Abstract
Regulatory T cells (Tregs) that express the transcription factor Foxp3 are critical for regulating intestinal inflammation. Candidate microbe approaches have identified bacterial species and strain-specific molecules that can affect intestinal immune responses, including species that modulate Treg responses. Because neither all humans nor mice harbor the same bacterial strains, we posited that more prevalent factors exist that regulate the number and function of colonic Tregs. We determined that short chain fatty acids (SCFA), gut microbiota-derived bacterial fermentation products, regulate the size and function of the colonic Treg pool and protect against colitis in a Ffar2(GPR43)-dependent manner in mice. Our study reveals that a class of abundant microbial metabolites underlies adaptive immune microbiota co-adaptation and promotes colonic homeostasis and health.
The intestinal immune system has co-evolved with the gut microbiota for the maintenance of intestinal health (1). Disruption of this homeostasis leads to intestinal inflammation and disease (2, 3). Colonic regulatory T cells (cTregs) expressing the transcription factor Foxp3 are critical for limiting intestinal inflammation and depend on microbiota-derived signals for proper development and function (4-7). Bacteroides fragilis and clostridial species induce Treg responses (6, 7); however, how the gut microbiota affect cTreg responses across mammalian hosts remains unclear. While polysaccharide A from B. fragilis modulates Treg responses (6), such effects are also likely mediated through more common factor(s) produced by many bacterial genera.
Humans and mice rely on bacteria to breakdown undigestible dietary components, such as fibers (8). Short chain fatty acids (SCFA) are bacterial fermentation products and range in concentration between 50-100mM in the colonic lumen (9). We examined SCFA concentrations in specific pathogen-free (SPF), gnotobiotic Altered Schaedler Flora (ASF)-colonized mice, and germ-free (GF) mice and found that GF mice had reduced concentrations of the three most abundant luminal SCFA, acetic acid, propionic acid and butyric acid (Table S1), as previously reported (10). The decrease of these SCFA in GF mice suggested that SCFA may contribute to their immune defects, specifically reduced cTreg numbers. We provided SCFA in the drinking water (150mM) to GF mice for three weeks and found that SCFA individually or in combination (SCFA mix) increased cTreg frequency and number (Fig. 1A) but did not increase the number or frequency of splenic, mesenteric lymph node (MLN), or thymic Tregs (fig. S1). These effects coincided with increased luminal SCFA (Table S1). SCFA increased CD4+ T cell frequency and number (fig. S2) but did not alter colonic Th1 or Th17 cell numbers significantly (fig. S3).
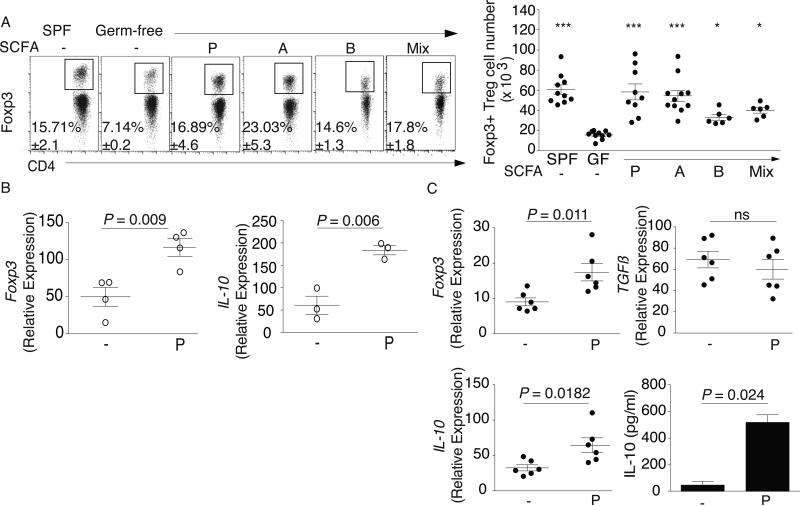
Fig. 1. SCFA restore colonic Treg populations and function in germ-free mice.
(A) Colonic lamina propria (LP) lymphocytes were isolated and stained for CD4 and Foxp3. Left panel: Representative dot plots and percentage of CD4+Foxp3+ within the CD45+CD3+ population from SPF, GF, and GF mice treated with propionate (P), acetate (A), butyrate (B), or the SCFA mix in the drinking water. Right panel: Numbers of Foxp3+ Tregs for the left panel. (B) cTregs were isolated from in vivo propionate-treated GF mice, sorted by CD4, CD127, and CD25, and examined ex vivo for expression of Foxp3 and IL-10 by RTqPCR. (C) cTregs were isolated from GF mice and purified as in 1B, cultured for 24 hrs in the presence or absence of 0.1 mM propionate and examined for expression of Foxp3, TGFβ, and IL-10 by RTqPCR and IL-10 protein production by ELISA. For panel A, symbols represent data from individual mice. Horizontal lines show the mean and error bars the SD. For panels B and C, each symbol or bar represents pooled cTregs from 3-5 mice. All data shown are representative of at least 3 independent experiments. A Kruskal-Wallis test with a Dunn's post hoc test was performed in panel A, *** P <0.001 and * P <0.05. A Mann-Whitney U test was performed in panels B and C.
Microbiota-induced cTreg development is associated with increases in de novo generation of inducible Tregs (iTregs) and not in Tregs of thymic origin (nTregs) (7). These populations can be distinguished by expression of Helios, which in vivo is restricted to nTregs (11). We found that Helios+ Treg frequency was similar between GF and SCFA-treated GF mice (fig. S4) but lower in SPF mice. SCFA-treatment increased Helios+ Treg numbers in GF mice, indicating that Tregs already present in the colonic lamina propria (cLP) were expanding.
To test if SCFA could affect cTregs in a GF setting, we isolated cTregs from GF mice treated with propionate in vivo for three weeks and examined expression of Foxp3 and interleukin (IL)-10, a key cytokine in Treg-mediated suppression. We also isolated cTregs from GF mice and stimulated them in vitro with propionate. Both treatments significantly increased Foxp3 and IL-10 expression (Fig. 1B, C). In vitro treatment increased IL-10 production but not transforming growth factor-β (TGF-β), a Treg-mediated suppression factor, suggesting that SCFA specifically induce Foxp3+ IL-10-producing Tregs (Fig. 1B, C).
The antibiotic vancomycin, which targets Gram-positive bacteria and disrupts the gut microbiota (12), reduces cTregs to similar levels as observed in GF mice (7) (fig. S5). However, when SPF mice were treated with a combination of vancomycin and SCFA, the reduction in cTregs was completely restored (fig. S5). Collectively, these results suggest that SCFA, play a role in cTreg homeostasis.
We questioned whether SCFA would augment cTregs in SPF mice. SCFA treatment of SPF mice increased Foxp3+ and Foxp3+IL-10+ cTreg frequency and number (Fig. 2A-C) but not Foxp3+TGFβ+ cTregs (fig. S6). We did not observe changes with SCFA treatment in small intestinal Treg numbers (fig. S7). Neither colonic Th17 and Th1 nor MLN and splenic Treg frequency and number) from SPF mice were affected by SCFA (fig. S8-S11).
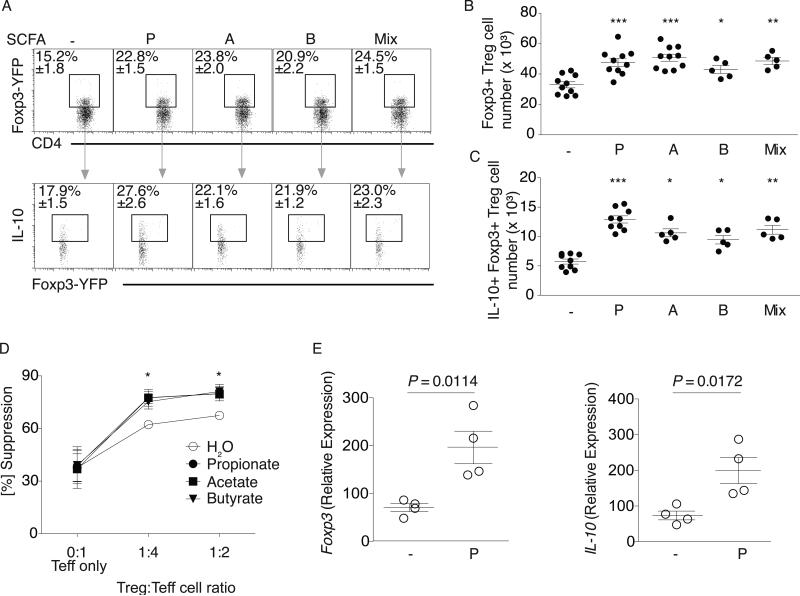
Fig. 2. SCFA augment colonic Treg population size and function in SPF mice.
(A) SPF Foxp3YFP-Cre mice were treated with water alone (-) or P, A, B, or the mix. Colonic LP lymphocytes were isolated and stained for CD4 and IL-10. Upper panel: Representative dot plots and percentage of CD4+ Foxp3-YFP+ within the CD45+CD3+ population. Lower panel: Representative dot plots and percentage of the CD4+Foxp3+IL-10+population. (B) Cell numbers for the data in (A) upper panel. (C) Cell numbers for the data in (A) lower panel. Symbols in B and C represent data from individual mice and represent 4 independent experiments. (D) cTregs were co-cultured with splenic effector T cells (Teff) and P, A, B, or media (sodium and pH matched) for 96 hrs. Percent suppression (y-axis), Treg:Teff ratios (x-axis). Symbols represent mean values and error bars SD for four independent experiments. * P < 0.01. (E) cTregs were isolated from the LP of in vivo propionate treated SPF Foxp3YFP-Cre mice, sorted for CD4 and YFP, and examined for ex vivo expression of Foxp3 and IL-10. Each symbol represents pooled cTregs from 3-5 mice, horizontal lines show the mean and error bars the SD. Four independent experiments were performed. A Kruskal-Wallis test with a Dunn's post hoc test was performed in panels B, C, D, *** P <0.0001, ** P <0.01, * P <0.05. A Mann-Whitney U test was performed for experiments in panel E and P-values are shown where significant.
These results may explain the benefits of dietary fibers and bacteria, such as clostridia and bifidobacteria (13) that can increase colonic luminal SCFA production and modulate inflammation in mice and humans. We measured SCFA production of species belonging to Clostridium cluster XI (Clostridium bifermentans), XIV (ASF 356 and 492), XVII (C. ramosum), and the bacteroides species, B. fragilis as Clostridium cluster XIV members and B. fragilis affect cTregs (6,7). ASF 356 and 492 and C. ramosum generated more propionate (14-62 vs 0.05-1.1 μmol/105 CFU) and acetate (118-220 vs 0.1-2 μmol/105 CFU) as compared to the other strains (Table S1).
cTregs regulate intestinal homeostasis and control inflammation by limiting proliferation of effector CD4+ T cells (Teff). Addition of SCFA to cTreg and Teff co-cultures increased cTreg suppressive capacity (Fig. 2D and fig. S12). In SPF mice, SCFA are taken up by colonic epithelial cells but also diffuse through the epithelium into the lamina propria where they can mediate their effects directly (9, 14). To determine if SCFA directly affect cTregs, we isolated cTregs from SCFA-treated SPF mice. In vivo treatment increased cTreg Foxp3 and IL-10 expression (Fig. 2E). We also isolated cTregs from SPF mice and incubated them with SCFA in vitro. Foxp3 expression as well as IL-10 expression and protein production increased (fig. S13), whereas TGFβ levels remained unchanged (fig. S14). As enhanced suppressive activity (Fig. 2D) could be attributed not only to higher IL-10 levels per cTreg but also to increased cTreg proliferation, we examined cTreg proliferation. cTregs exhibited enhanced proliferation when cultured in the presence of propionate (fig. S15).
In addition, we analyzed the expression patterns of Treg trafficking molecules. Although levels of the chemokine receptor CCR9 or α4β7 integrin were not altered in propionate-treated GF and SPF mice, levels of the cTreg homing receptor, GPR15 (15), did increase (fig. S16). Taken together, these data indicate that SCFA may have a beneficial effect in SPF mice through their ability to increase Foxp3+IL-10 producing cTregs and cTreg proliferative capacity, as well as alter cTreg GPR15 expression.
Considering that SCFA can influence cTregs directly, we asked if this was a receptor-mediated process. G coupled protein receptor (GPR) 43 (Ffar2 is the gene that encodes GPR43) binds SCFA and through its expression on innate immune cells mediates resolution of inflammatory responses (3, 16). We found that intestinal Tregs and, particularly cTregs, had significantly higher levels of Ffar2 than Tregs isolated from spleen or MLN (Fig. 3A, B), which was largely dependent upon microbiota-derived signals. As a reference, we compared cTreg Ffar2 expression levels to colonic myeloid (CD11b+) cells, which are known to express Ffar2 (3), and found that on average CD11b+ cells expressed 1.6 fold more Ffar2 than cTregs (fig. S17).
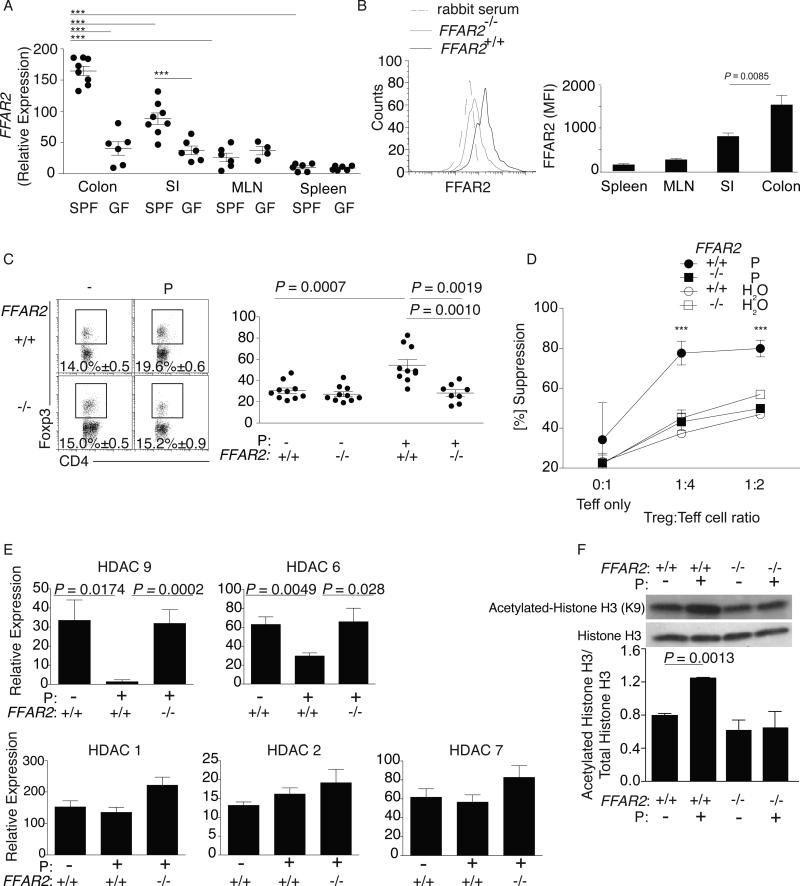
Fig 3. Ffar2 mediates SCFA effects on cTregs.
(A) Tregs were isolated from the colon, small intestine, spleen and MLN of SPF and GF BALB/c mice, purified as in 1B and Ffar2 expression examined by qPCR. Each symbol represents data from 3-5 individual mice, horizontal lines show the mean and error bars the SD. Data reflect 3-7 independent experiments. (B) Lymphocytes were isolated from the colon, small intestine, spleen, and MLN of SPF Ffar2−/− and littermate Ffar2+/+ mice. Cells were stained for CD4, Foxp3, and Ffar2. Left panel depicts a representative flow cytometry histogram comparing colonic Ffar2 expression in Ffar2−/− vs littermate Ffar2+/+ mice. Right panel shows the MFI for Ffar2 for Tregs from the indicated sites. Bars show the mean, error bars SD, and data reflect 4 independent experiments. (C) Colonic LP lymphocytes were isolated from Ffar2−/−and littermate Ffar2+/+ mice exposed to propionate (P) or water alone and stained for CD4 and Foxp3. Left panel: Representative dot plots with percentage of CD4+Foxp3+ within the CD45+CD3+ population. Right panel: Foxp3+ Treg number for the left panel. Each symbol represents data from individual mice, horizontal lines show the mean and error bars the SD. (D) Ffar2−/− and littermate Ffar2+/+ cTregs were co-cultured with splenic Teff cells in media with or without propionate for 96 hrs. Percent suppression (y-axis) and Treg:Teff ratios (x-axis). Symbols represent the mean of 3 independent experiments and error bars show the SD. (E) cTregs were isolated from the LP of Ffar2−/− and littermate Ffar2+/+ mice, purified as in 1B, cultured in the presence of 0.1 mM propionate or media (pH and sodium matched) for 24hrs and examined for expression of HDAC 1,2,6,7 and 9 by RTqPCR. Bars show the mean and error bars the SD of 3 independent experiments (F) Whole cell extracts were generated from cTregs isolated from the LP of Ffar2−/− and littermate Ffar2+/+ mice, purified as in 1B, and cultured in the presence of 0.1 mM propionate or media (pH and sodium matched) for 24hrs. Samples were analyzed by western blotting for histone acetylation by examining levels of acetylated histone (H3K9), total histone levels were used as a loading control. The western blot shown is representative of two independent experiments with cTregs cell lysates pooled from 10-12 mice per group. A bar graph of densitometry ratios of acetylated Histone H3:total Histone H3 is shown. Bars represent the mean and error bars the SD. A Kruskal-Wallis test with a Dunn's post hoc test was performed for panels A and D, *** P < 0.001. The Mann-Whitney U test was performed for panels C and E. The student's t-test was performed for panel B and F.
To determine if Ffar2 contributes to cTreg homeostasis, we treated Ffar2−/− mice and Ffar2+/+ littermates with propionate, which has the highest affinity for Ffar2 (17), and measured cTreg numbers. Propionate enhanced cTreg frequency and number in Ffar2+/+ but not Ffar2−/−mice (Fig. 3C). SCFA-mediated, enhanced cTreg suppressive capacity was also dependent on Ffar2 (Fig. 3D). In addition, propionate enhanced Foxp3 and IL-10 expression and IL-10 protein levels in Ffar2+/+, but not in Ffar2−/−, cTregs (fig. S18). Furthermore, we examined whether propionate could restore cTreg populations and numbers in the setting of vancomycin treatment and found that Ffar2 was necessary (fig. S19).
One mechanism by which SCFA mediate their effects is histone deacetylase (HDAC) inhibition (18). The HDAC inhibitor trichostatin-A (TSA) increases Treg gene expression and suppressive capacity (19); and HDAC6 and 9 down-regulate nTreg function (20). Given that SCFA promote cTreg homeostasis in our studies, we hypothesized that SCFA mediate their effect through HDAC inhibition. We treated Ffar2+/+ and Ffar2−/− mice with propionate and measured HDAC expression. Propionate treatment of Ffar2+/+ mice reduced cTreg HDAC6 and HDAC9 (class IIb and IIa, respectively) expression, but did not reduce HDAC1 and HDAC2 (class I) or HDAC7 (class IIa) expression (Fig. 3E). Western blot analysis demonstrated that propionate treatment of cTregs enhanced histone acetylation, all of which were dependent on Ffar2 expression (Fig. 3F). These results suggest that SCFA via Ffar2 may affect cTregs through HDAC inhibition.
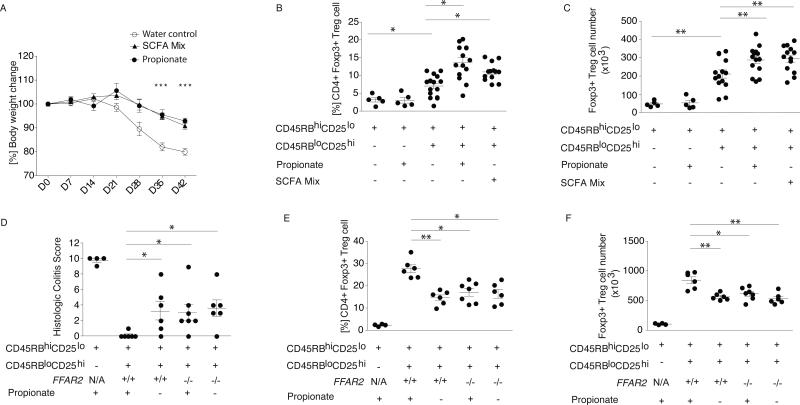
To further evaluate the effects of SCFA on cTregs, we utilized the T cell transfer model of colitis (21). In this model, lymphopenic mice (e.g. Rag2−/−) are injected either with naïve T cells, which results in severe colitis, or injected with naïve T cells in combination with Tregs, which reduces colitis severity. Propionate or SCFA mix-treated mice injected with naïve T cells and Tregs had less severe weight loss, reduced disease score, and lower levels of colonic pro-inflammatory cytokines than mice that received water alone (Fig. 4A and fig. S20). SFCA mix or propionate did not affect colitis severity in mice that received only naïve T cells (fig. S20). The frequency and number of cLP Foxp3+ Tregs in mice receiving propionate and SCFA mix increased (Fig. 4B, C). SCFA, however, did not result in conversion of naïve T cells to cTregs (Fig. 4B, C: group 1 vs 2). To evaluate whether these effects were cTreg intrinsic and dependent upon Ffar2, we performed the T cell transfer colitis model using Rag2−/− recipients, wild type naïve T cells, and Ffar2+/+ or Ffar2−/− Tregs with or without propionate in the drinking water (Fig. 4D, fig. S21). The effect of propionate on intestinal inflammation was dependent upon Ffar2 expression in Tregs as indicated by the colitis scores (Fig. 4D). Treg cell populations and cell numbers further substantiated that the propionate effects on cTregs were dependent on Ffar2 (Fig. 4E, F).
Fig. 4. SCFA exposure ameliorates T cell transfer colitis in a Treg-intrinsic, Ffar2-dependent manner.
BALB/c Rag2−/− mice were injected with CD4+CD45RBhiCD25lo naive T cells alone or in combination with Tregs. Following injection, mice received propionate, SCFA mix, or pH and sodium-matched drinking water. (A) Weekly percentage body weight change is shown across the experimental groups from experimental d0-d42. Symbols show the mean and error bars the SD. Data reflect three independent experiments. Colonic LP lymphocytes were isolated and stained for CD4 and Foxp3 and (B) percentage and (C) number of CD4+Foxp3+ within the CD45+CD3+ population are shown. Symbols represent data from individual mice, horizontal lines show the mean and error bars the SD. (D-F) C57BL/6 Rag2−/− mice were injected with CD4+CD45RBhiCD25lo naive T cells alone or in combination with Ffar2+/+ or Ffar2−/− Tregs. Following injection mice received propionate or pH and sodium-matched drinking water. (D) Histologic colitis score is shown along the y-axis, the treatment group and experimental conditions are shown along the x-axis. Colonic LP lymphocytes were isolated and (E) percentage and (F) number of CD4+Foxp3+ within the CD45+CD3+ population are shown. Symbols represent data from individual mice. Horizontal lines show the mean and error bars the SD. Panels D-F represent data from 2 independent experiments. The Kruskal-Wallis test with a Dunn's post hoc test was performed for panels A-F. ** P <0.01, * P <0.05.
Gut microbiota-host immune misadaptation has been implicated in the rising incidence of IBD, other inflammatory diseases, and obesity (22). The western dietary pattern, specifically reduced ingestion of plant-based fibers, may be a critical factor that links the gut microbiome to disease (23). While the gut microbiota composition is divergent across individuals, functional gene profiles are quite similar (24, 25), and alterations to common gut microbial metabolic pathways may affect production of symbiotic factors, such as SCFA, which regulate intestinal adaptive immune responses and promote health.
Supplementary Material
Acknowledgements
We thank members of the Garrett Lab for discussion, K. Sigrist for embryo re-derivation, C. Gallini for genotyping and J. Ramirez for animal husbandry. We thank Dr. Alexander Rudensky (Memorial Sloan Kettering Cancer Center) for Foxp3YFP-Cre mice. The data presented in this study are tabulated in the main paper and the supplementary materials. This study was supported by a NRSA F32DK095506 to P.S, NRSA F32DK098826 to M.H. and R01CA154426, K08AI078942, a Burroughs Wellcome Career in Medical Sciences Award, a Searle Scholars Award and a Cancer Research Institute Investigator Award to W.S.G. A patent has been filed on T regulatory cells, GPR43, and GPR43 agonists by Harvard University.
Footnotes
Author notes: P. S., M.H. and W.G. designed and carried out the experiments and wrote the manuscript. M.M., C.G. and N.P. carried out experiments. J.N.G. performed all histological assessment. M.B. provided Ffar2+/− embryos.
References and Notes
- 1.Lee YK, Mazmanian SK. Has the microbiota played a critical role in the evolution of the adaptive immune system. Science. 2010;330:1768–73. doi: 10.1126/science.1195568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hooper LV, Littman DR, Macpherson AJ. Interactions between the microbiota and the immune system. Science. 2012;336:1268–1273. doi: 10.1126/science.1223490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maslowski KM, et al. Regulation of inflammatory responses by gut microbiota and chemoattract receptor GPR43. Nature. 2009;461:1282–1286. doi: 10.1038/nature08530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Atarashi K, Umesaki Y, Honda K. Microbiotal influence on T cell subset development. Semin. Immunol. 2011;23:146–153. doi: 10.1016/j.smim.2011.01.010. [DOI] [PubMed] [Google Scholar]
- 5.Geuking MB, et al. Intestinal bacterial colonization of germ-free mice induces mutualistic regulatory T cell responses. Immunity. 34:794–806. doi: 10.1016/j.immuni.2011.03.021. [DOI] [PubMed] [Google Scholar]
- 6.Round JL, Mazmanian SK. Inducible Foxp3+ regulatory T-cell development by a commensal bacterium of the intestinal microbiota. Proc. Natl. Acad. Sci. U.S.A. 2010;107:12204–12209. doi: 10.1073/pnas.0909122107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Atarashi K, et al. Induction of colonic regulatory T cells by indigenous Clostridium species. Science. 2011;331:337–341. doi: 10.1126/science.1198469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ley RE, Peterson DA, Gordon JI. Ecological and evolutionary forces shaping microbial diversity in the human intestine. Cell. 2006;124:837–848. doi: 10.1016/j.cell.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 9.Cummings JH, Pomare EW, Branch WJ, Naylor CP, Macfarlane GT. Short chain fatty acids in human large intestine, portal, hepatic, and venous blood. Gut. 1987;28:1221–1227. doi: 10.1136/gut.28.10.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Høverstad T, Midtvedt T. Short-chain fatty acids in germfree mice and rats. J. Nutr. 1986;116:1772–1776. doi: 10.1093/jn/116.9.1772. [DOI] [PubMed] [Google Scholar]
- 11.Thornton AM, et al. Expression of Helios, an Ikaros transcription factor family member, differentiates thymic-derived from peripherally induced Foxp3+ T regulatory cells. J. Immunol. 2010;184:3433–3441. doi: 10.4049/jimmunol.0904028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ubeda C, et al. Vancomycin-resistant Enterococcus domination of intestinal microbiota is enable by antibiotic treatment in mice and precedes bloodstream invasion in humans. J. Clin. Invest. 2010;120:4332–4341. doi: 10.1172/JCI43918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fukuda S, et al. Bifidobacterium can protect from enteropathogenic infection through production of acetate. Nature. 2011;469:543–547. doi: 10.1038/nature09646. [DOI] [PubMed] [Google Scholar]
- 14.Vinolo MAR, Rodrigues HG, Nachbar RT, Curi R. Regulation of inflammation by short chain fatty acids. Nutrients. 2011;3:858–876. doi: 10.3390/nu3100858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim SV, et al. GPR15-mediated homing controls immune homeostasis in the large intestine mucosa. Science. 2013;340:1456–1459. doi: 10.1126/science.1237013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nilsson NE, Kotarsky K, Owman C, Olde B. Identification of a free fatty acid receptor, FFA2R. Biochem. Biophys. Res. Commun. 2003;303:1047–1052. doi: 10.1016/s0006-291x(03)00488-1. [DOI] [PubMed] [Google Scholar]
- 17.Le Poul E, et al. Functional characterization of human receptors for short chain fatty acids and their role in polymorphonuclear cell activation. J. Biol. Chem. 2003;278:25481–25489. doi: 10.1074/jbc.M301403200. [DOI] [PubMed] [Google Scholar]
- 18.Waldecker M, Kautenburger T, Daumann H, Busch C, Schrenk D. Inhibition of histone deacetylase activity by short-chain fatty acids and some polyphenol metabolites formed in the colon. J. Nutr. Biochem. 2008;19:587–593. doi: 10.1016/j.jnutbio.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 19.Tao R, et al. Deacetylase inhibition promotes the generation and function of regulatory T cells. Nat. Med. 2007;13:1299–1307. doi: 10.1038/nm1652. [DOI] [PubMed] [Google Scholar]
- 20.Beier UH, et al. Histone deacetylases 6 and 9 and sirtuin-1 control Foxp3+ regulatory T cell function through shared and isoform-specific mechanisms. Sci Signal. 2012;5:ra45. doi: 10.1126/scisignal.2002873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Powrie F, Leach MW, Mauze S, Caddle LB, Coffman RL. Phenotypically distinct subsets of CD4+ T cells induce or protect form chronic intestinal inflammation in C. B-17 scid mice. Int. Immunol. 1993;5:1461–1471. doi: 10.1093/intimm/5.11.1461. [DOI] [PubMed] [Google Scholar]
- 22.Lozupone CA, Stombaugh JI, Gordon JI, Jansson JK, Knight R. Diversity, stability and resilience of the human gut microbiota. Nature. 2012;489:220–230. doi: 10.1038/nature11550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.De Filippo C, et al. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc. Natl. Acad. Sci. U.S.A. 2010;107:14691–14696. doi: 10.1073/pnas.1005963107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Human Microbiome Project Consortium Structure, function and diversity of the healthy human microbiome. Nature. 2012;486:207–214. doi: 10.1038/nature11234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Qin J, et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464:59–65. doi: 10.1038/nature08821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rubtsov YP, et al. Regulatory T cell-derived interleukin-10 limits inflammation at environmental interfaces. Immunity. 2008;28:546–558. doi: 10.1016/j.immuni.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 27.Bjursell M, et al. Improved glucose control and reduced body fat mass in free fatty acid receptor 2-deficient mice fed a high-fat diet. Am. J. Physiol. Endocrinol. Metab. 2011;300:E211–20. doi: 10.1152/ajpendo.00229.2010. [DOI] [PubMed] [Google Scholar]
- 28.Garrett WS, et al. Communicable ulcerative colitis induced by T-bet deficiency in the innate immune system. Cell. 2007;131:33–45. doi: 10.1016/j.cell.2007.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.