Summary
Background
Tuberculosis in children is increasingly recognised as an important component of the global tuberculosis burden, with an estimated 1 million cases in 2015. Although younger children are vulnerable to severe forms of tuberculosis disease, no age-disaggregated estimates of paediatric tuberculosis mortality exist, and tuberculosis has never been included in official estimates of under-5 child mortality. We aimed to produce a global mortality burden estimate in children using a complementary approach not dependent on vital registration data.
Methods
In this mathematical modelling study, we estimated deaths in children younger than 5 years and those aged 5–14 years for 217 countries and territories using a case-fatality-based approach. We used paediatric tuberculosis notification data and HIV and antiretroviral treatment estimates to disaggregate the WHO paediatric tuberculosis incidence estimates by age, HIV, and treatment status. We then applied systematic review evidence on corresponding case-fatality ratios.
Findings
We estimated that 239 000 (95% uncertainty interval [UI] 194 000–298 000) children younger than 15 years died from tuberculosis worldwide in 2015; 80% (191 000, 95% UI 132 000–257 000) of these deaths were in children younger than 5 years. More than 70% (182 000, 140 000–239 000) of deaths occurred in the WHO southeast Asia and Africa regions. We estimated that 39 000 (17%, 23 000–73 000) paediatric tuberculosis deaths worldwide were in children with HIV infections, with 31 000 (36%, 19 000–59 000) in the WHO Africa region. More than 96% (230 000, 185 000–289 000) of all tuberculosis deaths occurred in children not receiving tuberculosis treatment.
Interpretation
Tuberculosis is a top ten cause of death in children worldwide and a key omission from previous analyses of under-5 mortality. Almost all these deaths occur in children not on tuberculosis treatment, implying substantial scope to reduce this burden.
Funding
UNITAID, National Institutes of Health, and National Institute for Health Research.
Introduction
In 2015, under-5 mortality—deaths in children younger than 5 years—was estimated to be 5·9 million.1 This health statistic was a key indicator in the Millennium Development Goals2 and the subsequent Sustainable Development Goals.3 The UN Inter-agency Group on Child Mortality Estimation (IGME) tracks overall under-5 mortality, and breakdowns by cause of death have been estimated by the study group formerly known as the Child Health Epidemiology Reference Group (CHERG), most recently in 2016.1 These estimates have been important in assessing progress towards targets, directing public health funding and spending, and for advocacy. However, tuberculosis has never been explicitly mentioned in these reports.
Tuberculosis, an infectious disease caused by bacteria and spread through the air, is the leading infectious cause of death worldwide. Although tuberculosis is curable, diagnostic methods for tuberculosis are imperfect and perform poorly in children because of difficulties obtaining samples and low bacillary loads. WHO first estimated total, both HIV-negative and HIV-positive, childhood tuberculosis mortality in 2015 and estimated that 136 000 children died from tuberculosis in 2014.4 This estimate increased substantially to 210 000 deaths for 2015, because of data updates in countries informing the imputation model used to disaggregate mortality by age and the overall increase of the envelope of total mortality for all ages.5 This mortality estimate is based on vital registration data when available, and regression models incorporating national indicators when vital registration data are unavailable. IGME and CHERG methods are also based on civil and vital registration data, but also use survey data (including verbal autopsy for cause of death) when these are not available.
Direct approaches for estimating tuberculosis mortality use either vital registration data, adjusting for ill-defined causes of death, or verbal autopsy data; both are problematic for children. Recent estimates of childhood tuberculosis incidence suggest a substantially larger incidence of tuberculosis in children than previously appreciated or directly reflected in notification data, with more than 60% of cases unreported or undiagnosed—almost twice the corresponding proportion in adults.4, 6, 7, 8 Large numbers of undiagnosed tuberculosis cases in children present difficulties for vital-registration-based estimates of mortality since the cause of death in children who die from undiagnosed tuberculosis is likely to be recorded as something else, usually another common disease with which the signs and symptoms of tuberculosis overlap. For example, many deaths attributed to pneumonia in places with high tuberculosis burdens might actually be due to tuberculosis.9, 10 Furthermore, even if a child is known to have died from tuberculosis, this might not be correctly recorded by weak or absent vital registration systems, common in many countries with high tuberculosis burdens.11, 12 Even with good vital registration systems, the rules of the International Statistical Classification of Diseases and Related Health Problems-10 (ICD-10) coding system state that tuberculosis should not be registered as the primary cause of death when another comorbidity such as HIV is present; moreover, recorded secondary causes of death are often not relayed to supranational bodies.13 Finally, verbal autopsy has limited sensitivity for tuberculosis deaths,14, 15 especially in children because of diverse and non-specific signs and symptoms.16 Thus, both the current magnitude of underdiagnosis in children and restrictions in the availability and quality of direct population-level data lead to substantial uncertainty that is difficult to quantify in estimates based on these data sources.
Research in context.
Evidence before this study
We searched PubMed without language or date restrictions for all records matching “(TB or tuberc*) and (child* or paed* or ped*) and (mortality or death*) and (burden or estimate or epidem* or global)” in any field, returning 1231 records. We found no articles estimating the global mortality due to tuberculosis in children younger than 15 years. Patton and colleagues estimated 17 000 deaths due to tuberculosis in the 10–14 age group for 2004. In 2012, WHO first produced estimates of tuberculosis mortality in children younger than 15 years, estimating 64 000 HIV-negative children died from tuberculosis in 2011. In 2016, using vital registration data, WHO estimated that 210 000 children younger than 15 years (HIV-positive and HIV-negative) died from tuberculosis in 2015. No estimates exist for tuberculosis mortality in children younger than 5 years.
Added value of this study
We produced a global mortality burden estimate in children younger than 15 years using a complementary approach not dependent on vital registration data. We estimated that tuberculosis caused nearly a quarter of a million deaths in children younger than 15 years of age in 2015. More than 80% of these deaths are in children younger than 5 years of age, which would make tuberculosis a top ten cause of death in this age group that is missing from all previous analyses. More than 96% of deaths occurred in children not receiving anti-tuberculosis treatment.
Implications of all the available evidence
Tuberculosis is a key unrecognised component of child mortality. Mortality could be drastically reduced with increased treatment coverage.
An orthogonal and complementary method to estimate childhood tuberculosis mortality is based on multiplying incidence estimates by case-fatality ratios. This approach requires a good understanding of case-fatality ratios for tuberculosis in children, by age, tuberculosis-treatment status and HIV status (both on and off antiretroviral therapy [ART]), and credible estimates of incidence. An understanding of the effects of HIV and ART status on tuberculosis risk in children is also needed to disaggregate incidence by these factors. Although such an approach makes generalisations about the application of standard case-fatality ratios to different settings, it is robust in its relative independence from data sources of inconsistent availability and quality.
In this analysis, we reassess mortality due to tuberculosis in children younger than 5 years and those aged 5–14 years using a case-fatality approach, taking advantage of recent tuberculosis incidence estimates, systematic reviews on case-fatality ratios, and the effects of HIV and ART on tuberculosis incidence in children.
Methods
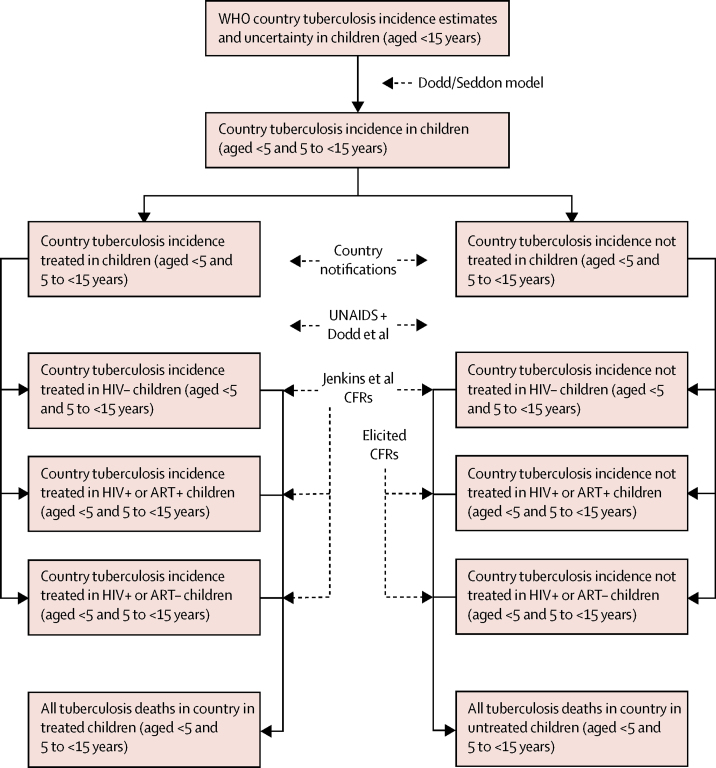
For every country, we used WHO estimates of tuberculosis incidence in children, WHO-compiled notification data from National Tuberculosis Programmes, and UNAIDS-estimated paediatric HIV and ART prevalence, with recent findings of case-fatality ratios.17 Figure 1 shows the modelling process. Table 1 presents corresponding parameters, data sources, and distributional assumptions characterising input uncertainty.
Figure 1.
Modelling data sources and stages
CFRs=Case-fatality ratios.
Table 1.
Data inputs, sources, and distributional characterisation of uncertainty
| Source | Value (95% CI) | Distribution | |
|---|---|---|---|
| Tuberculosis country incidence in children (aged <15 years) | WHO5 | Country-specific | Gamma |
| Tuberculosis notifications in children (aged <5 years and 5–14 years) | WHO5 | Country-specific | NA |
| Fraction of incidence in children <5 years for country | Dodd and colleagues7 | Country-specific | Beta |
| HIV prevalence in children aged <15 years | UNAIDS | Country-specific | Gamma |
| ART coverage in children aged <15 years | UNAIDS | Country-specific | Gamma |
| Incidence rate ratio for tuberculosis if HIV-positive | Dodd and colleagues18 | 77·8 (8·4–736·6)* | LN (4·36, 1·14) |
| Hazard ratio for tuberculosis if HIV-positive on ART | Dodd and colleagues18 | 0·3 (0·21–0·39) | LN (–1·20, 0·15) |
| Case-fatality ratio children aged <5 years, treated for tuberculosis, HIV-negative | Jenkins and colleagues17 | 1·9 (0·5–7·1) | LN (–3·96, 0·65) |
| Case-fatality ratio children aged <5 years, untreated, HIV-negative | Jenkins and colleagues17 | 43·6 (36·8–50·6) | LN (–0·83, 0·08) |
| Case-fatality ratio children aged <5 years, untreated, HIV-positive, no ART | Elicited | 90·0 (77·8–96·9) | Beta (33·18, 3·98) |
| Case-fatality ratio children aged <5 years, untreated, HIV-positive, on ART | Elicited | 63·5 (30·0–90·1) | Beta (5·08, 3·98) |
| Case-fatality ratio children aged 5–14 years, treated for tuberculosis, HIV-negative | Jenkins and colleagues17 | 0·8 (0·3–2·1) | LN (–4·83, 0·48) |
| Case-fatality ratio children aged 5–14 years, untreated, HIV-negative | Jenkins and colleagues17 | 14·9 (11·5–19·1) | LN (–1·90, 0·13) |
| Case-fatality ratio children aged 5–14 years, untreated, HIV-positive, no ART | Elicited | 87·4 (67·4–97·2) | Beta (16·18, 2·64) |
| Case-fatality ratio children aged 5–14 years, untreated, HIV-positive, on ART | Elicited | 45·7 (12·4–82·6) | Beta (2·89, 3·33) |
| Odds ratio of death on tuberculosis treatment (HIV-positive or no ART vs no HIV) | Jenkins and colleagues17 | 13·9 (5·4–36·2) | logit-multivariate normal (appendix) |
| Odds ratio of death on tuberculosis treatment (HIV-positive or on ART vs no HIV) | Jenkins and colleagues17 | 7·8 (2·3–27·0) | logit-multivariate normal (appendix) |
Data are n (95% CI) unless stated otherwise. LN=log-normal distribution. NA=not applicable. ART=antiretroviral therapy.
From a random-effects meta-analysis of data from Dodd and colleagues.18 When not otherwise stated, distribution parameters were established by matching median and variance.
We started with WHO-estimated tuberculosis incidence in children younger than 15 years for every country for 2015.5 Since the 2015 Global tuberculosis report,4 these have been based on an ensemble combination of methods proposed by Dodd and colleagues6 and Jenkins and colleagues.8 We used country point-estimates and uncertainty intervals to parameterise gamma distributions representing country paediatric tuberculosis incidence. We disaggregated these estimates for the younger than 15 years age group into younger than 5 years and those aged 5–14 years using the country-level incidence fraction in each age group from Dodd and colleagues,7 modelled as a country-specific beta distribution.
The notification data represent tuberculosis cases reported to WHO by each country in the two age groups (<5 years and 5 to <15 years). In our main analysis, we assumed all notified child cases had received treatment, and that the difference between the estimated number of incident cases and those notified by the programme represented untreated cases.
To disaggregate tuberculosis incidence by HIV and ART status, we combined country-level UNAIDS paediatric HIV prevalence estimates (h) and ART coverage in children with HIV (α) (appendix shows missing data assumptions) with data from a recent child-specific systematic review on the incidence rate ratio for incident tuberculosis with respect to UNAIDS country HIV prevalence estimates (ρ), and the protective effect of ART against tuberculosis disease (hazard ratio τ; not country specific).18 Specifically, we assumed that the fraction of the tuberculosis incidence in each HIV and ART category was proportional to (1–h) for HIV-negative children, to ρ.h.(1–α) for HIV-positive children not on ART, and to ρ.h.α.τ for HIV-positive children on ART. We assumed that levels of treatment for tuberculosis did not vary by HIV or ART status.
For children who did not receive tuberculosis treatment, we took case-fatality ratios for HIV-negative children younger than 5 years and those aged 5–14 years from a recent systematic review and meta-analysis.17 We assumed case-fatality ratios in untreated HIV-negative children were the same as case-fatality ratios from the pre-chemotherapy era. Estimates were unavailable for case-fatality ratios in HIV-positive children with tuberculosis who did not receive tuberculosis treatment. For this group, we therefore elicited beta distribution parameters characterising the case-fatality ratios in children younger than 5 years and those aged 5–14 years (stratified by ART status) from six clinicians with relevant experience using the roulette method provided by MATCH (appendix).19
For children who did receive tuberculosis treatment, case-fatality ratio estimates from the same meta-analysis17 were available for HIV-negative children younger than 5 years and those aged 5–14 years. This review also presented US data stratified by HIV status from before and after the introduction of ART. We used a regression model for these data to produce a joint posterior for the odds ratios of death given HIV and ART status, accounting for time period (table 1 and appendix).
Uncertainty in parameters and inputs was included using 10 000 samples with a Latin hypercube design, and distributional assumptions (table 1). Results for 2015 were aggregated by WHO region, and presented by treatment type, age group, and HIV status using median values as point estimates, and the 95 percentile ranges as uncertainty intervals. Median country estimates of per-person mortality were mapped. For countries with UNAIDS estimates of paediatric HIV prevalence higher than 0·1%, we calculated the percentage of HIV infection among incident paediatric tuberculosis and among paediatric tuberculosis deaths.
All analyses were done with R.20 Our reporting follows the GATHER guidelines.21
Sensitivity analyses
To explore sensitivity to the assumption that the difference between estimated incidence and notified cases represents untreated cases, we considered the ten countries with highest estimated number of deaths from tuberculosis in our model and assumed a certain proportion of children were treated but not notified. These proportions were assumed to be the same as the fraction of all tuberculosis cases treated outside the National Tuberculosis Programme sector when data were available (appendix).
To explore the sensitivity of our results to elicited values for case-fatality ratio in untreated HIV-positive children, we also generated results using the conservative assumption that case-fatality ratios in untreated HIV-positive children are the same as those in HIV-negative children (irrespective of ART status).
Data sharing
Scripts for data cleaning, as well as all subsequent merging and data handling are available on github. This repository also includes all input data, model analysis scripts, and a set of test output graphs around representation of input parameter uncertainty. It also contains additional output data, including country-level estimates.
Role of the funding source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
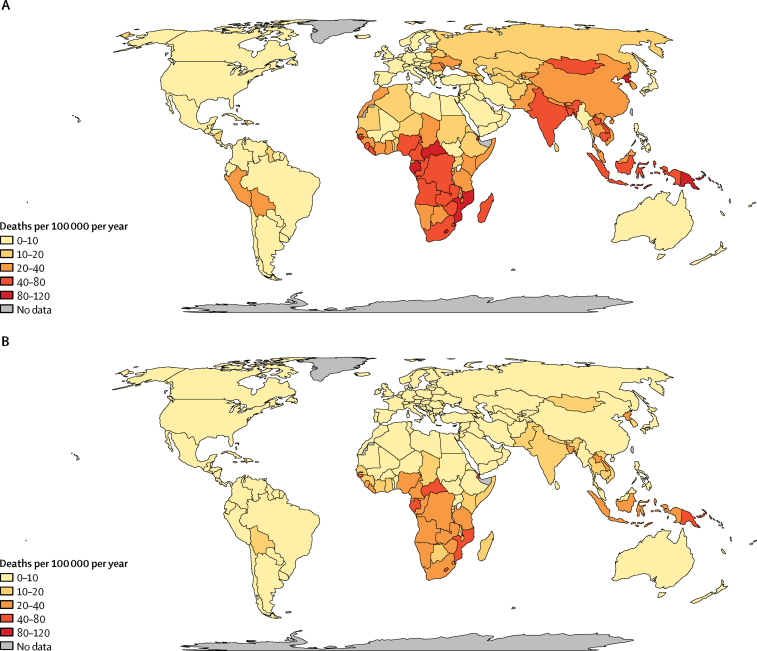
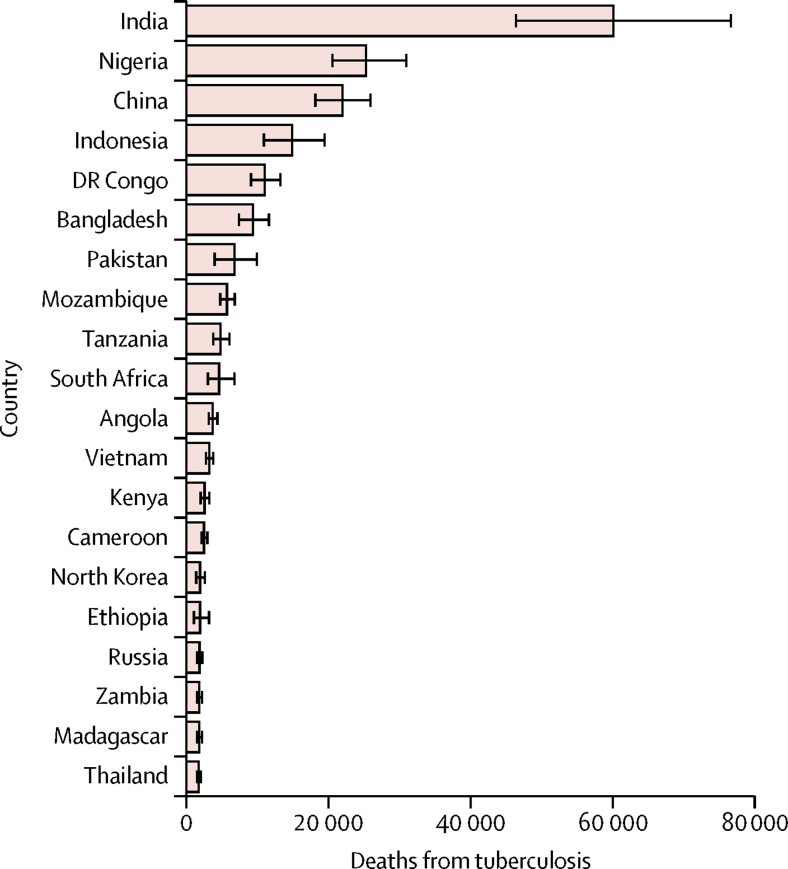
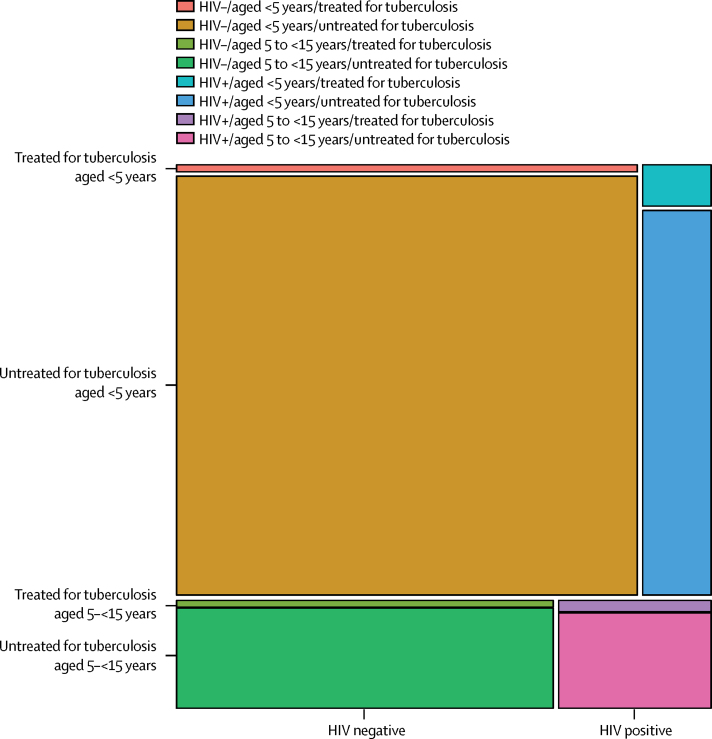
We estimated that 239 000 (95% UI 194 000–298 000) children younger than 15 years died from tuberculosis worldwide in 2015, with 191 000 (132 000–257 000) cases being in children younger than 5 years. This varied by region, with the WHO southeast Asia and Africa regions accounting for more than 70% of deaths (table 2). Per-person tuberculosis mortality in children was highest in countries in sub-Saharan Africa with high annual tuberculosis incidences (figure 2), whereas absolute burden was highest in populous countries, with the top five burdens being in India, Nigeria, China, Indonesia, and Democratic Republic of the Congo (figure 3). 191 000 (80%, 132 000–257 000) of tuberculosis deaths occurred in children younger than 5 years, and almost all (228 000 [96%]) occurred in children not on treatment (table 2, figure 4).
Table 2.
Number of deaths due to tuberculosis in children in 2015, by age group, WHO region, and HIV and tuberculosis treatment status, and mortality rate by age group
| Received tuberculosis treatment (HIV-negative) | Did not receive tuberculosis treatment(HIV-negative) | Received tuberculosis treatment (HIV-positive) | Did not receive tuberculosis treatment (HIV-positive) | Total mortality | Mortality rate per 100 000 | |
|---|---|---|---|---|---|---|
| Age <5 years | ||||||
| AFR | 651 (368– 1360) | 44 700 (30 600– 62 100) | 1900 (776 − 5500) | 173 00 (9160–37 500) | 66 100 (47 000–90 700) | 41 (29– 56) |
| AMR | 93 (61–152) | 3910 (2180– 5900) | 27 (6–152) | 84 (21–422) | 4180 (2380– 6210) | 5 (3–8) |
| EMR | 330 (163–837) | 10 500 (3510– 21 000) | 34 (7–180) | 185 (41–826) | 11 100 (4030– 21 800) | 13 (5–27) |
| EUR | 72 (47–134) | 4460 (2610–6500) | 1 (0–7) | 17 (4–101) | 4560 (2710– 6610) | 8 (4–11) |
| SEA | 1000 (506–2130) | 71 400 (25 300– 127 000) | 248 (53–1190) | 2620 (303–20 200) | 77 400 (27 700–137 000) | 43 (15–76) |
| WPR | 369 (140–1170) | 25 300 (10 500– 41 500) | 15 (2–110) | 219 (53 − 905) | 26 100 (11 300–42 300) | 22 (9–35) |
| Total | 2690 (1850– 4180) | 161 000 (108 000–223 000) | 2360 (1100– 6020) | 22 000 (11 800–47 600) | 191 000 (132 000–257 000) | 28 (19–38) |
| Age 5 to <15 years | ||||||
| AFR | 312 (216–480) | 9970 (5910–15800) | 937 (472–2090) | 10 200 (4760–24 200) | 21 900 (13 200–37 400) | 8 (5–14) |
| AMR | 52 (38–75) | 968 (430–1670) | 13 (3–67) | 51 (10–319) | 1120 (538–1880) | 0 (0–1) |
| EMR | 270 (150–566) | 976 (336–3880) | 25 (6–118) | 62 (7–390) | 1410 (649–4370) | 1 (0–3) |
| EUR | 61 (44–89) | 786 (338–1500) | 1 (0–6) | 7 (1–60) | 867 (406–1570) | 0 (0–1) |
| SEA | 1070 (612–2070) | 11 300 (2110–30400) | 302 (58–2080) | 889 (29–9910) | 14 400 (3700–38 000) | 3 (1–10) |
| WPR | 228 (128–438) | 6280 (1970–12400) | 18 (3–133) | 107 (20–572) | 6730 (2370–12 800) | 3 (1–5) |
| Global | 2050 (1510–3100) | 31 500 (18600–51400) | 1430 (738–3380) | 12 200 (5920–28 800) | 48 400 (30 900–75 800) | 3 (2–6) |
| Age 0 to <15 years | ||||||
| AFR | 978 (640– 1710) | 54 900 (42 200– 70 400) | 2890 (1320–7160) | 28 200 (16 300–54 800) | 88 600 (71 700–113 000) | 21 (17–26) |
| AMR | 148 (111– 207) | 4890 (3610– 6460) | 42 (10–209) | 165 (58–543) | 5310 (3990–6920) | 2 (1–3) |
| EMR | 625 (381– 1190) | 11 600 (5380– 21 700) | 62 (15–286) | 277 (86–988) | 12 700 (6370–22900) | 5 (2–10) |
| EUR | 136 (103– 200) | 5250 (3870– 6960) | 3 (0–13) | 26 (8–143) | 5440 (4050– 7140) | 3 (2– 4) |
| SEA | 2140 (1360–3580) | 83 100 (48 200– 132 000) | 574 (123–3140) | 4050 (781–25 400) | 92 500 (55 100–145 000) | 17 (10–26) |
| WPR | 616 (337–1410) | 31 800 (21 000 − 44 800) | 35 (7–230) | 349 (98–1330) | 32900 (22 100–46 000) | 9 (6–13) |
| Total | 4810 (3710–6590) | 193 000 (151 000–247 000) | 3900 (1960–8670) | 35 000 (20 500–67 400) | 239 000 (194 000–298 000) | 12 (10–15) |
Data are n (95% UI). AFR=African Region. AMR=Region of the Americas. EMR=Eastern Mediterranean Region. EUR=European Region. SEA=Southeast Asia Region. WPR=Western Pacific Region. Rounding and the use of medians mean that the 0 to <5 and 5 to <15 entries will not add up to those in the 0 to <15 section.
Figure 2.
Mortality rate from tuberculosis for children (A) aged <5 years in 2015 (tuberculosis deaths in children per 100 000 children) and (B) <15 years in 2015 (tuberculosis deaths in children per 100 000 children)
Figure 3.
Number of deaths from tuberculosis in children aged <15 years in 2015, for the 20 countries with highest paediatric tuberculosis mortality
Error bars denote IQR.
Figure 4.
Mosaic plot with areas showing the proportion of tuberculosis deaths in children aged <15 years in 2015, by age group, tuberculosis treatment status, and HIV infection status
We estimated about 40 000 (17%) deaths from tuberculosis in HIV-positive children; most of these were in Africa, where 31 000 (36%, 19 000–59 000) child tuberculosis deaths were associated with HIV. For the 50 countries with UNAIDS paediatric HIV prevalence higher than 0·1%, the median paediatric HIV prevalence was 0·2%, the median prevalence among incident paediatric tuberculosis cases was 13%, and the median prevalence among paediatric tuberculosis deaths was 27% (appendix). For South Africa, with one of the estimated highest HIV prevalence among paediatric tuberculosis deaths, the paediatric HIV prevalence was 1·5% (240 000, 95% UI 210 000–260 000), and we estimated 35% HIV prevalence in incident tuberculosis (11 200, 1800–27 100) and 69% (2900, 95% UI 400–11 800) HIV prevalence in tuberculosis deaths.
Our sensitivity analysis in which case-fatality ratios were assumed to be the same in HIV-positive and HIV-negative children not receiving tuberculosis treatment did not substantially change the global estimates. Global estimates reduced to 218 000 (95% UI 176 000–270 000) for children younger than 15 years and 179 000 (124 000–240 000) for children younger than 5 years; an overall reduction of about 24 000, mostly in the WHO Africa region.
Changing the assumption that absence of case notification represents absence of treatment also did not have a large effect on the global estimates. The ten countries with highest paediatric tuberculosis mortality accounted for 71% (170 000, 126 000–228 000) of global tuberculosis deaths in children. The assumption that a country-specific percentage of treated children who received tuberculosis treatment were not notified in the ten countries with the highest estimated paediatric tuberculosis mortality (appendix shows country-specific percentages used) reduced the number of deaths in children younger than 15 years to 218 000 (95% UI 169 000–282 000) and deaths in children younger than 5 years to 177 000 (120 000–244 000).
Discussion
Our case-fatality-based analysis suggests that 239 000 children younger than 15 years died from tuberculosis in 2015, with 191 000 deaths occurring in children younger than 5 years (>80% of the deaths in children aged <15 years). Almost all of these deaths (>96%) occurred in children who were not receiving treatment for tuberculosis. Recent estimates of low mortality rates in children on treatment (<1% mortality)17 imply that the number of paediatric tuberculosis deaths could be roughly halved if the case-detection ratio for children were improved from its current value of approximately one in three to the two in three currently achieved in adults, or reduced by more than ten times if all children with tuberculosis were given treatment.
Most deaths were in the WHO Africa and southeast Asia regions, both for under-5 and under-15 mortality; the highest individual country mortality was found in India, Nigeria, China, Indonesia, and the Democratic Republic of the Congo. All these countries are on the current WHO list of 30 high tuberculosis burden countries; all are populous, with tuberculosis incidence rates under 400 per 100 000 per year. About 17% of deaths were in children with HIV. Per-person tuberculosis mortality was highest in sub-Saharan Africa, where roughly 36% of tuberculosis deaths were in children infected with HIV. The average case-fatality rate for tuberculosis in children globally was about 24%.
Our 191 000 estimated tuberculosis deaths in children younger than 5 years implies that tuberculosis should rank as a major cause of death in children younger than 5 years worldwide, despite not appearing in the 2016 CHERG estimates, which were based on both vital registration and verbal autopsy data. Directly comparing our estimate for 2015 of 191 000 tuberculosis deaths in children younger than 5 years with the estimates for 2015 (for children aged 1–59 months) reported by Liu and colleagues1 suggests that tuberculosis might be the sixth highest cause of death in the 1–59 month age group, causing more deaths than meningitis, AIDS, measles, and pertussis. Some deaths ascribed to pneumonia, meningitis, and AIDS may have been caused by tuberculosis. However, some tuberculosis deaths were probably not represented in these global mortality estimates, and thus that overall estimates are too low. The extent of reclassification, and implications for total child mortality are unclear. To achieve better estimates of the burden of tuberculosis mortality in children, new methods are needed that can leverage vital registration while accounting for its imperfections.
Our estimate for tuberculosis mortality in children younger than 15 years is similar to the WHO estimate of 210 000, which was based on an independent approach using vital registration data and imputation using child-to-adult mortality rate ratios. A complementary approach based on case-fatality ratio offers a way to avoid some of the major limitations of methods based on vital registration and verbal autopsy. In view of the current diagnostic capacity and the state of vital registration systems in settings with high tuberculosis burdens, estimates based on vital registration and verbal autopsy data are highly sensitive to the assumptions made in adjusting primary data for misclassification and underdiagnosis. Additionally, any biases resulting from these assumptions will be perpetuated during extrapolations of these vital registration and verbal autopsy data to countries that do not collect such data. Moreover, the uncertainty associated with these assumptions is not well characterised because population-based autopsy studies that establish the true causes of childhood mortality are rare. Thus, there has been substantial volatility in vital-registration-based estimates of childhood tuberculosis deaths; the increase from 136 000 estimated deaths in 2014 to 210 000 deaths in 2015 was largely attributable to new data availability from India, a change which then fed into extrapolations to many other countries, suggesting that the assumptions made for the 2014 estimates were probably inaccurate. By contrast, the case-fatality ratio approach uses assumptions based on decades of scientific literature whose uncertainty can be quantified. Therefore, we believe that such an approach is important for contributing to the development of more rigorous estimates, even as efforts are made to strengthen systems for diagnosis and mortality surveillance.
As with any method, the case-fatality ratio approach has limitations. First, given the excellent treatment outcomes in children (<1% mortality in children <15 years) and the high case-fatality ratios in children who do not receive treatment (22% mortality in children <15 years),17 our estimate is sensitive to the assumption that children who are not notified as having tuberculosis do not receive tuberculosis treatment. However, our sensitivity analysis assuming high rates of non-notified treatment in the highest burden reduced our estimates of total childhood and under-5 tuberculosis mortality by less than 10%, still making tuberculosis the sixth largest contributor to under-5 mortality worldwide. We also neglected the potential for overdiagnosis on clinical grounds to substantially impact on our estimates.
Our estimates are also limited by not having case-fatality ratios for every potentially meaningful subgroup of children with tuberculosis. Because case-fatality ratios for children with tuberculosis who do not receive tuberculosis treatment were not available from recent studies, we assumed that these children had the same risk of death as children in the era before effective treatment was available in populations in Europe and the USA. However, in view of potential differences in underlying child health, these case-fatality ratios may be different today and in other settings. The case-fatality ratios we used were based on death within 1 year of diagnosis; we modelled progression to disease and death as occurring within 1 year of infection, which neglects the influence of trends in annual risks of infection via children who take longer to progress and die. For comparison, the case-fatality ratio for untreated HIV-negative adult tuberculosis cases (over their longer disease course) is thought to be 70% in smear-positive and 20% in smear-positive, culture-positive cases.22 Finally, we have not considered any potential protection against mortality conferred by Bacillus Calmette-Guérin (BCG) vaccination in tuberculosis disease, independent of protection against incidence. The review by Jenkins and colleages17 was unable to estimate case-fatality ratios among BCG-vaccinated children and a recent review and meta-analysis23 was suggestive of only a weak or absent additional effect of BCG against mortality independent of disease.
We assumed a uniform prevalence of HIV and ART coverage by age among children, and that the likelihood of tuberculosis diagnosis and notification is not affected by HIV status. We used an incident rate ratio for incident tuberculosis given HIV derived from comparing systematic review data on HIV prevalence in child tuberculosis cases with UNAIDS paediatric HIV prevalence. This might be subject to various biases, and is uncertain due to high between-country variability. However, it is specific to our input data, and yields HIV prevalence in child tuberculosis cases and deaths that accords with observations. Moreover, our estimates are within 10% of the vital-registration-based WHO estimates of paediatric HIV/tuberculosis mortality for the African and southeast Asia regions, which include most HIV and tuberculosis deaths. Data were also unavailable to inform case-fatality ratios for HIV-positive children who are not treated for tuberculosis, and these were based on expert opinion. However, our sensitivity analysis assuming HIV-negative case-fatality ratios applied to HIV-positive children resulted in a reduction in our total estimate of about 10%.
A major strength of our work is its independence from vital registration data, which is absent or of poor quality in many countries with a high tuberculosis burden and likely to miss a large and poorly quantified fraction of tuberculosis deaths. Instead, it makes use of recent evidence from updated childhood tuberculosis estimates, current data about HIV prevalence and ART coverage, and a systematic review and meta-analysis of case-fatality ratios. Another strength is our thorough treatment of uncertainty and investigation of the effect of key assumptions.
Reducing childhood deaths from tuberculosis requires more children to be reached, diagnosed, and treated. Developing an acceptable diagnostic test that reliably identified tuberculosis disease in children would be hugely beneficial, but an increasing willingness to treat children clinically for tuberculosis disease, without requiring confirmation of diagnosis, would also reduce mortality. However, it would probably mean an increase in the number of children treated unnecessarily for tuberculosis and we as a society would need to consider whether this is an approach we are prepared to follow.
Many other methods to reduce child deaths due to tuberculosis already exist, but to realise their protective benefit, health systems must invest in ensuring that they are fully implemented. For instance, household contact investigations can ensure that children with tuberculosis receive prompt treatment and that other exposed children can be given preventive treatment to reduce the risk of subsequent tuberculosis.24, 25 However, logistical and structural barriers have hampered the implementation of routine contact investigations,26 and only 7% of young child contacts received preventive treatment in 2015.5 Additionally, because children with HIV are at an increased risk of both developing tuberculosis and dying from it, prevention of mother-to-child transmission of HIV and ART initiation for children who are HIV-positive should reduce child tuberculosis deaths. However, in 2015, nearly a quarter of pregnant women living with HIV did not have access to services to prevent mother-to-child transmission, and half of all children living with HIV were not receiving ART.27 Finally, addressing other risk factors for tuberculosis, such as undernutrition, crowding, and poverty can all play a role in reducing tuberculosis deaths in children.
Tuberculosis is one of the top ten causes of death in children and a key missing component in previous analyses of under-5 mortality. Almost all these deaths occur in children who are not being treated for tuberculosis, implying that efforts to identify children at risk of tuberculosis, diagnose children with tuberculosis, and treat them appropriately must be implemented to substantially reduce tuberculosis mortality in children.
For all input and modelling data see https://github.com/petedodd/4PM
Acknowledgments
Acknowledgments
This work was funded from the STEP-TB grant from UNITAID to TB Alliance. HEJ was funded by US National Institutes of Health US NIH K01AI102944. The content of the article is solely the responsibility of the authors and does not necessarily represent the views of the National Institute of Allergy and Infectious Disease or the Office of the Director, NIH. CS is a staff member of the World Health Organization (WHO). He alone is responsible for the views expressed in this publication and they do not necessarily represent the decisions or policies of WHO. JAS was supported by an NIHR Academic Clinical Lectureship and also through a grant from the Academy of Medical Sciences. CMY was funded by a Charles H. Hood Foundation Child Health Research Award. Thanks to Veronica Mulenga, Chishala Chabala, Elisabetta Walters, Steve Graham, and Helena Rabie for participating in the elicitation exercise.
Contributors
PJD, CMY, JAS, and HEJ conceived and designed the study. PJD did the analysis and wrote first draft. All authors critically reviewed the methods and results and contributed to writing the report.
Declaration of interests
We declare no competing interests.
Supplementary Material
References
- 1.Liu L, Oza S, Hogan D. Global, regional, and national causes of under-5 mortality in 2000–15: an updated systematic analysis with implications for the Sustainable Development Goals. Lancet. 2016;388:3027–3035. doi: 10.1016/S0140-6736(16)31593-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.United Nations Millennium Development Goals and Beyond. 2015. http://www.un.org/millenniumgoals/bkgd.shtml (accessed Jan 23, 2017).
- 3.United Nations Transforming our world: the 2030 Agenda for Sustainable Development. 2015. http://www.un.org/ga/search/view_doc.asp?symbol=A/RES/70/1&Lang=E (accessed Jan 23, 2017).
- 4.WHO . Global tuberculosis report 2015. World Health Organization; Geneva: 2015. [Google Scholar]
- 5.WHO . Global tuberculosis report 2016. World Health Organization; Geneva: 2016. [Google Scholar]
- 6.Dodd PJ, Gardiner E, Coghlan R, Seddon JA. Burden of childhood tuberculosis in 22 high-burden countries: a mathematical modelling study. Lancet Glob Health. 2014;2:e453–e459. doi: 10.1016/S2214-109X(14)70245-1. [DOI] [PubMed] [Google Scholar]
- 7.Dodd PJ, Sismanidis C, Seddon JA. Global burden of drug-resistant tuberculosis in children: a mathematical modelling study. Lancet Infect Dis. 2016;16:1193–1201. doi: 10.1016/S1473-3099(16)30132-3. [DOI] [PubMed] [Google Scholar]
- 8.Jenkins HE, Tolman AW, Yuen CM. Incidence of multidrug-resistant tuberculosis disease in children: systematic review and global estimates. Lancet. 2014;383:1572–1579. doi: 10.1016/S0140-6736(14)60195-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oliwa JN, Karumbi JM, Marais BJ, Madhi SA, Graham SM. Tuberculosis as a cause or comorbidity of childhood pneumonia in tuberculosis-endemic areas: a systematic review. Lancet Respir Med. 2015;3:235–243. doi: 10.1016/S2213-2600(15)00028-4. [DOI] [PubMed] [Google Scholar]
- 10.Graham SM, Sismanidis C, Menzies HJ, Marais BJ, Detjen AK, Black RE. Importance of tuberculosis control to address child survival. Lancet. 2014;383:1605–1607. doi: 10.1016/S0140-6736(14)60420-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gupta M, Rao C, Lakshmi PV, Prinja S, Kumar R. Estimating mortality using data from civil registration: a cross-sectional study in India. Bull World Health Organ. 2016;94:10–21. doi: 10.2471/BLT.15.153585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rao C, Osterberger B, Anh TD, MacDonald M, Chuc NT, Hill PS. Compiling mortality statistics from civil registration systems in Viet Nam: the long road ahead. Bull World Health Organ. 2010;88:58–65. doi: 10.2471/BLT.08.061630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Glaziou P, Sismanidis C, Pretorius C, Floyd K. Methods used by WHO to estimate the Global burden of tuberculosis disease. https://arxiv.org/ftp/arxiv/papers/1603/1603.00278.pdf (accessed August 1, 2017).
- 14.Misganaw A, Mariam DH, Araya T, Aneneh A. Validity of verbal autopsy method to determine causes of death among adults in the urban setting of Ethiopia. BMC Med Res Methodol. 2012;12:130. doi: 10.1186/1471-2288-12-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang G, Rao C, Ma J. Validation of verbal autopsy procedures for adult deaths in China. Int J Epidemiol. 2006;35:741–748. doi: 10.1093/ije/dyi181. [DOI] [PubMed] [Google Scholar]
- 16.Setel PW, Whiting DR, Hemed Y. Validity of verbal autopsy procedures for determining cause of death in Tanzania. Trop Med Int Health. 2006;11:681–696. doi: 10.1111/j.1365-3156.2006.01603.x. [DOI] [PubMed] [Google Scholar]
- 17.Jenkins HE, Yuen CM, Rodriguez CA. Mortality in children diagnosed with tuberculosis: a systematic review and meta-analysis. Lancet Infect Dis. 2017;17:285–295. doi: 10.1016/S1473-3099(16)30474-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dodd PJ, Prendergast AJ, Beecroft C, Kampmann B, Seddon JA. The impact of HIV and antiretroviral therapy on tuberculosis risk in children: a systematic review and meta-analysis. Thorax. 2017;72:559–575. doi: 10.1136/thoraxjnl-2016-209421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morris DE, Oakley JE, Crowe JA. A web-based tool for eliciting probability distributions from experts. Environmental Modelling & Software. 2014;52:1–4. [Google Scholar]
- 20.R Core Team R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL http://www.R-project.org (accessed Jan 23, 2017).
- 21.Stevens GA, Alkema L, Black RE. Guidelines for Accurate and Transparent Health Estimates Reporting: the GATHER statement. PLoS Med. 2016;13:e1002056. doi: 10.1371/journal.pmed.1002056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tiemersma EW, van der Werf MJ, Borgdorff MW, Williams BG, Nagelkerke NJ. Natural history of tuberculosis: duration and fatality of untreated pulmonary tuberculosis in HIV negative patients: a systematic review. PLoS One. 2011;6:e17601. doi: 10.1371/journal.pone.0017601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abubakar I, Pimpin L, Ariti C. Systematic review and meta-analysis of the current evidence on the duration of protection by bacillus Calmette-Guerin vaccination against tuberculosis. Health Technol Assess. 2013;17:1–372. doi: 10.3310/hta17370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ayieko J, Abuogi L, Simchowitz B, Bukusi EA, Smith AH, Reingold A. Efficacy of isoniazid prophylactic therapy in prevention of tuberculosis in children: a meta-analysis. BMC Infect Dis. 2014;14:91. doi: 10.1186/1471-2334-14-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yuen CM, Jenkins HE, Chang R, Mpunga J, Becerra MC. Two methods for setting child-focused tuberculosis care targets. Public Health Action. 2016;6:83–96. doi: 10.5588/pha.16.0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hill PC, Rutherford ME, Audas R, van Crevel R, Graham SM. Closing the policy-practice gap in the management of child contacts of tuberculosis cases in developing countries. PLoS Med. 2011;8:e1001105. doi: 10.1371/journal.pmed.1001105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.UNAIDS Children and HIV. 2016. http://www.unaids.org/sites/default/files/media_asset/FactSheet_Children_en.pdf (accessed June 8, 2017).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.