Abstract
BACKGROUND
The diagnosis of active tuberculosis (TB)10 cases primarily relies on methods that detect Mycobacterium tuberculosis (Mtb) bacilli or their DNA in patient samples (e.g., mycobacterial culture and Xpert MTB/RIF assays), but these tests have low clinical sensitivity for patients with paucibacillary TB disease. Our goal was to evaluate the clinical performance of a newly developed assay that can rapidly diagnose active TB cases by direct detection of Mtb-derived antigens in patients’ blood samples.
METHODS
Nanoparticle (NanoDisk)-enriched peptides derived from the Mtb virulence factors CFP-10 (10-kDa culture factor protein) and ESAT-6 (6-kDa early secretory antigenic target) were analyzed by high-throughput mass spectrometry (MS). Serum from 294 prospectively enrolled Chinese adults were analyzed with this NanoDisk-MS method to evaluate the performance of direct serum Mtb antigen measurement as a means for rapid diagnosis of active TB cases.
RESULTS
NanoDisk-MS diagnosed 174 (88.3%) of the study’s TB cases, with 95.8% clinical specificity, and with 91.6% and 85.3% clinical sensitivity for culture-positive and culture-negative TB cases, respectively. NanoDisk-MS also exhibited 88% clinical sensitivity for pulmonary and 90% for extrapulmonary TB, exceeding the diagnostic performance of mycobacterial culture for these cases.
CONCLUSIONS
Direct detection and quantification of serum Mtb antigens by NanoDisk-MS can rapidly and accurately diagnose active TB in adults, independent of disease site or culture status, and outperform Mycobacterium-based TB diagnostics.
Tuberculosis (TB) is a highly prevalent and deadly infectious disease, with a worldwide incidence of more than 10.4 million new cases and 1.4 million deaths in 2015 according to the latest World Health Organization estimates (1). In China and other developing countries, TB diagnosis still heavily relies on microbiologic techniques, including smear microscopy for acid-fast bacilli (AFB) and the “gold standard” of mycobacterial culture (2, 3), which has a very long sample-to-answer timeframe. Both methods, however, have low clinical sensitivity for paucibacillary TB cases, which account for >60% of new TB cases each year in emerging TB-endemic areas such as China (1, 4, 5). The PCR-based Xpert MTB/RIF assay can increase the diagnosis speed and clinical sensitivity in multibacillary TB, but it still requires sputum or invasive biopsy specimens, exhibits moderate clinical sensitivity in patients with paucibacillary TB (e.g., Mycobacterium tuberculosis (Mtb) culture- or smear-negative TB cases), and cannot differentiate between live and nonviable Mtb bacilli to monitor response to anti-TB therapy (6–10). Blood-based IFN-γ release assays measure ex vivo immune responses to assay-introduced Mtb antigens and cannot distinguish between active TB and latent TB infection (11, 12).
Mtb bacilli robustly secrete CFP-10 (10-kDa culture filtrate antigen) and ESAT-6 (6-kDa early secretory antigenic target) to promote immunopathologic responses, and loss of either gene results in a significant reduction in virulence, strongly indicating these factors are specific for virulent mycobacteria (13–16). These factors appear to represent ideal biomarkers for active TB because their detection in any clinical patient sample constitutes evidence of active TB disease (17, 18). Current Mtb antigen immunoassays have poor diagnostic performance, however, which appears to result in part from antigen masking by host antibodies as well as potential interference by homologous antigens secreted by nontuberculous mycobacteria (NTM). We recently developed a peptide-based approach that overcomes both these issues by using antibody-conjugated porous discoidal nanoparticles (NanoDisks) to (a) bind Mtb-specific CFP-10 and ESAT-6 peptides present in digested serum samples of TB cases and (b) enhance their quantitative detection by bench-top MALDI-TOF mass spectrometry (MS) (19). In this study, we employed a new adult Chinese patient population to validate whether NanoDisk-MS assay results correlated with clinical diagnoses, outperformed culture and histology tests used to diagnose active TB in adults, and revealed differential clinical sensitivity for pulmonary and extrapulmonary TB cases or Mtb culture-positive and culture-negative TB cases.
Methods
PARTICIPANTS
A total of 376 adults (≥18 years of age) who visited the Shandong Chest Hospital in China for TB screening in 2013 were enrolled in this study. Eligibility criteria for participants were no history of prior TB treatment or HIV infection and written informed consent. Exclusion criteria for eligible subjects were missing or contaminated samples, incomplete procedures (lost to follow-up), and/or diagnosis of NTM infection. Study approvals were obtained from the Institutional Review Boards at Houston Methodist Hospital and Shandong Chest Hospital. Standard bacteriological and radiological tests were performed by an ISO15189-certified TB reference laboratory at Shandong Chest Hospital. Clinical services for sample and information collection were performed under contract on a fee-for-service basis.
CHARACTERIZATION OF PARTICIPANTS AND DEFINITIONS
Demographics, risk factors, clinical history, and findings were documented on a standardized case report form. The Diagnostic Criteria for Tuberculosis (WS288–2008) guidelines established by the People’s Republic of China were used as a general guideline to diagnose and classify all patients. Enrolled study participants were evaluated for risk of active TB based on their chest radiography, symptoms, and medical history results. Individuals suspected to have active TB disease based on one or more of these criteria were evaluated with AFB smears of 3 consecutive early morning sputa samples (≥24 h apart) and 8 weeks of mycobacterial cultures of sputum (3–5 mL) or specimens from other suspected infection sites. The Xpert MTB/RIF assay was not routinely available at the study site during this study. All enrolled participants had a 10-mL blood sample drawn before the start of anti-TB therapy. Venous blood samples were drawn into red top Vacutainer Tubes, which were gently inverted 5 times and incubated at room temperature for 30 min; then, they were centrifuged at 1000g for 10 min to isolate serum samples that were stored at −80 °C until thawed for analysis. Sputum samples were immediately processed and refrigerated within 4 h of collection. Decontamination of sputum specimens followed standard internationally recommended sodium hydroxide and N-acetyl-L-cysteine (NaOH-NALC) methodology (20, 21). Serum samples were not fixed or otherwise processed before digestion, and all sample preparation and handling steps were therefore conducted in a biosafety hood designated for this study in accordance with standard biosafety protocol for handling unfixed human blood samples. Experimental details are described in the Materials file in the Data Supplement that accompanies the online version of this article at http://www.clinchem.org/content/vol64/issue5).
Patients in whom the sites of disease were exclusively confined to lungs, pleura, and intrathoracic lymph nodes were classified as pulmonary TB. Patients whose disease extended to organs or tissues outside the thorax were considered to have extrapulmonary TB. Patients with both pulmonary and extrapulmonary disease findings were classified as pulmonary TB. A culture-positive TB case was defined as a mycobacterial culture-positive result, confirmed by Mtb-specific PCR amplification. A culture-negative TB case was diagnosed based on the physician’s clinical assessment and the patient’s response to treatment at the end of anti-TB therapy. Non-TB individuals were defined by: (a) negative chest radiography results and the absence of symptoms or history consistent with TB infection at the time of sample collection and follow-ups and/or (b) no anti-TB therapy administered, based on physician’s clinical recommendation.
NANODISK-MS ASSAYS
Serum samples (100 µL) were digested for 20 min with 10-µg sequencing-grade modified trypsin under microwave irradiation. NanoDisk particles functionalized with peptide-specific antibody were incubated with digested serum samples for 2 h at 25 °C with constant rotary mixing, after which peptide-loaded NanoDisk particles were directly spotted on the target for MALDI-TOF MS detection (19). Standard recombinant CFP-10 and ESAT-6 were trypsin-digested and analyzed on an AXIMA Resonance MALDI-TOF MS to select monovalently charged target peptide ions. CFP-10 and ESAT-6 peptide ions (m/z 1593.75 and 1900.95, respectively) were selected for diagnostic NanoDisk-MS assays owing to their high signal-to-noise ratios. Isotope-labeled versions of these 2 peptides with 10-Da mass increases (m/z 1603.60 and 1910.80) were synthesized as internal standards, and the MS intensity ratio of each target peptide and its internal standard in each sample was calculated for absolute quantification of serum CFP-10 and ESAT-6 concentration. Pooled human serum from non-TB cases was supplemented with different concentrations of CFP-10, ESAT-6, and internal standards and were processed with the aforementioned protocol to establish a standard curve in our previous study (19). Each clinical sample was analyzed in 3 replicate experiments, and the mean intensity ratio value for each target peptide was used to calculate the serum concentration of its corresponding Mtb biomarker antigen (see Materials file in the online Data Supplement for details).
ANALYTICAL PERFORMANCE
Clinical samples were pooled to generate a pooled “disease sample” (n = 20) and a pooled “healthy control” sample (n = 200) to assess the precision of the NanoDisk-MS assay. Five replicates of each of these samples were analyzed once a day for 5 days to determine intraassay and interassay precision, and the means of the 25 CFP-10 and ESAT-6 results for the pooled disease and control samples were used as reference concentrations for subsequent analytical evaluations. Limits of detection and quantification were determined as the lowest concentration that produced CFP-10 and ESAT-6 mass spectra peaks with signal-to-noise ratios ≥3 and ≥5, respectively, after serially diluting (2×) disease sample with analyte-free serum from a healthy donor. These samples were analyzed for stability effects after 30 days at −80 °C, after incubation at room temperature (20–24 °C) for 4 h, after refrigeration (4–8 °C) for 24 h, and after 2 freeze–thaw cycles. Sample interference effects were assessed by analyzing CFP-10 and ESAT-6 concentrations in disease sample aliquots supplemented with different levels of clinical interferents (Assurance Interference Test Kit, Sun Diagnostics) and by comparing these values to their respective reference concentrations (22) (see Materials file in the online Data Supplement for details).
DATA ANALYSIS
A NanoDisk-MS result indicative of active TB disease was defined as a positive signal for either CFP-10 or ESAT-6 target peptide above the limit of quantification (19). We therefore defined the NanoDisk-MS readout as the combined CFP-10 and ESAT-6 concentration in a serum sample. Definition for positive and negative results were established before the study team was unblinded to the patients’ information. NanoDisk-MS test results were compared to AFB smear and mycobacterial culture results for all cases and for pulmonary and extrapulmonary TB cases. NanoDisk-MS clinical sensitivity was also compared to evaluate assay performance between patients with positive and negative AFB smear or mycobacterial culture status. Differences between groups were tested for significance with the 2-sample χ2 test for binary variables and nonparametric Wilcoxon rank-sum tests for continuous variables, as normal distributions were not confirmed in these samples. GraphPad Prism software (v 5.0) was used for all statistical analysis and P values <0.05 were considered statistically significant.
Results
STUDY POPULATION
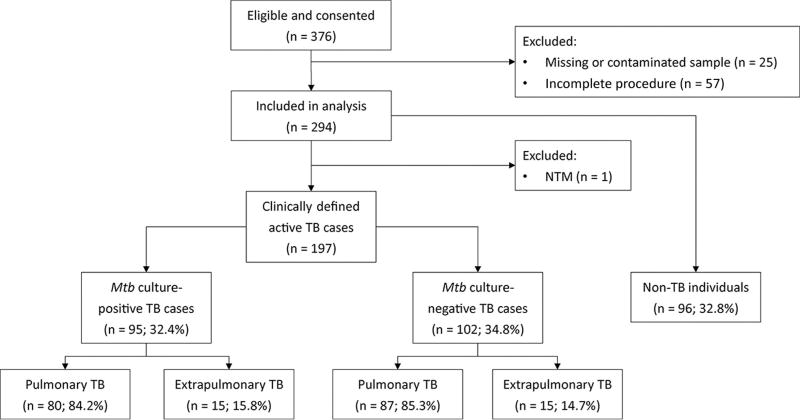
From a population of 376 individuals meeting study criteria, samples from 198 patients with active TB and 96 individuals without active TB were analyzed by our NanoDisk-MS assay (Fig. 1). Among patients with active TB, 95 had positive culture results (80 pulmonary TB and 15 extrapulmonary TB) and 102 had negative culture results (87 pulmonary TB and 15 extrapulmonary TB). One participant who was initially classified as an active TB case was later excluded from the study after analyses revealed that he was infected with an NTM. TB cases and non-TB cases did not differ by median age or gender distribution (Table 1; 44 vs 44 years of age and 65.5% vs 69.8% male, respectively). The TB group contained 167 pulmonary (84.2%) and 30 extrapulmonary (15.2%) cases (7 tuberculous meningitis, 6 peritonitis, 5 cervical lymphadenopathy, 3 genitourinary TB, 6 pericardial TB, and 3 spinal TB). Chest x-ray results revealed findings consistent with active TB disease in 82.2% of active TB cases and differed between pulmonary and extrapulmonary cases (94.0% vs 16.7%, respectively). Mycobacterial culture was positive in 48.2% of all TB patients, and the detection rate did not differ between pulmonary (47.9%) and extrapulmonary (50.0%) TB cases. AFB smear results demonstrated extremely poor overall clinical sensitivity (8.6%) and detected none of the extrapulmonary TB cases.
Fig. 1. Participant disposition flow chart.
Table 1.
Demographic, microbiology, and diagnostic data for study participants.
| All TB cases (n = 197) |
Pulmonary (n = 167) |
Extrapulmonary (n = 30) |
P value |
Non-TB cases (n = 96) |
|
|---|---|---|---|---|---|
| Demographics | |||||
| Male (%) | 129 (65.5%) | 109 (65.3%) | 20 (66.7%) | 0.882 | 67 (69.8%) |
| Median age (IQR) | 44 (26–60) | 45 (27–61) | 35.5 (23–57.5) | 0.246 | 44 (30.5–59.8) |
| Conventional TB diagnosis | |||||
| Chest x-ray positive (%) | 162 (82.2%) | 157 (94.0%) | 5 (16.7%) | <0.0001a | 0 (0.0%) |
| Mtb culture positive (%) | 95 (48.2%) | 80 (47.9%) | 15 (50.0%) | 0.832 | NA |
| AFB smear positive (%) | 17 (8.6%) | 17 (10.2%) | 0 (0.0%) | 0.140 | NA |
| NanoDisk-MS (CFP-10 + ESAT-6) | |||||
| Biomarker positive (%) | 174 (88.3%) | 147 (88.0%) | 27 (90.0%) | 0.999 | 4 (4.2%) |
| Median summed biomarker conc., nmol/L (IQR) | 2.567 (0.973, 4.613) | 2.604 (1.033, 4.816) | 1.929 (0.758, 3.531) | 0.309 | 0 (0.000, 0.000) |
•••.
OUTPERFORMANCE OF NANODISK-MS OVER SMEAR MICROSCOPY AND CULTURE IN OUR STUDY POPULATION
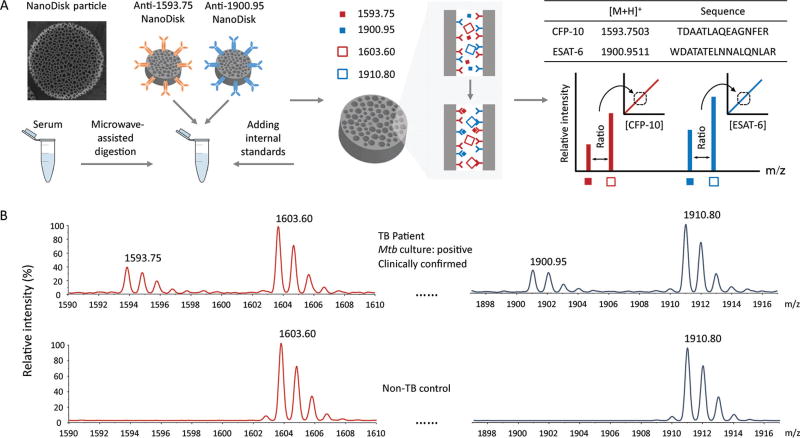
Current immunoassays do not accurately quantify Mtb antigens in blood samples owing to potential interference from host antibodies and homologous NTM proteins. However, we have previously reported that the Mtb CFP-10 peptide TDAATLAQEAGNFER (m/z 1593.75) and the Mtb ESAT-6 peptide WDATATELNNALQNLAR (m/z 1900.95) are highly specific for Mtb and can be used to directly quantify Mtb CFP-10 and ESAT-6 concentrations by MS (19). To quantify patient Mtb antigen concentrations, patient serum samples were trypsin-digested, supplemented with heavy-isotope variants of the Mtb target peptides as internal standards (m/z 1603.75 and 1910.95), and incubated with antibody-conjugated NanoDisks to selectively enrich these peptides and to enhance MALDI ionization to increase MS analytical sensitivity (Fig. 2A).
Fig. 2. NanoDisk-MS assay procedure.
Serum samples of suspected TB cases are trypsin digested and incubated with antibody-functionalized NanoDisk particles that bind Mtb-specific peptides (A). Serum Mtb CFP-10 and ESAT-6 concentration are determined by the mass spectra intensity ratios of the Mtb target peptides (1593.75 and 1900.95 m/z) and their isotope-labeled internal standards, respectively. Representative NanoDisk-MS results for the peaks of interest from a clinically confirmed active TB case (upper) and a control subject with a negative TB diagnosis (lower) (B).
Reference biomarker concentrations for pooled disease (CFP-10, 4.6 nmol/L and ESAT-6, 6.3 nmol/L) and control (CFP-10, 0 nmol/L and ESAT-6, 0 nmol/L) samples were determined as the mean of 25 replicates, and pooled disease sample was used for subsequent analytical validation. Intra- and interassay precision (CV) were found to be 13.1% and 14.8% for CFP-10, respectively, and 12.8% and 11.6% for ESAT-6, respectively. Dilution experiments of pooled disease sample revealed excellent CFP-10 and ESAT-6 linearity (R2 > 0.99, see Fig. 1 in the online Data Supplement) with limits of detection of 72 and 197 pmol/L and limits of quantification of 288 and 394 pmol/L, respectively (CV < 20%). NanoDisk-MS detected 88.5%–92.2% of the expected CFP-10 and ESAT-6 values after exposing aliquots of the disease sample to common storage and handling conditions, and 87.4%–94.7% of these values after contaminating disease sample aliquots with common clinical interferents (Table 2).
Table 2.
Analytical validation of the NanoDisk-MS assay.
| CFP-10 | ESAT-6 | |
|---|---|---|
| Disease pool:a | ||
| Mean concentration, nmol/L | 4.6 | 6.3 |
| Intraassay CV, % | 13.1 | 12.8 |
| Interassay CV, % | 14.8 | 11.6 |
| Limit of detection, nmol/L | 72 | 197 |
| Limit of quantification, nmol/L | 288 | 394 |
| Analyte stability after storage and handling (% untreated, mean ± SD) | ||
| 4 h @ 20–24 °C | 92.1 ± 0.7 | 91.5 ± 1.1 |
| 24 h @ 4–8 °C | 91.8 ± 0.4 | 92.2 ± 0.8 |
| 30 days @ −80 °C | 90.7 ± 0.6 | 91.4 ± 0.5 |
| 2 freeze-thaw cycles | 88.5 ± 0.9 | 89.3 ± 0.7 |
| Influence of clinical interferents (% untreated, mean ± SD) | ||
| Hemolysate | 90.8 ± 0.5 | 88.6 ± 0.6 |
| Triglycerides | 94.7 ± 1.0 | 91.3 ± 1.0 |
| Bilirubin | 91.1 ± 0.9 | 88.3 ± 0.3 |
| Abundant protein | 87.4 ± 0.8 | 91.7 ± 0.8 |
CFP-10 and ESAT-6 are undetectable in the healthy pool.
NanoDisk-MS spectra readily distinguished serum from selected individuals with and without active TB in a proof-of-principle test (Fig. 2B). Blinded analyses were thus performed on all study samples to determine the correspondence between NanoDisk-MS results and clinical TB diagnosis, with detectable Mtb antigen as the NanoDisk-MS criterion for active TB diagnosis. Subsequent analysis of unblinded data revealed that NanoDisk-MS detected CFP-10 and/or ESAT-6 signal in 174 of the 197 TB cases (88.3% clinical sensitivity; 95% CI, 83.1%–92.1%; Table 1) and 4 of the 96 study subjects without a clinical TB diagnosis (95.8% clinical specificity; 95% CI, 89.8%–98.4%; see Fig. 2 in the online Data Supplement). These results markedly outperformed mycobacterial culture (95 of 197, 48.2%; 95% CI, 41.4%–55.2%) and AFB smear (17 of 197, 8.6%; 95% CI, 5.5%–13.4%) detection rates in this population. Notably, 1 patient evaluated as TB-negative by NanoDisk-MS (ID: 50) was initially defined as a pulmonary TB case with multidrug resistance by culture and drug susceptibility testing but was reclassified as an NTM case (M. intracellulare) after confirmation by hsp65 gene sequencing; the patient was excluded from the study.
NANODISK-MS DETECTS ACTIVE TB CASES IRRESPECTIVE OF INFECTION SITE OR CULTURE STATUS
NanoDisk-MS detected 147 of 167 (88.0%; 95% CI, 82.2%–92.1%) pulmonary and 27 of 30 (90.0%; 95% CI, 74.4%–96.5%) extrapulmonary TB cases, revealing considerably better diagnostic performance than mycobacterial culture (47.9% and 50.0%, respectively) or AFB smear (10.2% and 0.0%, respectively) results, and detecting similar median-summed Mtb antigen concentrations in both groups (Table 1). NanoDisk-MS also detected 87 of 95 (91.6%; 95% CI, 84.3%–95.7%) culture-positive and 87 of 102 (85.3%; 95% CI, 77.2%–90.9%) culture-negative TB cases. Similar results were detected in study groups with AFB smear-positive and smear-negative results, in which NanoDisk-MS detected 17 of 17 (100%; 95% CI, 81.6%–100%) TB cases with positive results and 157 of 180 (87.2%; 95% CI, 81.6%–91.3%) TB cases with negative results (see Table 1 in the online Data Supplement).
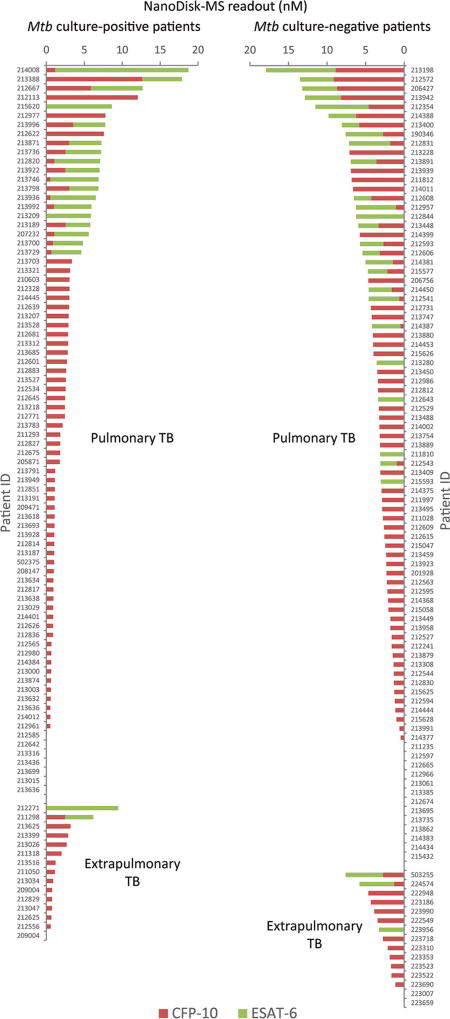
NanoDisk-MS also exhibited similar clinical sensitivity for culture-positive pulmonary (73 of 80, 91.3%; 95% CI, 83.0%–95.7%) and extrapulmonary (14 of 15, 93.3%; 95% CI, 70.2–98.8%) TB cases (Fig. 3), and culture negative pulmonary (74 of 87, 85.1%; 95% CI, 76.1%–91.1%) and extrapulmonary (13 of 15, 86.7%; 95% CI, 62.1%–96.3%) TB cases diagnosed with radiological findings, symptoms, and treatment outcome results.
Fig. 3. Serum CFP-10 (red) and ESAT-6 (green) concentrations (CFP-10 + ESAT-6) in each clinically confirmed TB case, with patients grouped according to their Mtb culture status and disease site.
Results indicate an average of 3 sample replicates per subject.
SERUM CFP-10 AND ESAT-6 CONCENTRATION DOES NOT DIFFER MARKEDLY BY SPECIFIC TB DIAGNOSIS
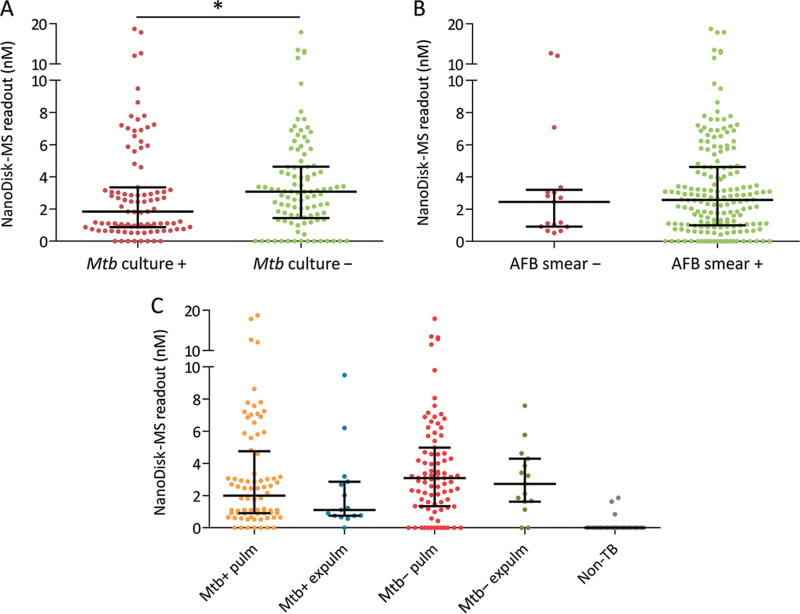
CFP-10 and ESAT-6 are frequently coproduced as heterodimers but were detected at different frequencies and different concentrations in active TB cases in this study (Fig. 3 and see Table 2 in the online Data Supplement). Most TB cases produced 1 or both antigens (174 of 197 cases); however, only 40 produced both CFP-10 and ESAT-6 (20.3%), with 125 individuals producing only CFP-10 (63.5%) and 9 producing only ESAT-6 (4.6%). NanoDisk-MS readout levels (summed concentrations of CFP-10 + ESAT-6) did not differ between pulmonary and extrapulmonary cases (Table 1) but significantly differed by culture status, with modestly higher combined antigen concentrations detected in culture-negative TB patients (Fig. 4A). No such difference was found between smear-positive and smear-negative cases (Fig. 4B). Secondary analysis of serum Mtb antigen concentrations in pulmonary and extrapulmonary cases by culture status also did not detect group differences (Fig. 4C).
Fig. 4. NanoDisk-MS results in different TB subgroups.
Mtb culture-positive vs culture-negative cases (A). AFB smear-positive vs smear-negative cases (B). Mtb culture-positive and culture-negative pulmonary and extrapulmonary TB cases and non-TB cases (C). Data points indicate the NanoDisk-MS readout for each study participant, while black lines indicate the median and interquartile range. NanoDisk-MS readout is the combined CFP-10 and ESAT-6 concentration.
Discussion
Rapid and reliable diagnostic assays to detect active TB cases are urgently needed for global TB control efforts. Bacteriologic (mycobacterial culture and AFB smear) and Xpert MTB/RIF TB assays commonly used for TB diagnosis worldwide require Mtb-rich specimens for accurate diagnosis. These methods thus exhibit suboptimal clinical sensitivity in paucibacillary patient populations, including patients with low mycobacterial loads or poor sputum production, and the majority of HIV-positive (23) and pediatric patients (24, 25). The collection of diagnostically useful samples can require invasive procedures (7) and technical variables that can influence test results. The Mtb virulence factors CFP-10 and ESAT-6 should always be detectable in active TB disease, however, regardless of the disease site or local Mtb concentrations. We therefore recently developed a NanoDisk-MS assay that rapidly and sensitively detects circulating Mtb antigens to allow robust diagnosis across the spectrum of TB cases. This assay allows for rapid TB diagnosis with several advantages over existing tests. These include (a) clinical sensitivity independent of disease site (pulmonary or extrapulmonary) or mycobacterial load (culture-negative TB cases), (b) use of 2 Mtb virulence factors to allow for case-to-case variation, (c) detection of serum biomarkers with low biosafety concerns due to lack of infectious aerosols, and (d) precise serum Mtb antigens quantification.
In our study population, NanoDisk-MS accurately detected CFP-10 and/or ESAT-6 in the serum of active TB cases, achieving 88.3% clinical sensitivity across all the disease-affected subgroups in this study, with 95.8% specificity. Patients with paucibacillary conditions that are missed by current TB tests are typically diagnosed subjectively on the basis of patient history, symptoms, and imaging results, as well as their observed response to anti-TB therapy. According to the 2015 World Health Organization Global Tuberculosis Report (1), mycobacterial culture, the current “gold standard” TB diagnostic, detects only about 30% of clinically diagnosed TB patients in China. AFB smear tests exhibit an extremely high false-negative rate (26), but due to the long turnaround time required for culture results, AFB smear is still widely used as a rapid initial screen for active TB. Due to this poor clinical sensitivity, suspected TB cases with negative culture and AFB smear results are often subjected to additional tests and/or empirical anti-TB therapy, resulting in additional costs and potentially unnecessary drug burden and toxicity for patients without TB.
In this study, Mtb culture and AFB smear results had 48.2% and 8.6% clinical sensitivity, respectively, across all TB cases, but their sensitivity decreased dramatically in extrapulmonary cases for AFB smear (0.0%). NanoDisk-MS clinical sensitivity did not differ for pulmonary vs extrapulmonary TB, nor was it impacted by culture or AFB status, strongly indicating that this blood-based biomarker approach can improve TB diagnosis in this diagnostically challenging patient cohort. Further, intergroup comparison of combined biomarker concentration indicated similar circulating levels regardless of bacterial load and disease site, supporting the utility of our serum biosignature across a broad spectrum of TB manifestations. Mtb culture, AFB smear, and radiographic results are qualitative and reflect bacilli present only in a given sample or image. NanoDisk-MS results, however, quantify circulating Mtb antigens as a measure of the systemic level of active Mtb bacilli, independent of the disease site(s), and should therefore differ among TB cases who have similar culture, smear, or radiographic findings, but different TB disease severity or extent.
The diagnosis of extrapulmonary and paucibacillary TB has not been greatly improved by the Xpert MTB/RIF assay, an automated molecular assay developed for rapid diagnosis of TB and rifampicin resistance (6–8). Indeed, the WHO has acknowledged the low-quality evidence for the use of Xpert to diagnose extrapulmonary TB, which accounts for about 25% of all TB cases (27). Further, previous publications suggest that Xpert MTB/RIF has low clinical sensitivity in culture-negative TB (28, 29). One study observed markedly reduced clinical sensitivity in culture-negative vs culture-positive pulmonary (25.0% vs 86.2%) and extrapulmonary (29.4% vs 67.7%) TB cases (26), suggesting that culture status has a profound effect on DNA-based diagnosis of both pulmonary and extrapulmonary TB infections. NanoDisk-MS thus appears to dramatically improve on culture and Xpert MTB/RIF both for its ability to utilize patient serum, rather than sputum or invasive biopsy specimens, and for its robust performance, which is unaffected by anatomical site (pulmonary vs extrapulmonary) or bacillary load (Mtb culture status).
NanoDisk-MS exhibited 95.8% clinical specificity in 96 non-TB individuals in this study. However, it is not clear why 4 of these patients had detectable Mtb antigen levels, as our MS-based method is highly specific for its target peptides. While these results may indicate actual false-positive events, it is also possible they represent subclinical infections with TB or specific strains of 2 NTM species (M. kansasii and M. marinum) with homology to the Mtb CFP-10 target peptide. Thus, clinical evaluation is still necessary for a final diagnosis of active TB based on positive NanoDisk-MS results. These 2 NTM should be rare (<0.5% suspected TB cases) in our population (30), but due to the lack of systematic clinical data for the non-TB individuals in this study we cannot provide definitive explanations for the NanoDisk-MS positive results in this group.
NTM infections, which have lower incidence than TB, can cause false-positive TB diagnosis owing to their similar clinical symptoms and bacteriological results. Further, several NTM species, including several of the more clinically common species, can also secrete homologs of CFP-10 and ESAT-6, which might complicate diagnosis. We have previously reported that the CFP-10 and ESAT-6 peptides analyzed by NanoDisk-MS are highly Mtb-specific, however, and our results correctly excluded 1 patient with a clinical diagnosis of active TB, who was later determined to have an NTM infection (M. intracellulare) by hsp65 gene sequencing.
The NanoDisk-MS assay was developed to permit the analytically sensitive multiplex quantification of serum CFP-10 and ESAT-6 concentrations in suspected TB cases to allow rapid diagnosis of active TB with high clinical sensitivity and specificity, irrespective of disease manifestation or Mtb concentration at the disease site(s). We detected both these Mtb virulence factors in our study population, but CFP-10 was observed with markedly higher frequency than ESAT-6. We did not observe any correlation between the CFP-10 and ESAT-6 concentrations within each patient. This might be a result of variable expression of ESAT-6 and CFP-10 in different strains of Mtb (31, 32). A recent study has also demonstrated that the host protein χ2-microglobulin can bind ESAT-6 and mask a tryptic cleavage site (33) that is required to generate the ESAT-6 1900.95 m/z peptide recognized by our NanoDisk-MS assay (34). The differences in CFP-10 and ESAT-6 detection rates may be due to a combination of these 2 factors.
NanoDisk-MS differs from other current TB diagnostic tests by providing quantitative results. We anticipate that longitudinal NanoDisk-MS results may therefore be useful for rapidly evaluating treatment response and predicting clinical outcomes of TB patients, which currently rely on culture conversion or nonstandardized physician evaluations. Serial quantification of serum Mtb antigens may also be useful for predicting the likelihood of drug resistance and disease recurrence. We did not observe correlation between CFP-10 and ESAT-6 concentrations, and diagnosis and treatment monitoring thus require separate assessment of these 2 biomarkers. Many hospitals and public health laboratories routinely employ MALDI-TOF MS for microbial identification (35–37), which should ease the translation of this method. However, development and optimization of a long-term storage method for antibody-conjugated NanoDisks is required before this platform is suitable for use in clinical studies. Ongoing development of less expensive, portable MS platforms, designed for use in resource-limited areas, may allow this approach to achieve widespread clinical application in all areas with high TB-burden (38, 39). Overall, our results support the further evaluation of NanoDisk-MS to detect circulating CFP-10 and ESAT-6 concentrations in patients with suspected TB as a means for rapid and accurate TB disease diagnosis in adults and in children who have paucibacillary disease.
Supplementary Material
Acknowledgments
The authors thank the Shandong Provincial Chest Hospital of China for their support and advice in the conduct of this study. The authors are grateful to the participants of this study.
Research Funding: Y. Hu, Arizona State University. Support for the performance of this study was provided by the US National Institute of Allergy and Infectious Diseases grants R01 Al113725-01A1 and R01 AI122932-01A1, and the US Eunice Kennedy Shriver National Institute of Child Health & Human Development grant R01 HD090927-01A1.
Role of Sponsor: The funding organizations played no role in the design of study, choice of enrolled patients, review and interpretation of data, or final approval of manuscript.
Footnotes
Nonstandard abbreviations: TB, tuberculosis; AFB, acid-fast bacilli; Mtb, Mycobacterium tuberculosis; NTM, nontuberculous mycobacteria; MS, mass spectrometry.
Author Contributions: All authors confirmed they have contributed to the intellectual content of this paper and have met the following 3 requirements: (a) significant contributions to the conception and design, acquisition of data, or analysis and interpretation of data; (b) drafting or revising the article for intellectual content; and (c) final approval of the published article.
Authors’ Disclosures or Potential Conflicts of Interest: Upon manuscript submission, all authors completed the author disclosure form. Disclosures and/or potential conflicts of interest:
Employment or Leadership: C. Liu, Arizona State University; Y. Hu, Arizona State University, Houston Methodist Research Institute.
Consultant or Advisory Role: None declared.
Stock Ownership: None declared.
Honoraria: None declared.
Expert Testimony: None declared.
Patents: None declared.
References
- 1.The World Health Organization. Global tuberculosis report 2015. 2015 [Google Scholar]
- 2.Monkongdee P, McCarthy KD, Cain KP, Tasaneeyapan T, Nguyen HD, Nguyen TN, et al. Yield of acid-fast smear and mycobacterial culture for tuberculosis diagnosis in people with human immunodeficiency virus. Am J Respir Crit Care Med. 2009;180:903– 8. doi: 10.1164/rccm.200905-0692OC. [DOI] [PubMed] [Google Scholar]
- 3.The World Health Organization. [Accessed11/29/17];Systematic screening for active tuberculosis: Principles and recommendations. 2013 Available at http://www.who.int/tb/publications/Final_TB_Screening_guidelines.pdf. [PubMed]
- 4.Dunlap NE, Bass J, Fujiwara P, Hopewell P, Horsburgh CR, Salfinger M, et al. Diagnostic standards and classification of tuberculosis in adults and children. Am J Respir Crit Care Med. 2000;161:1376–95. doi: 10.1164/ajrccm.161.4.16141. [DOI] [PubMed] [Google Scholar]
- 5.Swaminathan S, Rekha B. Pediatric tuberculosis: global overview and challenges. Clin Infect Dis. 2010;50:S184–S94. doi: 10.1086/651490. [DOI] [PubMed] [Google Scholar]
- 6.Evans CA. GeneXpert—a game-changer for tuberculosis control? PLoS Med. 2011;8:e1001064. doi: 10.1371/journal.pmed.1001064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ioannidis P, Papaventsis D, Karabela S, Nikolaou S, Panagi M, Raftopoulou E, et al. Cepheid GeneXpert MTB/RIF assay for Mycobacterium tuberculosis detection and rifampin resistance identification in patients with substantial clinical indications of tuberculosis and smear-negative microscopy results. J Clin Microbiol. 2011;49:3068–70. doi: 10.1128/JCM.00718-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pai M, Schito M. Tuberculosis diagnostics in 2015: landscape, priorities, needs, and prospects. J Infect Dis. 2015;211:S21–S8. doi: 10.1093/infdis/jiu803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boehme CC, Nabeta P, Hillemann D, Nicol MP, Shenai S, Krapp F, et al. Rapid molecular detection of tuberculosis and rifampin resistance. N Engl J Med. 2010;363:1005–15. doi: 10.1056/NEJMoa0907847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cowan JF, Chandler AS, Kracen E, Park DR, Wallis CK, Liu E, et al. Clinical impact and cost-effectiveness of Xpert MTB/RIF testing in hospitalized patients with presumptive pulmonary tuberculosis in the united states. Clin Infect Dis. 2017;64:482–9. doi: 10.1093/cid/ciw803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Munk ME, Arend SM, Brock I, Ottenhoff THM, Andersen P. Use of ESAT-6 and CFP-10 antigens for diagnosis of extrapulmonary tuberculosis. J Infect Dis. 2001;183:175–6. doi: 10.1086/317663. [DOI] [PubMed] [Google Scholar]
- 12.Wu-Hsieh BA, Chen CK, Chang JH, Lai SY, Wu CHH, Cheng WC, et al. Long-lived immune response to early secretory antigenic target 6 in individuals who had recovered from tuberculosis. Clin Infect Dis. 2001;33:1336–40. doi: 10.1086/323044. [DOI] [PubMed] [Google Scholar]
- 13.Sørensen AL, Nagai S, Houen G, Andersen P, Andersen AB. Purification and characterization of a low-molecular-mass T-cell antigen secreted by Mycobacterium tuberculosis. Infect Immun. 1995;63:1710–7. doi: 10.1128/iai.63.5.1710-1717.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berthet F-X, Rasmussen PB, Rosenkrands I, Andersen P, Gicquel B. A Mycobacterium tuberculosis operon encoding ESAT-6 and a novel low-molecular-mass culture filtrate protein (CFP-10) Microbiology. 1998;144:3195–203. doi: 10.1099/00221287-144-11-3195. [DOI] [PubMed] [Google Scholar]
- 15.Denkinger CM, Pai M, Patel M, Menzies D. Gamma interferon release assay for monitoring of treatment response for active tuberculosis: an explosion in the spaghetti factory. J Clin Microbiol. 2013;51:607–10. doi: 10.1128/JCM.02278-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brusasca PN, Colangeli R, Lyashchenko KP, Zhao X, Vogelstein M, Spencer JS, et al. Immunological characterization of antigens encoded by the RD1 region of the Mycobacterium tuberculosis genome. Scand J Immunol. 2001;54:448–52. doi: 10.1046/j.1365-3083.2001.00975.x. [DOI] [PubMed] [Google Scholar]
- 17.Feng TT, Shou CM, Shen L, Qian Y, Wu ZG, Fan J, et al. Novel monoclonal antibodies to ESAT-6 and CFP-10 antigens for ELISA-based diagnosis of pleural tuberculosis. Int J Tuberc Lung Dis. 2011;15:804–10. doi: 10.5588/ijtld.10.0393. [DOI] [PubMed] [Google Scholar]
- 18.Wu H-J, Li Y, Fan J, Deng Z, Hu Z, Liu X, et al. Antibody-free detection of Mycobacterium tuberculosis antigen using customized nanotraps. Anal Chem. 2014;86:1988–96. doi: 10.1021/ac4027669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu C, Zhao Z, Fan J, Lyon CJ, Wu H-J, Nedelkov D, et al. Quantification of circulating Mycobacterium tuberculosis antigen peptides allows rapid diagnosis of active disease and treatment monitoring. Proc Natl Acad Sci USA. 2017;114:3969–74. doi: 10.1073/pnas.1621360114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Caviedes L, Lee T-S, Gilman RH, Sheen P, Spellman E, Lee EH, et al. Rapid, efficient detection and drug susceptibility testing of Mycobacterium tuberculosis in sputum by microscopic observation of broth cultures. J Clin Microbiol. 2000;38:1203– 8. doi: 10.1128/jcm.38.3.1203-1208.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Apers L, Mutsvangwa J, Magwenzi J, Chigara N, Butterworth A, Mason P, Van der Stuyft P. A comparison of direct microscopy, the concentration method and the Mycobacteria Growth Indicator Tube for the examination of sputum for acid-fast bacilli. Int J Tuberc Lung Dis. 2003;7:376–81. [PubMed] [Google Scholar]
- 22.Grant RP, Hoofnagle AN. From lost in translation to paradise found: enabling protein biomarker method transfer by mass spectrometry. Clin Chem. 2014 doi: 10.1373/clinchem.2014.224840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reid MJA, Shah NS. Approaches to tuberculosis screening and diagnosis in people with HIV in resource-limited settings. Lancet Infect Dis. 2009;9:173– 84. doi: 10.1016/S1473-3099(09)70043-X. [DOI] [PubMed] [Google Scholar]
- 24.Starke JRT-WKT. Tuberculosis in the pediatric population of Houston, Texas. Pediatrics. 1989;84:28. [PubMed] [Google Scholar]
- 25.Montenegro SH, Gilman RH, Sheen P, Cama R, Caviedes L, Hopper T, et al. Improved detection of Mycobacterium tuberculosis in Peruvian children by use of a heminested IS6110 polymerase chain reaction assay. Clin Infect Dis. 2003;36:16–23. doi: 10.1086/344900. [DOI] [PubMed] [Google Scholar]
- 26.Zeka AN, Tasbakan S, Cavusoglu C. Evaluation of the GeneXpert MTB/RIF assay for rapid diagnosis of tuberculosis and detection of rifampin resistance in pulmonary and extrapulmonary specimens. J Clin Microbiol. 2011;49:4138–41. doi: 10.1128/JCM.05434-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.The World Health Organization. Xpert MTB/RIF: WHO policy update and implementation manual. 2014 [Google Scholar]
- 28.O’Grady J, Bates M, Chilukutu L, Mzyece J, Cheelo B, Chilufya M, et al. Evaluation of the Xpert MTB/RIF assay at a tertiary care referral hospital in a setting where tuberculosis and HIV infection are highly endemic. Clin Infect Dis. 2012;55:1171–8. doi: 10.1093/cid/cis631. [DOI] [PubMed] [Google Scholar]
- 29.Zar HJ, Workman L, Isaacs W, Dheda K, Zemanay W, Nicol MP. Rapid diagnosis of pulmonary tuberculosis in African children in a primary care setting by use of Xpert MTB/RIF on respiratory specimens: A prospective study. Lancet Glob Health. 2013;1:e97–e104. doi: 10.1016/S2214-109X(13)70036-6. [DOI] [PubMed] [Google Scholar]
- 30.Yu X, Liu P, Liu G, Zhao L, Hu Y, Wei G, et al. The prevalence of non-tuberculous mycobacterial infections in mainland China: systematic review and meta-analysis. J Infect. 2016;73:558–67. doi: 10.1016/j.jinf.2016.08.020. [DOI] [PubMed] [Google Scholar]
- 31.Uplekar S, Heym B, Friocourt V, Rougemont J, Cole ST. Comparative genomics of Esx genes from clinical isolates of Mycobacterium tuberculosis provides evidence for gene conversion and epitope variation. Infect Immun. 2011;79:4042–9. doi: 10.1128/IAI.05344-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arend SM, de Haas P, Leyten E, Rosenkrands I, Rigouts L, Andersen P, et al. ESAT-6 and CFP-10 in clinical versus environmental isolates of Mycobacterium kansasii. J Infect Dis. 2005;191:1301–10. doi: 10.1086/428950. [DOI] [PubMed] [Google Scholar]
- 33.Teutschbein J, Schumann G, Möllmann U, Grabley S, Cole ST, Munder T. A protein linkage map of the ESAT-6 secretion system 1 (ESX-1) of Mycobacterium tuberculosis. Microbiol Res. 2009;164:253–9. doi: 10.1016/j.micres.2006.11.016. [DOI] [PubMed] [Google Scholar]
- 34.Sreejit G, Ahmed A, Parveen N, Jha V, Valluri VL, Ghosh S, Mukhopadhyay S. The ESAT-6 protein of Mycobacterium tuberculosis interacts with beta-2-microglobulin (β2M) affecting antigen presentation function of macrophage. PLoS Pathog. 2014;10:e1004446. doi: 10.1371/journal.ppat.1004446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saleeb PG, Drake SK, Murray PR, Zelazny AM. Identification of mycobacteria in solid-culture media by matrix-assisted laser desorption ionization–time of flight mass spectrometry. J Clin Microbiol. 2011;49:1790–4. doi: 10.1128/JCM.02135-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.DeMarco ML, Ford BA. Beyond identification: emerging and future uses for MALDI-TOF mass spectrometry in the clinical microbiology laboratory. Clin Lab Med. 2013;33:611–28. doi: 10.1016/j.cll.2013.03.013. [DOI] [PubMed] [Google Scholar]
- 37.Patel R. MALDI-TOF mass spectrometry: transformative proteomics for clinical microbiology. Clin Chem. 2013;59:340–2. doi: 10.1373/clinchem.2012.183558. [DOI] [PubMed] [Google Scholar]
- 38.Ren Y, McLuckey MN, Liu J, Ouyang Z. Direct mass spectrometry analysis of biofluid samples using slug-flow microextraction nano-electrospray ionization. Angew Chem Int Ed Engl. 2014;53:14124–7. doi: 10.1002/anie.201408338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang X, Zhou X, Ouyang Z. Direct analysis of nonvolatile chemical compounds on surfaces using a handheld mass spectrometer with synchronized discharge ionization function. Anal Chem. 2016;88:826–31. doi: 10.1021/acs.analchem.5b03356. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.