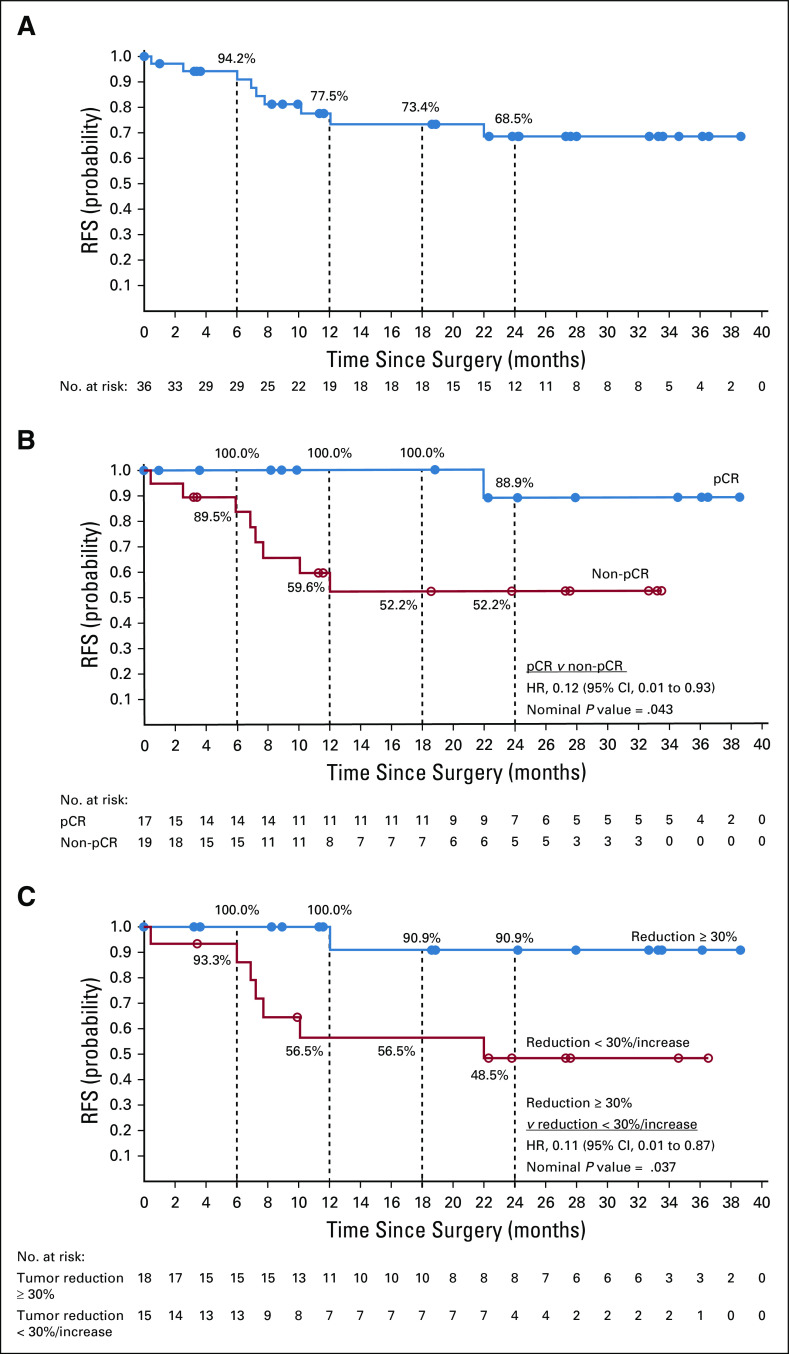
RFS and OS
Median RFS was not reached in 36 patients who underwent surgery (Fig 3A). At 12 and 24 months postoperatively, RFS rates were 77.5% (95% CI, 58.4% to 88.7%) and 68.5% (95% CI, 47.5% to 82.6%), respectively. Comparing patients with pCR versus without pCR by site review (n = 36), RFS at 12 months was 100.0% versus 59.6%, and at 24 months was 88.9% versus 52.2% (hazard ratio [HR], 0.12 [95% CI, 0.01 to 0.93]; Fig 3B). Similarly, comparing patients with pCR/MPR versus without pCR/MPR by central review (n = 26), RFS at 12 months was 100.0% versus 50.0%, and at 24 months was 88.9% versus 50.0% (HR, 0.09 [95% CI, 0.01 to 0.80]; Data Supplement). The single event observed among pathologic responders was a death unrelated to tumor relapse or study drug. Among 33 radiographically evaluable patients, comparing those with radiographic tumor reduction ≥ 30% versus those with reduction < 30% or progression, RFS at 12 months was 100.0% versus 56.5%, and at 24 months was 90.9% versus 48.5% (HR, 0.11 [95% CI, 0.01 to 0.87]; Fig 3C). There was no substantial difference in RFS between patient subgroups on the basis of tumor MCPyV status (n = 33) or PD-L1 expression (n = 25; Data Supplement).
FIG 3.
Recurrence-free survival (RFS) in (A) all patients with Merkel cell carcinoma who underwent surgery (n = 36), and for patients who underwent surgery, on the basis of (B) pathologic response by site review (n = 36) or (C) radiographic response per RECIST v1.1 (n = 33). Panel B shows that a single event occurred among patients with a pathologic complete response (pCR); this was a death unrelated to the study drug, in the absence of tumor relapse. After database lock, 1 case scored as non-pCR by site review was found to be not evaluable for pathologic response, because a planned subtotal tumor resection was performed. Note that radiographic responses per modified RECIST v1.1 were based on a single imaging scan before surgery, with no confirmatory scan performed. Median follow-up for 36 patients from date of surgery was 19.3 months (range, 1.0-38.7 months). Analyses were exploratory, and all reported P values are nominal. HR, hazard ratio.
Median OS was not reached in 39 treated patients (Fig 4A); at 12 and 24 months after the first nivolumab dose, OS rates were 93.2% (95% CI, 75.5% to 98.3%) and 79.4% (95% CI, 56.9% to 91.0%), respectively. Among patients who underwent surgery and were evaluable for pathologic response (n = 36) or radiographic response (n = 33), 100.0% and 88.9% of patients with pCR by site review and all patients with radiographic tumor reduction ≥ 30% were alive at 12 and 24 months, respectively (Figs 4B-4C). Similarly, among those with pCR/MPR by central review, OS rates were 100.0% and 88.9% at 12 and 24 months, respectively (Data Supplement). OS rates in patients who underwent surgery were comparable in subgroups on the basis of tumor MCPyV status or PD-L1 expression (Data Supplement).
FIG 4.
Overall survival (OS) in (A) all patients with Merkel cell carcinoma who received ≥ 1 dose of nivolumab (n = 39) and for subgroups of patients who underwent surgery, on the basis of (B) pathologic response by site review (n = 36) or (C) radiographic response per RECIST v1.1 (n = 33). After database lock, 1 case scored as non-pCR by site review was found to be not evaluable for pathologic response, because a planned subtotal tumor resection was performed. Note that radiographic responses per modified RECIST v1.1 were based on a single imaging scan before surgery, with no confirmatory scan performed. Analyses were exploratory, and all reported P values are nominal. (*) Because of the absence of events among patients with tumor reduction ≥ 30%, hazard ratios and P values are not available from statistical analyses. HR, hazard ratio; NA, not available; pCR, pathologic complete response.
Subsequent Anticancer Therapies
At database lock, 15 of the 39 treated patients (38.5%) had received anticancer therapies subsequent to neoadjuvant nivolumab (Data Supplement). Eight patients, including 5 with pCR, received planned standard-of-care adjuvant radiotherapy (7 received adjuvant radiotherapy alone, and 1 received adjuvant radiotherapy combined with systemic therapy). Six patients required subsequent treatment of disease relapse/progression: 3 received systemic therapy alone and 3 received systemic therapy combined with focal radiotherapy; 5 received anti–PD-(L)1 therapy (4 avelumab, 1 nivolumab). Another patient did not proceed to surgery because of an AE but received radiotherapy subsequent to nivolumab.
Tumor Genomic Analyses
A subset of 14 patients had pretreatment tumor specimens suitable for whole-genome sequencing; at database lock, 12 had data on both MCPyV status and pathologic response by site review, and 2 had pathologic response data only. MCPyV-negative tumors had notably higher TMB than did MCPyV-positive tumors (Data Supplement), consistent with literature reports.5 Similarly, higher UV mutational signature scores were seen in MCPyV-negative versus MCPyV-positive tumors (Data Supplement), consistent with reports by others3-5 and suggesting the potential oncogenic role of UV radiation in these cases. There was no apparent difference in TMB between patients achieving pCR versus non-pCR by site review (Data Supplement).
Multispectral Immunofluorescence Tumor Analysis
Eight patients (2 pCR, 3 MPR, and 3 non-pCR/MPR by central review) had paired pre- and post-nivolumab tumor specimens suitable for multispectral analysis of changes in immune cell subset proportions and activation, immune checkpoint expression, and histologic hallmarks of anti–PD-1–mediated tumor regression.22,23 Proportionate increases in CD79a+ B-lineage cells were found in 3 of 5 responders (Fig 5). Densities of intratumoral CD4+/PD-1+ and CD8+/PD-1+ cells increased in nearly every patient after anti–PD-1 therapy, regardless of pathologic response (Data Supplement); however, only CD8+ cells showed a notable increase in proliferative activity. Specifically, CD8+/PD-1+/Ki67+ cells (activated cytotoxic T cells) increased after nivolumab therapy in 6 of 8 patients. Densities of Ki67+ (ie, proliferating) tumor cells increased in the 3 patients with non-pCR/MPR and decreased in the 5 responders. The patient depicted in Fig 2A provided consent to the use of photographs and medical information.
FIG 5.
Immunologic and pathologic changes associated with neoadjuvant nivolumab therapy in paired pretreatment and post-treatment tumor biopsy specimens from 8 patients with Merkel cell carcinoma. Each pair of biopsy specimens was taken from the same tumor site. The pathologic response status of each patient by central review, and tumor Merkel cell polyomavirus (MCPyV) status, are displayed. Proportions of cell types in selected high-power fields indicate that patients experiencing a response to therapy have a decrease in tumor cell proportion, with measurable increases in CD79a+ cell populations (pan–B-cell marker including plasma cells) in 3 of 5 responders. Among the 3 patients with non–pathologic complete response (pCR)/major pathologic response (MPR), 80%-100% residual viable tumor was observed in the complete specimen. CD3, T cells; CD163, macrophages; CD79a, B-lineage cells; Other, additional cell types such as fibroblasts and endothelial cells.
DISCUSSION
Neoadjuvant immunotherapy, administered preoperatively with macroscopic tumor in place, holds the potential for inducing durable systemic antitumor immunity to prevent postsurgical relapse.34 Advanced, unresectable MCC is highly and rapidly responsive to anti–PD-(L)1 therapy, suggesting that a brief period of neoadjuvant therapy might suffice to mediate substantial tumor regression and potentially enable less extensive surgery, while mitigating risk from undue surgical delay.8-11 In the current study, patients with high-risk resectable MCC underwent brief presurgical anti–PD-1 therapy (approximately 4 weeks) designed to optimize risk:benefit in this relatively elderly population. Nevertheless, 3 patients did not undergo surgery because of disease progression or AEs. Among 36 patients who underwent surgery, the pCR rate approached 50%, exceeding rates observed in neoadjuvant anti–PD-1 trials in NSCLC (15%) and melanoma (19%-25%), which are not as responsive as MCC to anti–PD-1 monotherapy in the advanced disease setting.13,35,36 Moreover, pCR was significantly associated with prolonged RFS. Owing to rapid anti–PD-1 response kinetics in MCC,11 radiographic response within a 4-week period also significantly predicted RFS.
In this study, two thirds of patients had stage III (locoregional) MCC at enrollment. Comparing outcomes observed here with historical experience in stage III MCC, neoadjuvant anti–PD-1 may represent a treatment advance warranting continued follow-up and additional exploration in larger studies. There were no tumor relapses after pCR/MPR, suggesting that standard adjuvant radiotherapy may not be needed in responders. However, relapses occurred among patients with non-pCR/MPR, suggesting that a period of postoperative anti–PD-1 may be appropriate for some. The possibility of tailoring the extent of surgery and/or administration of postsurgical therapy according to radiographic and pathologic response status after neoadjuvant anti–PD-1 should be examined in future MCC trials, which have the potential to be practice changing.
Tumor markers predicting long-term clinical outcomes would be valuable to further characterize patients without pCR/MPR or radiographic regression, some of whom nevertheless have prolonged RFS and OS. Several unidimensional markers have been explored in advanced unresectable MCC; anti–PD-(L)1 efficacy did not correlate with baseline tumor viral status or infiltrating T-cell receptor diversity, although some studies showed a trend toward improved outcomes with PD-L1–positive tumors.8,11,37,38 In CheckMate 358, the efficacy of neoadjuvant anti–PD-1 did not correlate with baseline MCC viral status or PD-L1 expression. Also consistent with prior studies,5 MCPyV-positive tumors had low TMB, suggesting that a limited number of strong viral antigens can serve as tumor rejection antigens in MCC and potentially in other virus-associated cancers. Multiplex markers may provide increased sensitivity and specificity for treatment outcomes. For instance, we showed previously that the proximity of PD-1+ to PD-L1+ cells in the pretreatment MCC microenvironment correlates with the anti–PD-1 response.39 Here, multispectral immunofluorescence analysis of paired pre/post-treatment tumor specimens indicated shifting populations of tumor cells, B cells, and proliferating CD8+ T cells. Such studies, enabled by substantial quantities of tissue available from neoadjuvant surgical specimens, should help elucidate mechanism-of-action for anti–PD-(L)1 therapy in ways heretofore not possible.
ACKNOWLEDGMENT
We thank the patients and their families for making this study possible; the clinical study teams who participated in the study; Dako, an Agilent Technologies, Inc. company, for collaborative development of the PD-L1 IHC 28-8 pharmDx assay; and Bristol Myers Squibb (Princeton, NJ) and ONO Pharmaceutical Company Ltd. (Osaka, Japan). We gratefully acknowledge the efforts of Evan Lipson, MD, Trish Brothers, RN, and the clinical research team at the Johns Hopkins Kimmel Cancer Center, as well as Benjamin Green, Charles Roberts, Daphne Wang, and Morenike Jackson of the Tumor Microenvironment Technology Development Center in the Bloomberg∼Kimmel Institute for Cancer Immunotherapy at Johns Hopkins. Professional medical writing and editorial assistance were provided by Richard Daniel, PhD, of Parexel, funded by Bristol Myers Squibb.
PRIOR PRESENTATION
Presented in part at the American Society of Clinical Oncology 2018 Annual Meeting, Chicago, IL, June 1-5, 2018.
SUPPORT
Supported by Bristol Myers Squibb and ONO Pharmaceutical Company Limited (CheckMate 358 study); by The Mark Foundation for Cancer Research and National Cancer Institute R01 CA142779 (J.M.T.; multispectral immunofluorescence tissue staining and analysis at the Johns Hopkins Bloomberg∼Kimmel Institute for Cancer Immunotherapy); and by P30-CA015704 and P01-CA225517 (P.N.).
CLINICAL TRIAL INFORMATION
See accompanying Oncology Grand Rounds on page 2471
AUTHOR CONTRIBUTIONS
Conception and design: Suzanne L. Topalian, Bin Li, Paul Nghiem
Financial support: Robert L. Ferris
Administrative support: Julie E. Stein, Elizabeth L. Engle, Adam Barrows, Andrea Horvath, Paul Nghiem
Provision of study material or patients: Shailender Bhatia, Asim Amin, William H. Sharfman, Celeste Lebbé, Jean-Pierre Delord, Christine H. Chung, Paul Nghiem
Collection and assembly of data: Suzanne L. Topalian, Shailender Bhatia, Ragini R. Kudchadkar, William H. Sharfman, Celeste Lebbé, Jean-Pierre Delord, Lara A. Dunn, Michi M. Shinohara, Rima Kulikauskas, Christine H. Chung, Uwe M. Martens, Robert L. Ferris, Julie E. Stein, Elizabeth L. Engle, Lot A. Devriese, Bin Li, Adam Barrows, Andrea Horvath, Janis M. Taube, Paul Nghiem
Data analysis and interpretation: Suzanne L. Topalian, Asim Amin, Ragini R. Kudchadkar, William H. Sharfman, Celeste Lebbé, Michi M. Shinohara, Rima Kulikauskas, Christine H. Chung, Uwe M. Martens, Robert L. Ferris, Julie E. Stein, Elizabeth L. Engle, Lot A. Devriese, Christopher D. Lao, Junchen Gu, Bin Li, Tian Chen, Adam Barrows, Andrea Horvath, Janis M. Taube, Paul Nghiem
Manuscript writing: All authors
Final Approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Neoadjuvant Nivolumab for Patients With Resectable Merkel Cell Carcinoma in the CheckMate 358 Trial
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Suzanne L. Topalian
Stock and Other Ownership Interests: Aduro Biotech (I), Potenza Therapeutics (I), Five Prime Therapeutics, Tizona Therapeutics (I), DNAtrix (I), FLX Bio (I), WindMIL (I), Dragonfly Therapeutics, ERVAXX (I), Trieza Therapeutics (I)
Consulting or Advisory Role: Five Prime Therapeutics, Amgen (I), MedImmune (I), Merck, Compugen (I), DNAtrix (I), FLX Bio (I), Tizona Therapeutics (I), WindMIL (I), Dragonfly Therapeutics, Dynavax (I), ERVAXX (I), Immunomic Therapeutics (I), Janssen Oncology (I), Immunocore
Research Funding: Bristol Myers Squibb, Compugen (I), Potenza Therapeutics (I)
Patents, Royalties, Other Intellectual Property: Aduro Biotech (I), Bristol Myers Squibb (I), Immunomic Therapeutics (I), Arbor Pharmaceuticals (I), NexImmune (I), WindMIL (I)
Travel, Accommodations, Expenses: Bristol Myers Squibb, Five Prime Therapeutics, Merck
Shailender Bhatia
Honoraria: Genentech/Roche, EMD Serono, Bristol Myers Squibb, Sanofi/Regeneron, EMD Serono, Sanofi/Regeneron
Consulting or Advisory Role: Genentech/Roche, EMD Serono, Bristol Myers Squibb, Sanofi/Regeneron, EMD Serono, Sanofi/Regeneron (Inst)
Research Funding: Bristol Myers Squibb (Inst), Immune Design (Inst), Merck (Inst), EMD Serono (Inst), OncoSec (Inst), NantKwest (Inst), Novartis (Inst), Exicure (Inst), Nektar (Inst), Incyte (Inst)
Travel, Accommodations, Expenses: NantKwest, Sanofi/Regeneron
Asim Amin
Consulting or Advisory Role: Merck, Novartis
Speakers' Bureau: Bristol Myers Squibb, Merck, Exelixis, Bioarray Therapeutics, Sanofi/Regeneron, Novartis
Research Funding: Bristol Myers Squibb (Inst), Merck (Inst), Dynavax (Inst)
Ragini R. Kudchadkar
Honoraria: Bristol Myers Squibb, Array BioPharma
Consulting or Advisory Role: Bristol Myers Squibb, Novartis, Array BioPharma, Immunocore, Regeneron
Research Funding: Merck (Inst), Regeneron (Inst)
William H. Sharfman
Honoraria: Bristol Myers Squibb, Array BioPharma
Consulting or Advisory Role: Merck, Bristol Myers Squibb, Novartis, Regeneron, ION Pharma
Research Funding: Bristol Myers Squibb (Inst), Merck, Novartis
Celeste Lebbé
Honoraria: Roche, Bristol Myers Squibb, Novartis, Amgen, MSD, Pierre Fabre, Pfizer, Incyte
Consulting or Advisory Role: Bristol Myers Squibb, MSD, Novartis, Amgen, Roche, Merck Serono, Sanofi
Speakers' Bureau: Roche, Bristol Myers Squibb, Novartis, Amgen, MSD
Research Funding: Roche (Inst), Bristol Myers Squibb (Inst)
Travel, Accommodations, Expenses: Bristol Myers Squibb, MSD
Other Relationship: Avantis Medical Systems
Jean-Pierre Delord
Consulting or Advisory Role: Novartis, Roche/Genentech, Bristol Myers Squibb, MSD Oncology
Research Funding: Genentech (Inst), Bristol Myers Squibb (Inst), MSD Oncology (Inst)
Lara A. Dunn
Consulting or Advisory Role: Regeneron, CUE Biopharma
Research Funding: Pfizer, Regeneron, Eisai
Michi M. Shinohara
Research Funding: Soligenix, Elorac, Actelion, MiRagen
Rima Kulikauskas
Research Funding: Bristol Myers Squibb (Inst)
Christine H. Chung
Consulting or Advisory Role: Bristol Myers Squibb, CUE Biopharma, Ignyta, Mirati Therapeutics
Research Funding: MedImmune,, Bristol Myers Squibb, Lilly, Merck, Regeneron, Ignyta, IRX Therapeutics, Pfizer, Lion Biotechnologies
Travel, Accommodations, Expenses: MedImmune,, Mirati Therapeutics
Uwe M. Martens
Consulting or Advisory Role: MSD Oncology, Roche, Bristol Myers Squibb, Celgene
Travel, Accommodations, Expenses: Amgen, Bristol Myers Squibb, Celgene, Pierre Fabre
Robert L. Ferris
Consulting or Advisory Role: Bristol Myers Squibb, MedImmune,/MedImmune, Merck, Lilly, Pfizer, Amgen, EMD Serono, Tesaro, PPD, Bain Capital Life Sciences, GlaxoSmithKline, Iovance Biotherapeutics, Numab Therapeutics AG, Oncorus, ONO Pharmaceutical, Regeneron, TTMS, Aduro Biotech, Macrogenics, Nanobiotix, Torque Therapeutics, TTMS
Research Funding: Bristol Myers Squibb, VentiRx, MedImmune,/MedImmune, Merck, Tesaro, TTMS
Elizabeth L. Engle
Research Funding: Bristol Myers Squibb (Inst)
Lot A. Devriese
Honoraria: MSD B.V. the Netherlands (Inst)
Christopher D. Lao
Consulting or Advisory Role: Immunocore, BMS
Research Funding: Bristol Myers Squibb, Novartis, Dynavax, Genentech
Travel, Accommodations, Expenses: Immunocore, BMS
Junchen Gu
Employment: Bristol Myers Squibb, Janssen Research & Development
Stock and Other Ownership Interests: Universal Sequencing Technology Corporation
Bin Li
Employment: Bristol Myers Squibb, Pfizer (I)
Stock and Other Ownership Interests: Bristol Myers Squibb
Travel, Accommodations, Expenses: Bristol Myers Squibb
Tian Chen
Employment: Bristol Myers Squibb
Stock and Other Ownership Interests: Bristol Myers Squibb
Patents, Royalties, Other Intellectual Property: Combination therapy with anti–Il-8 antibodies and anti–PD-1 antibodies for treating cancer
Adam Barrows
Employment: Bristol Myers Squibb
Stock and Other Ownership Interests: Bristol Myers Squibb
Andrea Horvath
Employment: Bristol Myers Squibb
Stock and Other Ownership Interests: Bristol Myers Squibb
Travel, Accommodations, Expenses: Bristol Myers Squibb
Janis M. Taube
Consulting or Advisory Role: Bristol Myers Squibb, MedImmune,, Merck, Compugen, Akoya Biosciences
Research Funding: Bristol Myers Squibb, Akoya Biosciences (Inst)
Patents, Royalties, Other Intellectual Property: Image processing of multiplex IF/IHC slides (Inst)
Travel, Accommodations, Expenses: Bristol Myers Squibb, MedImmune,, Merck, Amgen
Paul Nghiem
Consulting or Advisory Role: EMD Serono, Pfizer, Merck Sharp & Dohme, 4SC, Regeneron/Sanofi
Research Funding: Bristol Myers Squibb, EMD Serono
Patents, Royalties, Other Intellectual Property: Patent pending for high affinity T-cell receptors that target the Merkel polyomavirus.
Travel, Accommodations, Expenses: Sanofi/Regeneron, Merck Sharp & Dohme
No other potential conflicts of interest were reported.
REFERENCES
- 1. Harms KL, Healy MA, Nghiem P, et al: Analysis of prognostic factors from 9387 Merkel cell carcinoma cases forms the basis for the new 8th edition AJCC staging system. Ann Surg Oncol 23:3564-3571, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harms PW, Harms KL, Moore PS, et al. The biology and treatment of Merkel cell carcinoma: Current understanding and research priorities. Nat Rev Clin Oncol. 2018;15:763–776. doi: 10.1038/s41571-018-0103-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harms PW, Vats P, Verhaegen ME, et al. The distinctive mutational spectra of polyomavirus-negative Merkel cell carcinoma. Cancer Res. 2015;75:3720–3727. doi: 10.1158/0008-5472.CAN-15-0702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wong SQ, Waldeck K, Vergara IA, et al. UV-associated mutations underlie the etiology of MCV-negative Merkel cell carcinomas. Cancer Res. 2015;75:5228–5234. doi: 10.1158/0008-5472.CAN-15-1877. [DOI] [PubMed] [Google Scholar]
- 5.Goh G, Walradt T, Markarov V, et al. Mutational landscape of MCPyV-positive and MCPyV-negative Merkel cell carcinomas with implications for immunotherapy. Oncotarget. 2016;7:3403–3415. doi: 10.18632/oncotarget.6494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iyer JG, Blom A, Doumani R, et al. Response rates and durability of chemotherapy among 62 patients with metastatic Merkel cell carcinoma. Cancer Med. 2016;5:2294–2301. doi: 10.1002/cam4.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lipson EJ, Vincent JG, Loyo M, et al. PD-L1 expression in the Merkel cell carcinoma microenvironment: Association with inflammation, Merkel cell polyomavirus and overall survival. Cancer Immunol Res. 2013;1:54–63. doi: 10.1158/2326-6066.CIR-13-0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nghiem PT, Bhatia S, Lipson EJ, et al. PD-1 blockade with pembrolizumab in advanced Merkel-cell carcinoma. N Engl J Med. 2016;374:2542–2552. doi: 10.1056/NEJMoa1603702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaufman HL, Russell J, Hamid O, et al. Avelumab in patients with chemotherapy-refractory metastatic Merkel cell carcinoma: A multicentre, single-group, open-label, phase 2 trial. Lancet Oncol. 2016;17:1374–1385. doi: 10.1016/S1470-2045(16)30364-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.D’Angelo SP, Russell J, Lebbé C, et al. Efficacy and safety of first-line avelumab treatment in patients with stage IV metastatic Merkel cell carcinoma: A preplanned interim analysis of a clinical trial. JAMA Oncol. 2018;4:e180077. doi: 10.1001/jamaoncol.2018.0077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nghiem P, Bhatia S, Lipson EJ, et al. Durable tumor regression and overall survival in patients with advanced Merkel cell carcinoma receiving pembrolizumab as first-line therapy. J Clin Oncol. 2019;37:693–702. doi: 10.1200/JCO.18.01896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hauschild A, Schadendorf D. Checkpoint inhibitors: A new standard of care for advanced Merkel cell carcinoma? Lancet Oncol. 2016;17:1337–1339. doi: 10.1016/S1470-2045(16)30441-7. [DOI] [PubMed] [Google Scholar]
- 13.Forde PM, Chaft JE, Smith KN, et al. Neoadjuvant PD-1 blockade in resectable lung cancer. N Engl J Med. 2018;378:1976–1986. doi: 10.1056/NEJMoa1716078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Necchi A, Anichini A, Raggi D, et al. Pembrolizumab as neoadjuvant therapy before radical cystectomy in patients with muscle-invasive urothelial bladder carcinoma (PURE-01): An open-label, single-arm, phase II study. J Clin Oncol. 2018;36:JCO1801148. doi: 10.1200/JCO.18.01148. [DOI] [PubMed] [Google Scholar]
- 15.Cloughesy TF, Mochizuki AY, Orpilla JR, et al. Neoadjuvant anti-PD-1 immunotherapy promotes a survival benefit with intratumoral and systemic immune responses in recurrent glioblastoma. Nat Med. 2019;25:477–486. doi: 10.1038/s41591-018-0337-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schalper KA, Rodriguez-Ruiz ME, Diez-Valle R, et al. Neoadjuvant nivolumab modifies the tumor immune microenvironment in resectable glioblastoma. Nat Med. 2019;25:470–476. doi: 10.1038/s41591-018-0339-5. [DOI] [PubMed] [Google Scholar]
- 17.Rozeman EA, Menzies AM, van Akkooi ACJ, et al. Identification of the optimal combination dosing schedule of neoadjuvant ipilimumab plus nivolumab in macroscopic stage III melanoma (OpACIN-neo): A multicentre, phase 2, randomised, controlled trial. Lancet Oncol. 2019;20:948–960. doi: 10.1016/S1470-2045(19)30151-2. [DOI] [PubMed] [Google Scholar]
- 18.Rozeman EA, Menzies AM, Krijgsman O, et al. 18-months relapse-free survival (RFS) and biomarker analyses of OpACIN-neo: A study to identify the optimal dosing schedule of neoadjuvant (neoadj) ipilimumab (IPI) + nivolumab (NIVO) in stage III melanoma. Ann Oncol. 2019;30:v910. [Google Scholar]
- 19.Edge SB, Byrd DR, Compton CC, et al. AJCC Cancer Staging Manual(ed 7)New York, NY: Springer; 2010 [Google Scholar]
- 20.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 21.National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) Version 4.0. https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm.
- 22.Cottrell TR, Thompson ED, Forde PM, et al. Pathologic features of response to neoadjuvant anti-PD-1 in resected non-small-cell lung carcinoma: A proposal for quantitative immune-related pathologic response criteria (irPRC) Ann Oncol. 2018;29:1853–1860. doi: 10.1093/annonc/mdy218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. doi: 10.1158/1078-0432.CCR-19-2379. Stein JE, Lipson EJ, Cottrell TR, et al: Pan-tumor pathologic scoring of response to PD-(L)1 blockade. Clin Cancer Res 26:545-551, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paulson KG, Carter JJ, Johnson LG, et al. Antibodies to merkel cell polyomavirus T antigen oncoproteins reflect tumor burden in merkel cell carcinoma patients. Cancer Res. 2010;70:8388–8397. doi: 10.1158/0008-5472.CAN-10-2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Paulson KG, Lewis CW, Redman MW, et al. Viral oncoprotein antibodies as a marker for recurrence of Merkel cell carcinoma: A prospective validation study. Cancer. 2017;123:1464–1474. doi: 10.1002/cncr.30475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shuda M, Arora R, Kwun HJ, et al. Human Merkel cell polyomavirus infection I. MCV T antigen expression in Merkel cell carcinoma, lymphoid tissues and lymphoid tumors. Int J Cancer. 2009;125:1243–1249. doi: 10.1002/ijc.24510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moshiri AS, Doumani R, Yelistratova L, et al. Polyomavirus-negative Merkel cell carcinoma: A more aggressive subtype based on analysis of 282 cases using multimodal tumor virus detection. J Invest Dermatol. 2017;137:819–827. doi: 10.1016/j.jid.2016.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chang H, Sasson A, Srinivasan S, et al. Bioinformatic methods and bridging of assay results for reliable tumor mutational burden assessment in non-small-cell lung cancer. Mol Diagn Ther. 2019;23:507–520. doi: 10.1007/s40291-019-00408-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tate JG, Bamford S, Jubb HC, et al. COSMIC: The catalogue of somatic mutations in cancer. Nucleic Acids Res. 2019;47:D941–D947. doi: 10.1093/nar/gky1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 31.Brookmeyer R, Crowley J. A confidence interval for the median survival time. Biometrics. 1982;38:29–41. [Google Scholar]
- 32.Klein JP, Moeschberger ML. Survival Analysis: Techniques for Censored and Truncated Data. New York, NY: Springer; 1997. [Google Scholar]
- 33.Greenwood M. The Natural Duration of Cancer: Reports of Public Health and Related Subjects. Vol. 33. London, United Kingdom: His Majesty’s Stationery Office; 1926. [Google Scholar]
- 34.Topalian SL, Taube JM, Pardoll DM. Neoadjuvant checkpoint blockade for cancer immunotherapy. Science. 2020;367:eaax0182. doi: 10.1126/science.aax0182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Amaria RN, Reddy SM, Tawbi HA, et al. Neoadjuvant immune checkpoint blockade in high-risk resectable melanoma. Nat Med. 2018;24:1649–1654. doi: 10.1038/s41591-018-0197-1. Erratum: Nat Med 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang AC, Orlowski RJ, Xu X, et al. A single dose of neoadjuvant PD-1 blockade predicts clinical outcomes in resectable melanoma. Nat Med. 2019;25:454–461. doi: 10.1038/s41591-019-0357-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miller NJ, Church CD, Fling SP, et al. Merkel cell polyomavirus-specific immune responses in patients with Merkel cell carcinoma receiving anti-PD-1 therapy. J Immunother Cancer. 2018;6:131. doi: 10.1186/s40425-018-0450-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kaufman HL, Russell JS, Hamid O, et al. Updated efficacy of avelumab in patients with previously treated metastatic Merkel cell carcinoma after ≥1 year of follow-up: JAVELIN Merkel 200, a phase 2 clinical trial. J Immunother Cancer. 2018;6:7. doi: 10.1186/s40425-017-0310-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Giraldo NA, Nguyen P, Engle EL, et al. Multidimensional, quantitative assessment of PD-1/PD-L1 expression in patients with Merkel cell carcinoma and association with response to pembrolizumab. J Immunother Cancer. 2018;6:99. doi: 10.1186/s40425-018-0404-0. [DOI] [PMC free article] [PubMed] [Google Scholar]