Abstract
Medicinal plants have been widely used to treat a variety of infectious and non-infectious ailments. According to one estimate, 25% of the commonly used medicines contain compounds isolated from plants. Several plants could offer a rich reserve for drug discovery of infectious diseases, particularly in an era when the latest separation techniques are available on one hand, and the human population is challenged by a number of emerging infectious diseases on the other hand. Among several other ailments, viral infections, particularly infections associated with human immunodeficiency virus type 1 (HIV-1) and 2 (HIV-2), and newly emerging infectious viruses have challenged mankind survival. Of importance, a variety of medicinal plants have shown promise to treat a number of viral infections, and some of them possess broad-spectrum antiviral activity. In the past, exploration into the antiviral activity of various promising medicinal plants was limited due to: (a) highly infectious nature of viruses and (b) lack of appropriate separation techniques for the identification of antiviral components from plants. Development of vector-based strategies, in which non-infectious molecular clone of a virus could be used for antiviral screening purposes, and advancement in separation technologies offers promise for medicinal plants usage in modern drug discovery. This article describes potential antiviral properties of medicinal plants against a diverse group of viruses, and suggests screening the potential of plants possessing broad-spectrum antiviral effects against emerging viral infections.
Keywords: Medicinal plants, Traditional medicine, Viral infections, Antivirals
1. Introduction
The history of medicinal plants dates back to the origin of human civilization on earth. Several of these may have been used to treat viral infections in the past, however, first recognized interest in their development as antiviral agent is the efforts of the Boots drug company (Nottingham, England) to screen 288 plants for anti-influenza activity (Chantrill et al., 1952). Later studies have reported the inhibitory effects of medicinal plants extracts on the replication of several viruses. Particularly herpes simplex virus type 2 (HSV-2) (Debiaggi et al., 1988), HIV (Asres and Bucar, 2005, Vermani and Garg, 2002), hepatitis B virus (HBV) (Huang et al., 2006, Kwon et al., 2005), and emerging viral infections associated with poxvirus and severe acute respiratory syndrome (SARS) virus (Kotwal et al., 2005) were strongly inhibited by various plants extracts. Most of these studies have utilized either water soluble or alcoholic extracts of medicinal plants, and limited efforts have been directed toward the identification of active natural ingredient exhibiting antiviral effects. Moreover, recent studies showing antiviral potential of plant extracts against viral strains resistant to conventional antiviral agents (Serkedjieva, 2003, Tolo et al., 2006) have challenged the modern drug discovery practices, and deem a very careful look toward exploring natural antiviral components of medicinal plants.
The molecular mechanisms associated with the antiviral effects of plant extracts may vary among different viruses. However, the potentials of plant extract to boost inherent antiviral defense of human body which involves an intricate immune system might utilize common pathways. In recent past, a number of studies have explored immunostimulatory properties of plant extracts having antiviral properties (Webster et al., 2006). The root extracts of medicinal plant Heracleum maximum Bartr. (Umbelliferae) which possess antiviral effects besides antifungal and antibacterial properties, stimulated Interleukin 6 (IL-6) production in the macrophage activation assay, confirming antiviral effects association with the immunostimulatory properties (Webster et al., 2006). Furthermore, Plantago major Linn. and P. asiatica Linn. (Plantaginaceae) commonly used plants as folk medicine in Taiwan for the treatment of infectious diseases, exhibited lymphocyte proliferation and secretion of interferon-gamma (IFN-γ) at low concentrations. Both lymphocyte proliferation activity and induced secretion of interferon-gamma (IFN-γ) are indicators of cell-mediated immune response modulation (Chiang et al., 2003). Along with similar lines, Sambucol, a product isolated from Sambucus nigra L., which is effective against various strains of influenza had shown to boost immune responses by secreting inflammatory cytokines (IL-1 beta, TNF-alpha, IL-6, and IL-8) (Barak et al., 2001).
Besides immunomodulatory effect, another intriguing finding is the broad-spectrum antiviral nature of plant extracts (Pompei et al., 1979). This could be associated with a single phytochemical, or a number of different plant constituents. Among several such observations few are: (i) an extract of the Trifollium species Secomet-V exhibited antiviral effect against a number of infectious viruses such as human papillomavirus, Marburg, influenza, HIV, HBV and HCV (Kotwal et al., 2005), (ii) Pandanin, a lectin isolated from the saline extract of the leaves of Pandanus amaryllifolius Roxb. showed antiviral effect against HSV-1 and influenza virus strain H1N1 (Ooi et al., 2004), (iii) crude extract of hop showed antiviral effect against a diverse group of viruses, suggesting the presence of broad-spectrum antiviral phytochemicals in various parts of the plants (Buckwold et al., 2004).
Medicinal plants have been used throughout the world, however, their wide usage had been limited to China, India, Japan, Pakistan, Sri Lanka, Thailand and a number of African countries. A detailed review has previously described national activities around the globe relevant to medicinal plants usage priorities (Hoareau and DaSilva, 1999). Developed countries are also turning to encourage the usage of plant-based natural medicinal product in their healthcare systems. The Natural Health Product Regulations of Canada promulgated in January 2004 is an important step toward modernization of plant-based product usage in healthcare. This regulation encourages usage of modern technology and evidence-based scientific support toward promoting medicinal plants and the associated products (Siow et al., 2005) (Table 1 ).
Table 1.
Partial list of viruses inhibited by medicinal plants
| Virus | Medicinal plant used | Antiviral effect | Reference |
|---|---|---|---|
| Herpes simplex virus (HSV) | Carissa edulis Vahl. | A medicinal plant exhibiting strong anti-HSV 1, and 2 activities both in vitro and in vivo | Tolo et al. (2006) |
| Phyllanthus urinaria L. | 1346TOGDG and geraniin isolated from Phyllanthus urinaria inhibited HSV-1 and HSV-2, respectively | Yang et al. (2007) | |
| Influenza virus | Geranium sanguineum L. | A medicinal plant reducing the infectivity of various influenza virus strains in vitro and in vivo | (Pantev et al. (2006) and Serkedjieva (1997) |
| Elderberry extract | A randomized, double-blinded placebo-controlled study revealed that elderberry extract seems to offer an efficient, safe and cost-effective treatment for influenza | Zakay-Rones et al. (2004) | |
| Hepatitis B virus | Boehmeria nivea L. | A root extract of Boehmeria nivea reduced HBV production in an in vitro and in vivo model | Huang et al. (2006) |
| Polygonum cuspidatum Sieb. & Zucc. | Inhibits hepatitis B virus in a stable HBV-producing cell line | Chang et al. (2005) | |
| Hepatitis C virus (HCV) | Saxifraga melanocentra Engl. & Irmsch. | A compound namely 1,2,3,4,6-penta-O-galloyl-beta-d-glucoside isolated from Saxifraga melanocentra | Zuo et al. (2005) |
| Poliovirus | Guazuma ulmifolia Lam. | Both plants extract inhibited poliovirus replication, as well as, blocked the synthesis of viral antigens in infected cell cultures | Felipe et al. (2006) |
| Stryphnodendron adstringens | |||
| Viral haemorrhagic septicaemia virus (VHSV) | Olea europaea L. | Leaf extract inhibited viral replication | Micol et al. (2005) |
| Severe acute respiratory syndrome-associated coronavirus (SARS-CoV) | Lycoris radiate | Lycorine, isolated from Lycoris radiate possesses anti-SARS-CoV | Li et al. (2005) |
| Human immunodeficiency virus | Phyllanthus amarus Schum. & Thonn. | Inhibits HIV replication both in vitro and in vivo | Notka et al. (2004) |
| Olive leaf extract (OLE) | Inhibits acute infection and cell-to-cell transmission of HIV-1 | Lee-Huang et al. (2003) | |
| Vesicular stomatitis virus (VSV) | Trichilia glabra L. | Leaves extract of Trichilia glabra inhibits VSV | Cella et al. (2004) |
| Human adenovirus type 1 | Black soybean extract | Inhibition of human adenovirus type 1 and coxsackievirus B1 in a dose-dependent manner | Yamai et al. (2003) |
| Dengue virus type-2 (DEN-2) | Azadirachta indica Juss. (Neem) | The aqueous extract of neem leaves inhibited DEN-2 both in vitro and in vivo | Parida et al. (2002) |
2. HIV/AIDS and medicinal plants
The first International Conference on Traditional Medicine and AIDS held in Dakar, Senegal in the year 1999, organized by the Association for the Promotion of Traditional Medicine (PROMETRA) generated considerable support for the usage of medicinal plants among HIV-infected individuals. Based on the recommendations of this meeting, parallel sessions of The Fifth Conference of Parties (COP-5) to the Convention on Biological Diversity and the International Conference on Medicinal Plants, Traditional Medicine and Local Communities in Africa for the first time placed the role of traditional medicine and HIV/AIDS on the international biodiversity agenda, and suggested the decade 2000–2010 as “Decade for the Development of African Traditional Medicine” (2000). Moreover, HIV/AIDS was selected as priority for future research and development in the area of medicinal plants in Africa. The most important recommendation of this conference was an HIV/AIDS Research Initiative on Traditional Healthcare in Africa (HARITHAF), entrusted with the responsibility of developing controlled clinical protocols for evaluating the safety and efficacy of potential phytomedicines for HIV/AIDS.
Another major interest in medicinal plants is efforts of the Canadian AIDS Treatment Information Exchange (CATIE), an organization involved in improving the health and quality of life for people living with HIV/AIDS in Canada. The CATIE has prepared a list of medicinal plants showing potential beneficial effects for HIV-infected individuals (Table 2 ) (2005b). The CATIE's “Practical Guide to Herbal Therapies for People Living with HIV” is very informative document, detailing potential role of medicinal plants in the lives of HIV-infected individuals. However, healthcare individuals should be very cautious in practicing plants/phytochemicals medicinal usage in AIDS afflicted individuals, as the information collected by the CATIE are anecdotal and lack scientific validity. Moreover, there is lack of qualified personnel in the field of medicinal plants. Appropriate clinical trials are also necessary for validating medicinal usage of plants among humans.
Table 2.
Plants and their products used among HIV-infected individualsa
| Common name | Scientific name | Activity/comments |
|---|---|---|
| Aloe | Aloe vera | (a) Jelly-like substance found in the leaves may be used to treat skin problems associated with HIV and anti-HIV drugs. (b) Acemannan, a complex sugar extracted from Aloe vera used for retroviral infection |
| Andrographis | Andrographis paniculata (Burm.f.) Nees. | (a) Antiviral effect through immunomodulation. (b) AndroVir, a drug extracted from Andrographis paniculata increased CD4+ counts and 30% decrease in viral load was recorded after 9 weeks in preliminary trials |
| Ashwagandha leaves | Withania somnifera (L.) Dunal. | Have been prescribed by Ayurvedic practitioners to rejuvenate the immune system of HIV-positive people, animal studies have confirmed immunostimulatory properties |
| Astragalus | Astragalus membranaceus Bunge. | A bone marrow stimulant and one of the first herb showing anti-HIV activity by the Chinese medicine practitioners |
| Atractylodes | Atractylodes macrocephala Koidz. | HIV-positive people have used roots of Atracylodes for improving body weight, muscle strength, also reduced immunodeficiency-associated diarrhea with immune function improvement |
| Cat's claw (inner bark) | Uncaria tomentosa Willd. | A small study showed an increase in CD4+ counts of individuals taking Uncaria tomentosa. Antioxidant and immunomodulatory properties have also been described |
| Garlic | Allium sativum L. | Amerliorate conditions associated with HIV infection such as fungal infections (thrush) and parasitic infections (cryptosporidium) |
| Ginger | Zingiber officinale Roscoe. | May be beneficial for nausea associated with antiretroviral treatment |
| Gingko (seeds) | Gingko biloba L. | Ingestion of Gingko biloba seeds itself is toxic, however, certain products isolated from seeds may prevent HIV-associated memory loss |
| Ginseng (roots) | Panax ginseng C. Meyer. (Asian); Panax quinquefolium L. (North American); Eleutherococcus senticosus (Rupr. & Maxim.) Maxim. (Siberian) | A tonic herb which improves cell-mediated immune system usually ravaged among HIV-infected individuals. Contraindicated during pregnancy |
| Goldenseal (roots) | Hydrastis canadensis L. | An alkaloid, Berberine present in Hydrastis Canadensis has potential of controlling diarrhea and weight loss among HIV-infected individuals |
| Greater Celandine (flower) | Chelidonium majus L. | Due to anticancer properties may be beneficial for HIV-associated Kaposi sarcoma |
| Hyssop (leaves and flowers) | Hyssopus officinalis L. | In vitro studies shows that Hyssopus officinalis inhibits the replication of HIV without any toxicities |
| Lemon balm | Melissa officinalis L. | In vitro antiviral properties against HIV and HSV |
| Licorice (roots) | Glycyrrhiza glabra L. | Glycyrrhizin present in Glycyrrhiza glabra have immunostimulatory properties and inhibit viral production |
| Lomatium | Lomatium dissectum (Nutt.) Mathias & Constance) Lomatium suksdorfii (Wats.) Coult. & Rose. | Both Lomatium dissectum and Lomatium suksdorfii inhibit HIV in vitro |
| Marijuana | Cannabis sativa L. | The production and sale of marijuana is illegal, however, Canadian federal government has a system to grant the legal rights to grow and possess. Medically, HIV people use it for preventing nausea and stimulating appetite |
| Olive leaf | Olea europaea L. | In vitro antiviral (against HIV) and antioxidant properties have been reported |
| Psyllium (seed and husk of plant) | Plantago ovata Forssk. | Psyllium fiber bars are useful for diarrhea, a side effect of protease inhibitors therapy among HIV-infected individuals |
| Sanguinaria | Sanguinaria canadensis | Have been used for Pneumocystis carinii pneumonia (PCP) usually encountered by HIV-infected individuals |
| Shatvari | Asparagus racemosus Willd. | Immunostimulant, in vivo studies showed that Asparagus racemosus stimulate macrophages, the immune cells involved in controlling microorganisms |
| SPV30 (boxwood extract) | Buxus sempervirens L. | Mild antiretroviral effects |
| St. John's Wort | Hypericum perforatum L. | Antiretroviral effects associated with exposure to light (photosensitive antiretroviral component). Inactivate conventional antiretrovirals and as such contraindicated for HIV patients on other medications |
| Tea tree oil | Melaleuca alternifolia | Controls HIV-associated thrush (fungal infections) |
Information based on CATIE's “Practical Guide to Herbal Therapies for People Living with HIV”.
The CATIE's “Practical Guide to Herbal Therapies for People Living with HIV” is a good start highlighting the potentials of phytochemicals extracted from plants. Further exploration and identification of natural products for controlling HIV and associated infections is necessary due to several reasons. First, efforts in the past to find an effective cure for HIV infection has met with disappointing results. Second, development of vaccine for HIV pandemic seems a far-fetched dream. The most effective therapeutic regimen for HIV-infected individuals is highly active antiretroviral therapy (HAART) which is a combination of protease inhibitors and nucleoside or non-nucleoside reverse transcriptase inhibitors. HAART can only control HIV infection among individuals continuously on therapeutic regimen and withdrawal of medication leads to reemergence of the diseases. Furthermore, based on HIV pathogenesis efforts have been made to target every step of viral life cycle starting from entry to viral morphogenesis as shown in Fig. 1 , however, none of these strategies have led to cure.
Fig. 1.
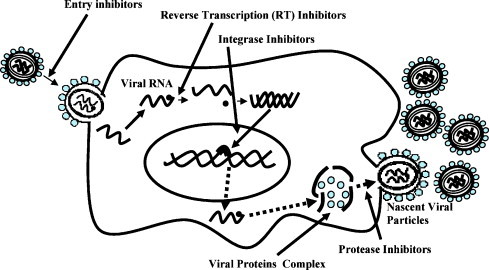
HIV life cycle and potential antiviral targets.
The plant kingdom is highly diverse and ranges from unicellular microscopic plants to long-lived huge trees. Screening of each and every plant or their individual parts (stem, leaves, roots, flowers, bark) for the identification of antiviral components is impossible. Current knowledge relevant to antiviral properties of medicinal plants is based on subjective information. There are also examples of antiviral properties of plants, or phytochemically screened products based on the properties of newly identified phytocomponents. One such example is a potential antiviral compound, cyanovirin N (CV-N), an 11-kDa protein isolated from the cyanobacterium Nostoc ellipsosporum. The CV-N possesses broad-spectrum antiviral activity besides inhibiting HIV-1 infection (Boyd et al., 1997) and is on the list of potentially emerging antiviral compounds (De Clercq, 2005, Witvrouw et al., 2005). The antiviral activity of CV-N has been dissected at molecular levels and involves highly specific interactions with the viral envelope glycoprotein gp120 (Barrientos et al., 2003, Esser et al., 1999). The viral envelope plays a major role in the infectivity of permissive cells and mediates interactions with permissive cells CD4 receptors in concert with chemokine receptors, CXCR4, CCR5. There is also a class of antiviral compounds known as HIV entry inhibitors, which interact either with viral envelope or host cell receptors mediating viral entry. Besides CV-N, Baicalin, another natural product originating from plant source interacts with chemokine receptors and inhibits entry of HIV (Kitamura et al., 1998, Li et al., 2000, Wang et al., 2004, Zhang et al., 1991). Now a days effort have been directed to characterize the anti-HIV activity of medicinal plants at molecular level. A recent study compared various plants and their individual parts (stem, leaves, roots, etc.) in inhibiting viral reverse transcriptase (RT) and integrase, the two essential enzymes in HIV infection (Bessong et al., 2006). In comparing organic solvents and aqueous fractions of various medicinal plants, the alcoholic, n-butanol fraction of the Bridelia micrantha (Hochst) showed highest anti-RT activity. None of the six potential antiviral medicinal plants tested in this study inhibited integration step. This study also identified a potential anti-RT component of a medicinal plant which requires further investigation. RT inhibitors are already in the armamentarium of anti-HIV agents. As RT plays a major role in controlling viral infectivity and replication, in the past, several reports have linked medicinal plants capability to inhibit RT activity an indirect measurement of anti-HIV effect (Fu et al., 2006, Kostova, 2006, Woradulayapinij et al., 2005).
Medicinal plants have also shown inhibitory effects on viral protease (PR), an enzyme essential for proteolytic processing of polyprotein precursor into proteins essential for the assembly of viral particles. Of importance, HAART, the most favorable therapeutic regimen for treating HIV-infected individuals also contain protease inhibitors as part of the combination drugs cocktail. In the past, phytochemicals, such as triterpene derivatives from a number of medicinal plants have shown inhibitory effects on PR (Huang and Chen, 2002, Hussein et al., 1999, Park et al., 2005, Yu et al., 2005, Yu et al., 2006). Ongoing efforts are crucial toward further development of previously characterized protease inhibitors and identification of new compounds with such activity. Naturally occurring RT and protease inhibitors in various medicinal plants also offer a reserve of unexplored antiviral compounds.
3. Human herpesviruses and medicinal plants
Several members of the human herpesviruses (HHV) family are causative agent for human diseases. The majority of HHV infections are associated with HSV-1 and HSV-2. The other members of herpesviruses causing different illnesses are: HHV-3 (varicella zoster virus), HHV-4 (Epstein Barr virus), HHV-5 (Cytomegalovirus), HHV-6, HHV-7 and HHV-8.
In the past, most of the medicinal plants have been explored against a single member of herpes family, i.e. HSV. For example, aqueous extract of plant material has been used to treat HSV-associated infections. The phytochemical, Podophyllotoxin, isolated from the aqueous extract of Podophyllum peltatum L. inhibited HSV type 1 (HSV-1) (Bedows and Hatfield, 1982). Besides aqueous extracts, organic solvents extracts of various plants have also shown anti-HSV activity, suggesting varied nature of antivirals present in medicinal plants. Two compounds namely geraniin and 1,3,4,6-tetra-O-galloyl-beta-d-glucose (1346TOGDG), isolated from the acetone extract of another plant Phyllanthus urinaria, also suppressed HSV-2 and HSV-1 (Yang et al., 2007). HSV infections are usually serious problems among immunocompromised individuals, particularly HIV-infected individuals. Moreover, development of resistant herpes strains refractory to conventional antiviral drugs is also a major issue. There has always been a quest for antiviral which can overcome resistant strains of viruses or suppress emergence of viral resistance. Based on available information one can easily speculate about the presence of natural antiviral compounds effective against resistant strains of viruses. One such precedent is an aqueous extract from the roots of Carissa edulis (Forssk.) Vahl (Apocynaceae), a medicinal plant locally growing in Kenya, showing remarkable anti-HSV activity in vitro and in vivo for both wild type and resistant strains of HSV (Tolo et al., 2006).
4. Anti-influenza virus activity of medicinal plants
The quest for influenza virus natural inhibitors is very ancient. In the past century, several scientific efforts have been directed toward identifying phytochemicals capable of inhibiting influenza virus (Cochran et al., 1966, May and Willuhn, 1978). Studies in the past and recent literature suggest that a variety of natural products isolated from several plants inhibit influenza virus both in vitro (Mothana et al., 2006, Pantev et al., 2006, Prajoubklang et al., 2005) and in vivo (Ivanova et al., 2005, Prahoveanu et al., 1986). An intriguing observation is the presence of anti-influenza activity in a wide variety of phytochemicals, such as alkaloids, flavonoids, glucosides, polyphenols, saponins (Wang et al., 2006). It is being hoped that in near future, we will be able to see an effective phytochemical for controlling the influenza virus.
5. Medicinal plants in viral hepatitis
Viral hepatitis or inflammation of the liver is caused by a number of different viruses named hepatitis A, B, C, D and E. Although exposure to any of these viruses leads to acute infection, however, type B, C, and D are unique in causing chronic infection. Traditional medicine for viral hepatitis has mainly focused on plants belonging to the genus Phyllanthus of the Euphorbiaceae family. Clinical studies were also designed to compare anti-hepatitis B effects for different species of Phyllanthus, i.e. P. amarus (L.), P. niruri (L.) and P. urinaria (L.) (Wang et al., 1995). Molecular studies on antiviral potentials of P. amarus (L.) showed its inhibitory effect on HBV polymerase activity and mRNA transcription due to interactions with HBV enhancer I and C/EBP alpha and beta transcription factors (Lee et al., 1996, Ott et al., 1997). Microarray analyses revealed the anti-HBV activity of ethanolic extract of another member of Phyllanthus (P. nanus) due to over expression of several genes particularly annexin 7 (Anx7) (Lam et al., 2006).
Usually, screening of antiviral compounds has been snagged due to the lack of representative smaller animal model. However, in the case of HBV infection in humans, duck hepatitis B virus (DHBV) model has served as an excellent screening system (Niu et al., 1990). The DHBV system has been used for screening anti-HBV effect of various medicinal plants. Based on previous observations, that suggested anti-HBV effect of Phyllanthus species, various members of this plant family were screened for their antiviral effects in DHBV model. Initial findings revealed that Phyllanthus amarus has no significant inhibitory effect on DHBV DNA replication in vivo (Niu et al., 1990). Further studies compared antiviral activity of P. amarus and P. maderas (Munshi et al., 1993a), P. maderaspatensis (Munshi et al., 1993b) in DHBV model and concluded that these medicinal plants do not have any therapeutic potential against HBV in humans as previously thought. Although P. nanus, another member of the Phyllanthus genus of plants showed strong inhibitory effect in controlling DHBV in primary cell culture model (Lam et al., 2006). Such controversial data need further investigation at molecular level to validate initial clinical data in the DHBV model. Besides resolving initial observation relevant to therapeutic development of Phyllanthus species plants, DHBV model has been very helpful in identifying a number of other plants which could be potentially utilized for isolation of candidate therapeutics for appropriate clinical trials. For example screening of 56 different Chinese medicinal herbs led to the identification of two potentials anti-DHBV plant extracts, i.e. Ardisia chinensis, and Pithecellobium clypearia (Leung et al., 2006). The identification of anti-HBV potentials of Oenanthe javanica Blume DC flavones (OjF), as a strong inhibitor of HBsAg and HBeAg secretion (involved in viral pathogenesis) in 2.2.15 cells and reducing DHBV-DNA levels in the HBV-infected duck model is also credited to this valuable model (Wang et al., 2005).
Sometimes several medicinal plants are mixed together to develop a combination therapy for treating a particular ailment. For viral hepatitis such combination therapies have also been tried. For example, liquid fermentation broth of Ganoderma lucidum on supplementation with aqueous extract of Radix Sophorae flavescentis (Chinese herbal medicine) showed strong anti-hepatitis B virus activity in vitro and in vivo. Furthermore, co-fermentation of both these medicinal plant for the development of antiviral broth showed superior antiviral effect compared with simple mixing (Li et al., 2006).
Hepatitis C is another member of the viruses causing viral hepatitis. A study reviewed the beneficial and adverse effects of herbal preparations used for the treatment of viral hepatitis in the Cochrane Collaboration, MEDLINE, EMBASE, and BIOSIS databases. Data from manual searches for five Chinese and one Japanese journals were also included besides information from randomized clinical trials (Liu et al., 2003). Evaluation of previously completed 13 randomized trials analysis included in this study showed that only four have appropriate methodologies. Disappointingly, few herbs/phytochemicals used in clinical trials have anti-HCV activity, like Silybin, and Oxymatrine, which showed a significant reduction of serum aspartate aminotransferase (AST) and gamma glutamyltranspeptidase levels and HCV clearance, respectively. A number of Chinese herbal mix such as Bing Gan Tang, Yi Zhu decoction, and Yi Er Gan Tang individually or in combination with antivirals showed beneficial effects on clearing HCV RNA and normalizing serum alanine aminotransferase (ALT). Various herbal products adverse events were also summarized in this information collection report (Liu et al., 2003). Such reports strongly suggest appropriate vigilance programs as well as involvement of international regulatory agencies for assessing the benefits of herbal medicine.
Screening programs aimed at the identification of anti-HCV medicinal plants are ongoing, particularly due to the prevalence of infection in the resource poor countries and lack of good vaccine. The methanolic extracts of Acacia nilotica L. Willd ex Delile, Boswellia carterii, Embelia schimperi, Quercus infectoria, Trachyspermum ammi L. and aqueous extracts of Piper cubeba L., Q. infectoria and Syzygium aromaticum L., showed potentials to inhibit HCV (Hussein et al., 2000). Moreover, a number of phytochemical products such as catechin, glycyrrhizin, polysterols, silymarin potentials as anti-HCV agents have also been reviewed (Jassim and Naji, 2003, Patrick, 1999).
6. Medicinal plants in emerging viral infections
Emerging viral infections are posing a major threat to the mankind. Medicinal plants exhibiting broad antiviral effects could be brought into the antiviral discovery programs for such infection. One such example is Glycyrrhizin, a bioactive component of liquorice (Glycyrrhiza uralensis Fisch), and lycorine isolated from Lycoris radiata L. initially used for certain other indication, showed strong anti-SARS-CoV activity (Li et al., 2005). A number of plants previously shown to possess broad-spectrum antiviral effects could be screened for newly emerging/resistant viral strains.
7. Medicinal plants in miscellaneous viral infections
A variety of herbal (medicinal) preparations have shown potentials for inhibiting viruses causing serious infections among humans such as measles viruses (Olila et al., 2002, Sindambiwe et al., 1999), human rotaviruses (HRV) (Husson et al., 1994, Takahashi et al., 2001), respiratory syncytial virus (RSV), human rhinoviruses (Glatthaar-Saalmuller et al., 2001), coxsackie group of viruses (Evstropov et al., 2004, Su et al., 2006), neurotropic Sindbis virus (NSV) (Paredes et al., 2001) and various strains of poliovirus (Andrighetti-Frohner et al., 2005, Melo et al., 2006, Vilagines et al., 1985). To prove/disprove antiviral effect of any herbal preparation it is essential to molecularly dissect its effects. Very few studies have addressed this important aspect relevant to the therapeutic development of phytochemicals. One such example is the molecular study of the hot water extracts of Stevia rebaudiana L. which blocked entry of various infectious serotypes of HRV into the permissive cells by an anionic polysaccharide having a molecular weight of 9800 with uronic acid as a major sugar constituent (Takahashi et al., 2001). Similarly an alkaloid extract of Haemanthus albiflos bulbs inhibited RNA synthesis of HRV propagated in MA-104 cells (Husson et al., 1994).
Based on all the above information, it can be fairly concluded that medicinal plants offer a variety of anti-infectious compounds, particularly antiviral agents. Screening programs aimed at identifying potential anti-infectious agent from medicinal plants offer a great potential in the field of pharmaceutical developments.
8. Isolation and characterization of plant components exhibiting medicinal characteristics
The very first and basic step towards evaluating the therapeutic potential of a medicinal plant is preparation of crude cellular lysate of the plant matrix followed by extraction of various components having potential medicinal value. There are several books and reviews describing standardized extraction procedures from medicinal plants. A few to start with could be: Plant Drug Analysis: A Thin Layer Chromatography Atlas (Wagner et al., 1996), Modern Phytomedicine: Turning Medicinal Plants into Drugs (Ahmad et al., 2007) and Laboratory Handbook for the Fractionation of Natural Extracts (Houghton and Raman, 1998). Most of the books published in phytomedicine have mainly paid attention to classical isolation procedures. Unfortunately, some of these classical procedures have limitations of reproducibility and quality, thus compromising the safety and efficacy of phytomedicinal preparations. As such there is an urgent need to refine and further develop classical methodologies to obtain procedural consistency and highly pure plant components exhibiting medicinal value. In recent past, increased interest in traditional medicine has complemented quality awareness and refinement in extraction methodologies and standardization of procedures for phytochemedicinal products isolation (Atta-ur-Rahman and Choudhary, 1998, Ong, 2004). To ensure high quality herbal preparations, efforts are ongoing to replace traditional methodologies with modern sample preparation and extraction procedures. Classical solvent separation of phytomedicinal products is being complemented with modern techniques like microwave-assisted extraction, pressurized-liquid extraction, matrix-assisted laser desorption/ionization mass spectrometry (Wu et al., 2007) and several others. To further facilitate plant-based drug discovery efforts are also being directed toward standardization of methodologies which can be used to study pharmacokines/pharmacodynamics behavior of phytomedicinal products (Lin et al., 2005).
9. Future directions in antiviral potentials of medicinal plants
The importance of medicinal plants can be ascertained from the fact that according to the World Health Organization (WHO) estimates, 80% of the World's population fulfills their healthcare needs from phytomedicinal sources (2005a). Programs aimed at inclusion of medicinal plants into the healthcare need of the people should be sponsored in countries where plants and their products are practiced for medical needs. The countries having heavy usage of traditional medicine should sponsor scientific programs devoted toward modern drug discovery from phytochemicals. Improved separation technologies offer potentials to screen medicinal plant's anti-infectious/antiviral nature. Several problems previously thought to be hurdle in the medicinal plants antiviral drug discovery program are no longer an issue. For example screening of the antiviral potentials of plant extracts always posed a threat of incidental infection to the workers. Vector-based assay techniques have been very helpful in overcoming such screening hurdles, i.e. recombinant viral vectors mimicking the infection and expressing firefly luciferase marker gene have been widely used to screen a variety of antivirals (Esimone et al., 2005).
A clever usage of plants is the production of vaccines and protein-based therapeutics. Several scientific reports suggest that plant could offer a good source for the production of pharmaceutical grade peptides/proteins (Glenz and Warzecha, 2006, Koprowski and Yusibov, 2001). Since the expression of first subunit vaccine for HBV surface antigen in 1992, several other different vaccine antigens have been successfully expressed in plants and their safety has been assessed in animal and humans (Glenz and Warzecha, 2006, Ma et al., 2003, Thanavala et al., 2005). While considering this aspect of plants for treating human viral diseases one has to be careful, as majority of viral vaccines are constituted of attenuated or inactivated viral particles. Due to these limitations efforts have been directed toward expressing coat proteins of different viruses which are presumed to assemble as virus-like-particles (VLP) in plants and are antigenic in nature. Several other issues like appropriate processing of protein to be expressed in plants are important aspects to be considered.
There are other issues to be resolved before the translational usage of medicinal plants in the developed world. One such issue is the isolation of active ingredient associated with the medicinal characteristics of a particular plant. In certain parts of the world crude plant extracts are used in healthcare and their efficacy is well-documented without any side effects. Although it will be hard to get these plant extracts approved through international regulatory agencies such as the Food and Drug Administration (FDA) of the United States of America or other equivalent European counterparts, however, for countries with limited resources, government sponsored explorations will serve as a gateway for merging of modern drug discovery with conventional Chinese/Eastern medicine. Moreover, considering the problems faced by the developed world such as drug resistance and failure in finding an effective vaccine for deadly infectious agent such as HIV, a causative agent for deadly AIDS, phytomedicinal products may provide a hope.
Although, herbal (medicinal plants) preparations are widely used in several parts of the world, individually or in combination, data about the interactions of medicinal plants on living system is non-existent. It is only experience of the indigenous people using a particular plant/phytochemical product for treating an ailment. Clinical findings such as co-administration of medicinal plants kava-kava and St. John's Wort leading to hepatotoxicity (Musch et al., 2006) should be made available to the healthcare providers practicing traditional medicine. Generally, herbal remedies are perceived as harmless, however, several reports suggest hepatotoxicity associated with herbal medication (Pak et al., 2004, Schiano, 2003). Publication of scientific reports relevant to the cytotoxicities of medicinal plants usage should be encouraged and incorporated into a universal database system. Moreover, larger randomized, double-blind, placebo-controlled multicenter clinical trials should be conducted before incorporation of a particular herbal remedy in treating people.
Acknowledgements
MM received support from the Department of Microbiology & Immunology, Drexel University College of Medicine, Philadelphia, PA, USA and the Higher Education Commission of Pakistan. ZP and BW are funded from the National Institutes of Health.
References
- Ahmad I., Aqil F., Owais M. 1st ed. Wiley-VCH, Verlag GmbH & Co; KGaA, Weinheim: 2007. Modern Phytomedicine: Turning Medicinal Plants into Drugs. [Google Scholar]
- Andrighetti-Frohner C.R., Sincero T.C., da Silva A.C., Savi L.A., Gaido C.M., Bettega J.M., Mancini M., de Almeida M.T., Barbosa R.A., Farias M.R., Barardi C.R., Simoes C.M. Antiviral evaluation of plants from Brazilian Atlantic Tropical Forest. Fitoterapia. 2005;76(3/4):374–378. doi: 10.1016/j.fitote.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Anon Traditional Medicine & HIV/AIDS in Africa. A report from the international conference on medicinal plants, traditional medicine & local communities in Africa. A Parallel Session to the Fifth Conference of the Parties to the Convention on Biological Diversity; Nairobi, Kenya, May 16–19, 2000; 2000. [DOI] [PubMed] [Google Scholar]
- Anon., 2005a. Medicinal plants—time to harness the potential of neglected genetic resources? Plant Genetic Resources: Characterization and Utilization. http://www.cabipublishing.org/pgr.
- Anon., 2005b. A Practical Guide to Herbal Therapies for People Living with HIV. Canadian AIDS Treatment Information Exchange (CATIE).
- Asres K., Bucar F. Anti-HIV activity against immunodeficiency virus type 1 (HIV-I) and type II (HIV-II) of compounds isolated from the stem bark of Combretum molle. Ethiop. Med. J. 2005;43(1):15–20. [PubMed] [Google Scholar]
- Atta-ur-Rahman, Choudhary M.I. New natural products from medicinal plants of Pakistan. Pure Appl. Chem. 1998;70:385–389. [Google Scholar]
- Barak V., Halperin T., Kalickman I. The effect of Sambucol, a black elderberry-based, natural product, on the production of human cytokines: I. Inflammatory cytokines. Eur. Cytokine Netw. 2001;12(2):290–296. [PubMed] [Google Scholar]
- Barrientos L.G., O’Keefe B.R., Bray M., Sanchez A., Gronenborn A.M., Boyd M.R. Cyanovirin-N binds to the viral surface glycoprotein, GP120 and inhibits infectivity of Ebola virus. Antiviral Res. 2003;58(1):47–56. doi: 10.1016/s0166-3542(02)00183-3. [DOI] [PubMed] [Google Scholar]
- Bedows E., Hatfield G.M. An investigation of the antiviral activity of Podophyllum peltatum. J. Nat. Prod. 1982;45(6):725–729. doi: 10.1021/np50024a015. [DOI] [PubMed] [Google Scholar]
- Bessong P.O., Rojas L.B., Obi L.C., Tshisikawe P.M., Igunbor E.O. Further screening of Venda medicinal plants for activity against HIV type 1 reverse transcriptase and integrase. Afr. J. Biotechnol. 2006;5(6):526–528. [Google Scholar]
- Boyd M.R., Gustafson K.R., McMahon J.B., Shoemaker R.H., O’Keefe B.R., Mori T., Gulakowski R.J., Wu L., Rivera M.I., Laurencot C.M., Currens M.J., Cardellina J.H., II, Buckheit R.W., Jr., Nara P.L., Pannell L.K., Sowder R.C., II, Henderson L.E. Discovery of cyanovirin-N, a novel human immunodeficiency virus-inactivating protein that binds viral surface envelope glycoprotein gp120: potential applications to microbicide development. Antimicrob. Agents Chemother. 1997;41(7):1521–1530. doi: 10.1128/aac.41.7.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckwold V.E., Wilson R.J., Nalca A., Beer B.B., Voss T.G., Turpin J.A., Buckheit R.W., III, Wei J., Wenzel-Mathers M., Walton E.M., Smith R.J., Pallansch M., Ward P., Wells J., Chuvala L., Sloane S., Paulman R., Russell J., Hartman T., Ptak R. Antiviral activity of hop constituents against a series of DNA and RNA viruses. Antiviral Res. 2004;61(1):57–62. doi: 10.1016/s0166-3542(03)00155-4. [DOI] [PubMed] [Google Scholar]
- Cella M., Riva D.A., Coulombie F.C., Mersich S.E. Virucidal activity presence in Trichilia glabra leaves. Rev. Argent Microbiol. 2004;36(3):136–138. [PubMed] [Google Scholar]
- Chang J.S., Liu H.W., Wang K.C., Chen M.C., Chiang L.C., Hua Y.C., Lin C.C. Ethanol extract of Polygonum cuspidatum inhibits hepatitis B virus in a stable HBV-producing cell line. Antiviral Res. 2005;66(1):29–34. doi: 10.1016/j.antiviral.2004.12.006. [DOI] [PubMed] [Google Scholar]
- Chantrill B.H., Coulthard C.E., Dickinson L., Inkley G.W., Morris W., Pyle A.H. The action of plant extracts on a bacteriophage of Pseudomonas pyocyanea and on influenza A virus. J. Gen. Microbiol. 1952;6(1–2):74–84. doi: 10.1099/00221287-6-1-2-74. [DOI] [PubMed] [Google Scholar]
- Chiang L.C., Chiang W., Chang M.Y., Lin C.C. In vitro cytotoxic, antiviral and immunomodulatory effects of Plantago major and Plantago asiatica. Am. J. Chin. Med. 2003;31(2):225–234. doi: 10.1142/S0192415X03000874. [DOI] [PubMed] [Google Scholar]
- Cochran K.W., Nishikawa T., Beneke E.S. Botanical sources of influenza inhibitors. Antimicrob. Agents Chemother. (Bethesda) 1966;6:515–520. [PubMed] [Google Scholar]
- De Clercq E. Emerging anti-HIV drugs. Expert Opin. Emerg. Drugs. 2005;10(2):241–273. doi: 10.1517/14728214.10.2.241. [DOI] [PubMed] [Google Scholar]
- Debiaggi M., Pagani L., Cereda P.M., Landini P., Romero E. Antiviral activity of Chamaecyparis lawsoniana extract: study with herpes simplex virus type 2. Microbiologica. 1988;11(1):55–61. [PubMed] [Google Scholar]
- Esimone C.O., Grunwald T., Wildner O., Nchinda G., Tippler B., Proksch P., Uberla K. In vitro pharmacodynamic evaluation of antiviral medicinal plants using a vector-based assay technique. J. Appl. Microbiol. 2005;99(6):1346–1355. doi: 10.1111/j.1365-2672.2005.02732.x. [DOI] [PubMed] [Google Scholar]
- Esser M.T., Mori T., Mondor I., Sattentau Q.J., Dey B., Berger E.A., Boyd M.R., Lifson J.D. Cyanovirin-N binds to gp120 to interfere with CD4-dependent human immunodeficiency virus type 1 virion binding, fusion, and infectivity but does not affect the CD4 binding site on gp120 or soluble CD4-induced conformational changes in gp120. J. Virol. 1999;73(5):4360–4371. doi: 10.1128/jvi.73.5.4360-4371.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evstropov A.N., Burova L.G., Orlovskaia I.A., Grek O.R., Zakharova L.N., Volkhonskaia T.A. Anti-enterovirus and immunostimulating activity of the polyphenol complex extracted from Pethaphylloides fruticosa (L.) O. Schwarz. Vopr. Virusol. 2004;49(6):30–33. [PubMed] [Google Scholar]
- Felipe A.M., Rincao V.P., Benati F.J., Linhares R.E., Galina K.J., de Toledo C.E., Lopes G.C., de Mello J.C., Nozawa C. Antiviral effect of Guazuma ulmifolia and Stryphnodendron adstringens on Poliovirus and Bovine Herpesvirus. Biol. Pharm. Bull. 2006;29(6):1092–1095. doi: 10.1248/bpb.29.1092. [DOI] [PubMed] [Google Scholar]
- Fu M., Ng T.B., Jiang Y., Pi Z.F., Liu Z.K., Li L., Liu F. Compounds from rose (Rosa rugosa) flowers with human immunodeficiency virus type 1 reverse transcriptase inhibitory activity. J. Pharm. Pharmacol. 2006;58(9):1275–1280. doi: 10.1211/jpp.58.9.0015. [DOI] [PubMed] [Google Scholar]
- Glatthaar-Saalmuller B., Sacher F., Esperester A. Antiviral activity of an extract derived from roots of Eleutherococcus senticosus. Antiviral Res. 2001;50(3):223–228. doi: 10.1016/s0166-3542(01)00143-7. [DOI] [PubMed] [Google Scholar]
- Glenz K., Warzecha H. New medicinal plants for the production of vaccines. J. Verbr. Lebensm. 2006;1:126–130. [Google Scholar]
- Hoareau L., DaSilva E.J. Medicinal plants: a re-emerging health aid. Electr. J. Biotechnol. 1999;2(2) http://www.ejb.org/content/vol2/issue2/full2 [Google Scholar]
- Houghton P.J., Raman A. Chapman and Hall; New York: 1998. Laboratory Handbook for the Fractionation of Natural Extracts. [Google Scholar]
- Huang K.L., Lai Y.K., Lin C.C., Chang J.M. Inhibition of hepatitis B virus production by Boehmeria nivea root extract in HepG2 2.2.15 cells. World J. Gastroenterol. 2006;12(35):5721–5725. doi: 10.3748/wjg.v12.i35.5721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L., Chen C.H. Molecular targets of anti-HIV-1 triterpenes. Curr. Drug Targets Infect. Disord. 2002;2(1):33–36. doi: 10.2174/1568005024605936. [DOI] [PubMed] [Google Scholar]
- Hussein G., Miyashiro H., Nakamura N., Hattori M., Kakiuchi N., Shimotohno K. Inhibitory effects of Sudanese medicinal plant extracts on hepatitis C virus (HCV) protease. Phytother. Res. 2000;14(7):510–516. doi: 10.1002/1099-1573(200011)14:7<510::aid-ptr646>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Hussein G., Miyashiro H., Nakamura N., Hattori M., Kawahata T., Otake T., Kakiuchi N., Shimotohno K. Inhibitory effects of Sudanese plant extracts on HIV-1 replication and HIV-1 protease. Phytother. Res. 1999;13(1):31–36. doi: 10.1002/(SICI)1099-1573(199902)13:1<31::AID-PTR381>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Husson G.P., Vilagines P., Sarrette B., Vilagines R. Study of antiviral action of total alkaloids from Haemanthus albiflos. Ann. Pharm. Fr. 1994;52(6):311–322. [PubMed] [Google Scholar]
- Ivanova E., Toshkova R., Serkedjieva J. A plant polyphenol-rich extract restores the suppressed functions of phagocytes in influenza virus-infected mice. Microbes Infect. 2005;7(3):391–398. doi: 10.1016/j.micinf.2004.11.013. [DOI] [PubMed] [Google Scholar]
- Jassim S.A.A., Naji M.A. Novel antiviral agents: a medicinal plant perspective. J. Appl. Microbiol. 2003;95:412–427. doi: 10.1046/j.1365-2672.2003.02026.x. [DOI] [PubMed] [Google Scholar]
- Kitamura K., Honda M., Yoshizaki H., Yamamoto S., Nakane H., Fukushima M., Ono K., Tokunaga T. Baicalin, an inhibitor of HIV-1 production in vitro. Antiviral Res. 1998;37(2):131–140. doi: 10.1016/s0166-3542(97)00069-7. [DOI] [PubMed] [Google Scholar]
- Koprowski H., Yusibov V. The green revolution: plants as heterologous expression vectors. Vaccine. 2001;19:2735–2741. doi: 10.1016/s0264-410x(00)00511-9. [DOI] [PubMed] [Google Scholar]
- Kostova I. Coumarins as inhibitors of HIV reverse transcriptase. Curr. HIV Res. 2006;4(3):347–363. doi: 10.2174/157016206777709393. [DOI] [PubMed] [Google Scholar]
- Kotwal G.J., Kaczmarek J.N., Leivers S., Ghebremariam Y.T., Kulkarni A.P., Bauer G., C D.E.B., Preiser W., Mohamed A.R. Anti-HIV, anti-Poxvirus, and anti-SARS activity of a nontoxic, acidic plant extract from the Trifollium Species Secomet-V/anti-Vac suggests that it contains a novel broad-spectrum antiviral. Ann. NY Acad. Sci. 2005;1056:293–302. doi: 10.1196/annals.1352.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon D.H., Kwon H.Y., Kim H.J., Chang E.J., Kim M.B., Yoon S.K., Song E.Y., Yoon D.Y., Lee Y.H., Choi I.S., Choi Y.K. Inhibition of hepatitis B virus by an aqueous extract of Agrimonia eupatoria L. Phytother. Res. 2005;19(4):355–358. doi: 10.1002/ptr.1689. [DOI] [PubMed] [Google Scholar]
- Lam W.Y., Leung K.T., Law P.T., Lee S.M., Chan H.L., Fung K.P., Ooi V.E., Waye M.M. Antiviral effect of Phyllanthus nanus ethanolic extract against hepatitis B virus (HBV) by expression microarray analysis. J. Cell Biochem. 2006;97(4):795–812. doi: 10.1002/jcb.20611. [DOI] [PubMed] [Google Scholar]
- Lee C.D., Ott M., Thyagarajan S.P., Shafritz D.A., Burk R.D., Gupta S. Phyllanthus amarus down-regulates hepatitis B virus mRNA transcription and replication. Eur. J. Clin. Invest. 1996;26(12):1069–1076. doi: 10.1046/j.1365-2362.1996.410595.x. [DOI] [PubMed] [Google Scholar]
- Lee-Huang S., Zhang L., Huang P.L., Chang Y.T., Huang P.L. Anti-HIV activity of olive leaf extract (OLE) and modulation of host cell gene expression by HIV-1 infection and OLE treatment. Biochem. Biophys. Res. Commun. 2003;307(4):1029–1037. doi: 10.1016/s0006-291x(03)01292-0. [DOI] [PubMed] [Google Scholar]
- Leung K.T., Chiu L.C., Lam W.S., Li Y., Sun S.S., Ooi V.E. In vitro antiviral activities of Chinese medicinal herbs against duck hepatitis B virus. Phytother. Res. 2006;20(10):911–914. doi: 10.1002/ptr.1969. [DOI] [PubMed] [Google Scholar]
- Li B.Q., Fu T., Dongyan Y., Mikovits J.A., Ruscetti F.W., Wang J.M. Flavonoid baicalin inhibits HIV-1 infection at the level of viral entry. Biochem. Biophys. Res. Commun. 2000;276(2):534–538. doi: 10.1006/bbrc.2000.3485. [DOI] [PubMed] [Google Scholar]
- Li S.Y., Chen C., Zhang H.Q., Guo H.Y., Wang H., Wang L., Zhang X., Hua S.N., Yu J., Xiao P.G., Li R.S., Tan X. Identification of natural compounds with antiviral activities against SARS-associated coronavirus. Antiviral Res. 2005;67(1):18–23. doi: 10.1016/j.antiviral.2005.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Yang Y., Fang L., Zhang Z., Jin J., Zhang K. Anti-hepatitis activities in the broth of Ganoderma lucidum supplemented with a Chinese herbal medicine. Am. J. Chin. Med. 2006;34(2):341–349. doi: 10.1142/S0192415X06003874. [DOI] [PubMed] [Google Scholar]
- Lin Z.J., Qiu S.X., Wufuer A., Shum L. Simultaneous determination of glycyrrhizin, a marker component in radix Glycyrrhizae, and its major metabolite glycyrrhetic acid in human plasma by LC–MS/MS. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2005;814(2):201–207. doi: 10.1016/j.jchromb.2004.10.026. [DOI] [PubMed] [Google Scholar]
- Liu J., Manheimer E., Tsutani K., Gluud C. Medicinal herbs for hepatitis C virus infection: a Cochrane hepatobiliary systematic review of randomized trials. Am. J. Gastroenterol. 2003;98(3):538–544. doi: 10.1111/j.1572-0241.2003.07298.x. [DOI] [PubMed] [Google Scholar]
- Ma J.K., Drake P.M., Christou P. The production of recombinant pharmaceutical proteins in plants. Nat. Rev. Genet. 2003;4:794–805. doi: 10.1038/nrg1177. [DOI] [PubMed] [Google Scholar]
- May G., Willuhn G. Antiviral effect of aqueous plant extracts in tissue culture. Arzneimittelforschung. 1978;28(1):1–7. [PubMed] [Google Scholar]
- Melo F.L., Benati F.J., Junior W.A., de Mello J.C., Nozawa C., Linhares R.E. The in vitro antivirial activity of an aliphatic nitro compound from Heteropteris aphrodisiaca. Microbiol. Res. 2006 doi: 10.1016/j.micres.2006.03.011. (PMID: 16735108) [DOI] [PubMed] [Google Scholar]
- Micol V., Caturla N., Perez-Fons L., Mas V., Perez L., Estepa A. The olive leaf extract exhibits antiviral activity against viral haemorrhagic septicaemia rhabdovirus (VHSV) Antiviral Res. 2005;66(2/3):129–136. doi: 10.1016/j.antiviral.2005.02.005. [DOI] [PubMed] [Google Scholar]
- Mothana R.A., Mentel R., Reiss C., Lindequist U. Phytochemical screening and antiviral activity of some medicinal plants from the island Soqotra. Phytother. Res. 2006;20(4):298–302. doi: 10.1002/ptr.1858. [DOI] [PubMed] [Google Scholar]
- Munshi A., Mehrotra R., Panda S.K. Evaluation of Phyllanthus amarus and Phyllanthus maderaspatensis as agents for postexposure prophylaxis in neonatal duck hepatitis B virus infection. J. Med. Virol. 1993;40(1):53–58. doi: 10.1002/jmv.1890400111. [DOI] [PubMed] [Google Scholar]
- Munshi A., Mehrotra R., Ramesh R., Panda S.K. Evaluation of antihepadnavirus activity of Phyllanthus amarus and Phyllanthus maderaspatensis in duck hepatitis B virus carrier Pekin ducks. J. Med. Virol. 1993;41(4):275–281. doi: 10.1002/jmv.1890410404. [DOI] [PubMed] [Google Scholar]
- Musch E., Chrissafidou A., Malek M. Acute hepatitis due to kava-kava and St. John's Wort: an immune-mediated mechanism? Dtsch. Med. Wochenschr. 2006;131(21):1214–1217. doi: 10.1055/s-2006-941754. [DOI] [PubMed] [Google Scholar]
- Niu J.Z., Wang Y.Y., Qiao M., Gowans E., Edwards P., Thyagarajan S.P., Gust I., Locarnini S. Effect of Phyllanthus amarus on duck hepatitis B virus replication in vivo. J. Med. Virol. 1990;32(4):212–218. doi: 10.1002/jmv.1890320404. [DOI] [PubMed] [Google Scholar]
- Notka F., Meier G., Wagner R. Concerted inhibitory activities of Phyllanthus amarus on HIV replication in vitro and ex vivo. Antiviral Res. 2004;64(2):93–102. doi: 10.1016/j.antiviral.2004.06.010. [DOI] [PubMed] [Google Scholar]
- Olila D., Olwa O., Opuda-Asibo J. Screening extracts of Zanthoxylum chalybeum and Warburgia ugandensis for activity against measles virus (Swartz and Edmonston strains) in vitro. Afr. Health Sci. 2002;2(1):2–10. [PMC free article] [PubMed] [Google Scholar]
- Ong E.S. Extraction methods and chemical standardization of botanicals and herbal preparations. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2004;812(1/2):23–33. doi: 10.1016/j.jchromb.2004.07.041. [DOI] [PubMed] [Google Scholar]
- Ooi L.S., Sun S.S., Ooi V.E. Purification and characterization of a new antiviral protein from the leaves of Pandanus amaryllifolius (Pandanaceae) Int. J. Biochem. Cell Biol. 2004;36(8):1440–1446. doi: 10.1016/j.biocel.2004.01.015. [DOI] [PubMed] [Google Scholar]
- Ott M., Thyagarajan S.P., Gupta S. Phyllanthus amarus suppresses hepatitis B virus by interrupting interactions between HBV enhancer I and cellular transcription factors. Eur. J. Clin. Invest. 1997;27(11):908–915. doi: 10.1046/j.1365-2362.1997.2020749.x. [DOI] [PubMed] [Google Scholar]
- Pak E., Esrason K.T., Wu V.H. Hepatotoxicity of herbal remedies: an emerging dilemma. Prog. Transplant. 2004;14(2):91–96. doi: 10.1177/152692480401400203. [DOI] [PubMed] [Google Scholar]
- Pantev A., Ivancheva S., Staneva L., Serkedjieva J. Biologically active constituents of a polyphenol extract from Geranium sanguineum L. with anti influenza activity. Z Naturforsch. [C] 2006;61(7/8):508–516. doi: 10.1515/znc-2006-7-807. [DOI] [PubMed] [Google Scholar]
- Paredes A., Hasegawa M., Prieto F., Mendez J., Rodriguez M., Rodriguez-Ortega M. Biological activity of Guatteria cardoniana fractions. J. Ethnopharmacol. 2001;78(2/3):129–132. doi: 10.1016/s0378-8741(01)00315-4. [DOI] [PubMed] [Google Scholar]
- Parida M.M., Upadhyay C., Pandya G., Jana A.M. Inhibitory potential of neem (Azadirachta indica Juss) leaves on dengue virus type-2 replication. J. Ethnopharmacol. 2002;79(2):273–278. doi: 10.1016/s0378-8741(01)00395-6. [DOI] [PubMed] [Google Scholar]
- Park J.C., Kim S.C., Choi M.R., Song S.H., Yoo E.J., Kim S.H., Miyashiro H., Hattori M. Anti-HIV protease activity from rosa family plant extracts and rosamultin from Rosa rugosa. J. Med. Food. 2005;8(1):107–109. doi: 10.1089/jmf.2005.8.107. [DOI] [PubMed] [Google Scholar]
- Patrick L. Hepatitis C: epidemiology and review of complementary/alternative medicine treatments. Altern. Med. Rev. 1999;4:220–238. [PubMed] [Google Scholar]
- Pompei R., Flore O., Marccialis M.A., Pani A., Loddo B. Glycyrrhizic acid inhibits virus growth and inactivates virus particles. Nature. 1979;281(5733):689–690. doi: 10.1038/281689a0. [DOI] [PubMed] [Google Scholar]
- Prahoveanu E., Esanu V., Anton G., Frunzulica S. Prophylactic effect of a Beta vulgaris extract on experimental influenza infection in mice. Virologie. 1986;37(2):121–123. [PubMed] [Google Scholar]
- Prajoubklang A., Sirithunyalug B., Charoenchai P., Suvannakad R., Sriubolmas N., Piyamongkol S., Kongsaeree P., Kittakoop P. Bioactive deoxypreussomerins and dimeric naphthoquinones from Diospyros ehretioides fruits: deoxypreussomerins may not be plant metabolites but may be from fungal epiphytes or endophytes. Chem. Biodivers. 2005;2(10):1358–1367. doi: 10.1002/cbdv.200590108. [DOI] [PubMed] [Google Scholar]
- Schiano T.D. Hepatotoxicity and complementary and alternative medicines. Clin. Liver Dis. 2003;7(2):453–473. doi: 10.1016/s1089-3261(03)00030-8. [DOI] [PubMed] [Google Scholar]
- Serkedjieva J. Anti-infective activity of a plant preparation from Geranium sanguineum L. Pharmazie. 1997;52(10):799–802. [PubMed] [Google Scholar]
- Serkedjieva J. Influenza virus variants with reduced susceptibility to inhibition by a polyphenol extract from Geranium sanguineum L. Pharmazie. 2003;58(1):53–57. [PubMed] [Google Scholar]
- Sindambiwe J.B., Calomme M., Cos P., Totte J., Pieters L., Vlietinck A., Vanden Berghe D. Screening of seven selected Rwandan medicinal plants for antimicrobial and antiviral activities. J. Ethnopharmacol. 1999;65(1):71–77. doi: 10.1016/s0378-8741(98)00154-8. [DOI] [PubMed] [Google Scholar]
- Siow Y.L., Gong Y., Au-Yeung K.K., Woo C.W., Choy P.C., O K. Emerging issues in traditional Chinese medicine. Can. J. Physiol. Pharmacol. 2005;83(4):321–334. doi: 10.1139/y05-029. [DOI] [PubMed] [Google Scholar]
- Su M., Li Y., Leung K.T., Cen Y., Li T., Chen R., Ooi V.E. Antiviral activity and constituent of Ardisia chinensis benth against coxsackie B3 virus. Phytother. Res. 2006;20(8):634–639. doi: 10.1002/ptr.1912. [DOI] [PubMed] [Google Scholar]
- Takahashi K., Matsuda M., Ohashi K., Taniguchi K., Nakagomi O., Abe Y., Mori S., Sato N., Okutani K., Shigeta S. Analysis of anti-rotavirus activity of extract from Stevia rebaudiana. Antiviral Res. 2001;49(1):15–24. doi: 10.1016/s0166-3542(00)00134-0. [DOI] [PubMed] [Google Scholar]
- Thanavala Y., Mahoney M., Pal S., Scott A., Richter L., Natarajan N., Goodwin P., Arntzen C., Mason H. Immunogenicity in humans of an edible vaccine for hepatitis B. Proc. Natl. Acad. Sci. U.S.A. 2005;102:3378–3382. doi: 10.1073/pnas.0409899102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolo F.M., Rukunga G.M., Muli F.W., Njagi E.N., Njue W., Kumon K., Mungai G.M., Muthaura C.N., Muli J.M., Keter L.K., Oishi E., Kofi-Tsekpo M.W. Anti-viral activity of the extracts of a Kenyan medicinal plant Carissa edulis against herpes simplex virus. J. Ethnopharmacol. 2006;104(1/2):92–99. doi: 10.1016/j.jep.2005.08.053. [DOI] [PubMed] [Google Scholar]
- Vermani K., Garg S. Herbal medicines for sexually transmitted diseases and AIDS. J. Ethnopharmacol. 2002;80(1):49–66. doi: 10.1016/s0378-8741(02)00009-0. [DOI] [PubMed] [Google Scholar]
- Vilagines P., Delaveau P., Vilagines R. Inhibition of poliovirus replication by an extract of Matricaria chamomilla (L) C. R. Acad. Sci. III. 1985;301(6):289–294. [PubMed] [Google Scholar]
- Wagner H., Bladt S., Ricki V. 2nd ed. Springer; New York: 1996. Plant Drug Analysis: A Thin Layer Chromatography. [Google Scholar]
- Wang M., Cheng H., Li Y., Meng L., Zhao G., Mai K. Herbs of the genus Phyllanthus in the treatment of chronic hepatitis B: observations with three preparations from different geographic sites. J. Lab. Clin. Med. 1995;126(4):350–352. [PubMed] [Google Scholar]
- Wang Q., Wang Y.T., Pu S.P., Zheng Y.T. Zinc coupling potentiates anti-HIV-1 activity of baicalin. Biochem. Biophys. Res. Commun. 2004;324(2):605–610. doi: 10.1016/j.bbrc.2004.09.093. [DOI] [PubMed] [Google Scholar]
- Wang W.N., Yang X.B., Liu H.Z., Huang Z.M., Wu G.X. Effect of Oenanthe javanica flavone on human and duck hepatitis B virus infection. Acta Pharmacol. Sin. 2005;26(5):587–592. doi: 10.1111/j.1745-7254.2005.00055.x. [DOI] [PubMed] [Google Scholar]
- Wang X., Jia W., Zhao A., Wang X. Anti-influenza agents from plants and traditional Chinese medicine. Phytother. Res. 2006;20(5):335–341. doi: 10.1002/ptr.1892. [DOI] [PubMed] [Google Scholar]
- Webster D., Taschereau P., Lee T.D., Jurgens T. Immunostimulant properties of Heracleum maximum Bartr. J. Ethnopharmacol. 2006;106(3):360–363. doi: 10.1016/j.jep.2006.01.018. [DOI] [PubMed] [Google Scholar]
- Witvrouw M., Fikkert V., Hantson A., Pannecouque C., O’Keefe B.R., McMahon J., Stamatatos L., de Clercq E., Bolmstedt A. Resistance of human immunodeficiency virus type 1 to the high-mannose binding agents cyanovirin N and concanavalin A. J. Virol. 2005;79(12):7777–7784. doi: 10.1128/JVI.79.12.7777-7784.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woradulayapinij W., Soonthornchareonnon N., Wiwat C. In vitro HIV type 1 reverse transcriptase inhibitory activities of Thai medicinal plants and Canna indica L. rhizomes. J. Ethnopharmacol. 2005;101(1–3):84–89. doi: 10.1016/j.jep.2005.03.030. [DOI] [PubMed] [Google Scholar]
- Wu W., Liang Z., Zhao Z., Cai Z. Direct analysis of alkaloid profiling in plant tissue by using matrix-assisted laser desorption/ionization mass spectrometry. J. Mass Spectrom. 2007;42(1):58–69. doi: 10.1002/jms.1138. [DOI] [PubMed] [Google Scholar]
- Yamai M., Tsumura K., Kimura M., Fukuda S., Murakami T., Kimura Y. Antiviral activity of a hot water extract of black soybean against a human respiratory illness virus. Biosci. Biotechnol. Biochem. 2003;67(5):1071–1079. doi: 10.1271/bbb.67.1071. [DOI] [PubMed] [Google Scholar]
- Yang C.M., Cheng H.Y., Lin T.C., Chiang L.C., Lin C.C. The in vitro activity of geraniin and 1,3,4,6-tetra-O-galloyl-beta-d-glucose isolated from Phyllanthus urinaria against herpes simplex virus type 1 and type 2 infection. J. Ethnopharmacol. 2007;110(3):555–558. doi: 10.1016/j.jep.2006.09.039. [DOI] [PubMed] [Google Scholar]
- Yu D., Sakurai Y., Chen C.H., Chang F.R., Huang L., Kashiwada Y., Lee K.H. Anti-AIDS agents 69. Moronic acid and other triterpene derivatives as novel potent anti-HIV agents. J. Med. Chem. 2006;49(18):5462–5469. doi: 10.1021/jm0601912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu D., Wild C.T., Martin D.E., Morris-Natschke S.L., Chen C.H., Allaway G.P., Lee K.H. The discovery of a class of novel HIV-1 maturation inhibitors and their potential in the therapy of HIV. Expert Opin. Investig. Drugs. 2005;14(6):681–693. doi: 10.1517/13543784.14.6.681. [DOI] [PubMed] [Google Scholar]
- Zakay-Rones Z., Thom E., Wollan T., Wadstein J. Randomized study of the efficacy and safety of oral elderberry extract in the treatment of influenza A and B virus infections. J. Int. Med. Res. 2004;32(2):132–140. doi: 10.1177/147323000403200205. [DOI] [PubMed] [Google Scholar]
- Zhang X., Tang X., Chen H. Inhibition of HIV replication by baicalin and S. baicalensis extracts in H9 cell culture. Chin. Med. Sci. J. 1991;6(4):230–232. [PubMed] [Google Scholar]
- Zuo G.Y., Li Z.Q., Chen L.R., Xu X.J. In vitro anti-HCV activities of Saxifraga melanocentra and its related polyphenolic compounds. Antivir. Chem. Chemother. 2005;16(6):393–398. doi: 10.1177/095632020501600606. [DOI] [PubMed] [Google Scholar]


