Abstract
Purpose
To provide a population-based estimate of the incidence of herpes zoster ophthalmicus (HZO) with comparisons across racial, gender, and age groups, as well as to estimate the frequency of postherpetic neuralgia (PHN).
Design
Retrospective, population-based cohort study
Participants
All patients enrolled in the Kaiser Permanente Hawaii health plan during the study period (N=217,061).
Methods
All patient encounters between January 1, 2006 and December 31, 2007 in the electronic medical record of Kaiser Permanente Hawaii were queried for International Classification of Diseases, 9th Edition (ICD9) codes corresponding to HZO. Charts were reviewed to confirm a diagnosis of HZO and to collect information on specific ocular manifestations. Demographic data and information on PHN were collected electronically. Incidence rates were calculated per 100,000 person-years for the entire population, as well as for age-, gender-, and race-specific subgroups.
Main Outcome Measure
Clinical diagnosis of HZO during the study period.
Results
One hundred thirty-four cases of HZO were identified in this population of 217,061 people. The overall incidence was 30.9 per 100,000 person-years (95% confidence interval (CI): 25.9–36.6). The incidence rate for the population 65 years of age and over was 104.6 per 100,000 person-years (95% CI: 79.0–135.9), approximately five times the remainder of the population (p<0.001). The most common manifestation of HZO was dermatitis, followed by keratitis and conjunctivitis. The incidence of HZO for Pacific Islanders was 19.0 per 100,000 person-years (95% CI: 12.4– 28.3), which was significantly lower than the rate for non-Pacific Islanders (p=0.007). Twenty-one percent of HZO patients developed PHN. Older age and HZO with keratitis, conjunctivitis, and/or uveitis were found to be risk factors for PHN.
Conclusions
This study provides a population-based estimate of HZO and highlights differences across various age and racial groups. It also suggests that demographic characteristics may be useful in determining the risk of developing HZO.
Herpes zoster is a reactivation of the varicella zoster virus, most commonly seen in the elderly and immunosuppressed, with incidence estimates ranging from 1.25 to 5.25 per 1,000 person-years.1–12 Herpes zoster ophthalmicus (HZO), or herpes zoster involvement of the ophthalmic division of cranial nerve V, has been estimated to account for ten to twenty percent of all herpes zoster cases.2, 13, 14 Approximately half of these cases have more than skin involvement, most commonly keratitis, conjunctivitis and uveitis.2, 15–17 If patients are not treated promptly and aggressively, HZO with intraocular involvement can lead to significant visual impairment. While multiple studies have been conducted on the epidemiology of herpes zoster overall, the most recent population-based estimate of HZO was done more than forty years ago. 1–12, 18 Changes in the use of immunosuppressant drugs, epidemiology of associated systemic diseases, and implementation of both the varicella and zoster vaccines in recent years may all have an impact on the epidemiology of HZO.4–9, 19
Kaiser Permanente Hawaii provides an ideal setting for this population-based study as it is comprised of five centers throughout the state and serves more than fifteen percent of the Hawaiian population overall. Patients generally receive all of their medical care through this healthcare plan. Furthermore, Kaiser Permanente Hawaii contains a racially diverse population that has not been studied previously to ascertain the incidence of HZO. The aim of this study was to estimate the incidence of HZO and its specific ocular complications, including postherpetic neuralgia (PHN).
Methods
Patient encounters between January 1, 2006 and December 31, 2007 in the electronic medical record of Kaiser Permanente Hawaii were queried for International Classification of Diseases, 9th edition (ICD9) diagnoses codes corresponding to herpes zoster with ophthalmic complications (Table 1). All patient charts from this search were reviewed by a cornea and uveitis fellowship-trained ophthalmologist to verify that a diagnosis of HZO had been made by the patient’s physician during the study period. Patients with a confirmed diagnosis of HZO were further classified as having blepharitis, conjunctivitis, dermatitis, keratitis, or uveitis. If patients had more than one ocular manifestation, all were counted. Demographic data and occurrence of PHN were collected electronically. A diagnosis of PHN during or up to two years after the end of the study period was determined based on ICD9 coding (Table 1). Demographic data were compared between incident cases and the general Kaiser membership using Fisher’s exact test.
Table 1.
International Classification of Diseases, 9th Edition diagnosis codes used to identify herpes zoster ophthalmicus and postherpetic neuralgia cases
| ICD9 Diagnosis Code | Description |
|---|---|
| 53.20 | Herpes zoster dermatitis of eyelid |
| 53.21 | Herpes zoster keratoconjunctivitis |
| 53.22 | Herpes zoster iridocyclitis |
| 53.29 | Herpes zoster with ophthalmic complications, other |
| 53.12 | Herpes zoster, postherpetic trigeminal neuralgia |
| 53.19 | Herpes zoster with other nervous system complications |
ICD9 = International Classification of Diseases, 9th Edition
Incident cases were defined as patients experiencing their first known episode of herpes zoster during the study period and were confirmed by medical record review. All incidence rates were calculated per 100,000 person-years. Quarterly population data was used to calculate the total number of person-years of follow-up due to the constant flux of membership in the Kaiser Permanente health system. Incidence rates were calculated overall and by specific ocular manifestation, as well as by age and gender. Age was stratified into the following groups: 0–14, 15–24, 25–44, 45–64, and 65+ years of age, based on patient age at the middle of the study period, January 1, 2007. When calculating age- and gender-specific incidence rates, total number of person-years was adjusted to reflect quarterly membership data within each group. Incidence rates were also calculated by patient-reported race. Because race was only available for a portion of the incident cases and general Kaiser membership, incidence rates by race were adjusted to reflect the racial demographics among the subset of patients who reported their race.
Ninety-five percent confidence intervals (CI) were calculated for each incidence rate assuming a Poisson distribution. Age-, race-, and gender-specific incidence rates were compared using Poisson regression since the data met the assumptions for this regression method.20 A p-value less than 0.05 was considered statistically significant. All analyses were performed using STATA 11.0 (StataCorp, College Station, TX) and R statistical software (The R Foundation for Statistical Computing, Vienna, Austria). Institutional Review Board (IRB)/Ethics Committee approval was obtained. All work was Health Insurance Portability and Accountability Act-compliant and adhered to the tenets of the Declaration of Helsinki.
Results
The total mid-study period population of Kaiser Hawaii was 217,061 people on January 1, 2007, corresponding to 433,794.2 person-years during the study period. Within this population, 168 patient records were confirmed to have a diagnosis of HZO, and of these, 134 patients were confirmed incident cases.
Basic demographic information, including gender, age distribution, and race, for both the Kaiser Hawaii population and HZO cases is shown in Table 2. The HZO cases were significantly older than the general Kaiser population with most cases falling into the oldest two age groups. Among patients with available race information, a significantly lower proportion of HZO cases identified as native Pacific Islanders compared to the general Kaiser population (p=0.02).
Table 2.
Patient Demographics
| Kaiser Hawaii | Confirmed HZO Cases | P-value1 | |
|---|---|---|---|
| Total | 217, 0612 | 134 | |
|
| |||
| Female | 110,701 (51%) | 71 (53%) | 0.67 |
| Age Category (years) | |||
| 0–14 | 40,057 (18%) | 2 (1%) | <0.001 |
| 15–24 | 28,047 (13%) | 4 (3%) | <0.001 |
| 25–44 | 58,376 (27%) | 18 (13%) | <0.001 |
| 45–64 | 63,914 (29%) | 54 (40%) | 0.008 |
| ≥65 | 26,667 (12%) | 56 (42%) | <0.001 |
|
| |||
| Race | N=114,505 (53%)3 | N=102 (76%)4 | <0.001 |
| Alaskan/Native American | 1,463 (1%) | 1 (1%) | 1.00 |
| Asian | 46,024 (40%) | 47 (46%) | 0.23 |
| Black | 1,758 (2%) | 2 (2%) | 0.67 |
| Pacific Islander | 31,083 (27%) | 17 (17%) | 0.02 |
| White | 30,603 (27%) | 34 (33%) | 0.15 |
| Other | 3,574 (3%) | 1 (1%) | 0.38 |
HZO = herpes zoster ophthalmicus
Fisher’s exact test
General membership of Kaiser Permanente Hawaii on January 1, 2007
The race distribution for the subgroup of the population reporting race was used to calculate the person-time denominators for each race in all incidence calculations by racial subgroup.
The race distribution for the subgroup of incident cases reporting race was used to calculate an adjusted number of total incident cases for each race in all incidence calculations by racial subgroup.
The overall incidence of HZO in the Kaiser Hawaii population was 30.9 cases per 100,000 person-years (95% CI: 25.9–36.6, Table 3). Incidence rates increased with age overall as well as within the male and female subgroups. Specifically, the incidence rate of HZO in the 65 and over age group (Table 3) was significantly higher than the incidence rate for the rest of the population, 20.5 cases per 100,000 person-years (p<0.001). Similarly, the incidence rate of HZO in the 45–64 age group, the second oldest age group, (Table 3) was significantly higher than the incidence rate in the less than 45 age group, 9.5 cases per 100,000 person-years (p<0.001). There was no significant difference between the incidence rates for males and females, both overall (p=0.56, Table 3) and for age-specific subgroups.
Table 3.
Study period herpes zoster ophthalmicus incidence by presentation, age, and gender (January 1, 2006 to December 31, 2007)
| Age(years) | Female | Male | All Cases | |||
|---|---|---|---|---|---|---|
| All Presentations | N | Rate (95%CI)1 | N | Rate (95% CI)1 | N | Rate (95% CI)1 |
| Total | 71 | 32.4 (25.3–40.9) | 63 | 29.3 (22.5–37.5) | 134 | 30.9 (25.9–36.6) |
|
| ||||||
| 0–14 | 1 | 2.6 (0.1–14.3) | 1 | 2.4 (0.1–13.6) | 2 | 2.5 (0.3–9.0) |
| 15–24 | 1 | 3.6 (0.1–20.3) | 3 | 10.6 (2.2–31.0) | 4 | 7.2 (2.0–18.4) |
| 25–44 | 9 | 15.4 (7.0–29.2) | 9 | 15.5 (7.1–29.4) | 18 | 15.4 (9.1–24.4) |
| 45–64 | 29 | 45.0 (30.1–64.6) | 25 | 39.4 (25.5–58.2) | 54 | 42.2 (31.7–55.1) |
| ≥65 | 31 | 104.9 (71.3–148.9) | 25 | 104.2 (67.5–153.9) | 56 | 104.6 (79.0–135.9) |
|
| ||||||
| Specific Presentations2 | ||||||
|
| ||||||
| Blepharitis | 1 | 0.5 (0.0–2.5) | - | 0.0 (0.0–1.7) | 1 | 0.2 (0.0–1.3) |
| Conjunctivitis | 19 | 8.7 (5.2–13.5) | 13 | 6.1 (3.2–10.4) | 32 | 7.4 (5.0–10.4) |
| Dermatitis | 53 | 24.2 (18.1–31.7) | 50 | 23.3 (17.3–30.7) | 103 | 23.7 (19.4–28.8) |
| Keratitis | 20 | 9.1 (5.6–14.1) | 18 | 8.4 (5.0–13.2) | 38 | 8.8 (6.2–12.0) |
| Uveitis | 3 | 1.4 (0.3–4.0) | 5 | 2.3 (0.8–5.4) | 8 | 1.8 (0.8–3.6) |
Incidence rates are reported per 100,000 person-years. Range in parentheses indicates 95% confidence interval (CI).
Patients were counted more than once if presenting with more than one manifestation of herpes zoster ophthalmicus.
Table 3 also reports incidence rates of HZO by anatomical site. Dermatitis was the most common presentation of HZO in this population, followed by keratitis, and conjunctivitis. Eighty-seven patients (64.9%) had dermatitis without any other manifestations of HZO. Additionally, twenty-four patients had keratoconjunctivitis, seven patients had keratouveitis, and one patient had blepharoconjunctivitis.
Incidence rate estimates by race are displayed in Table 4. The lowest incidence rate was in the Pacific Islander subgroup (19.0 cases per 100,000 person-years, 95% CI: 12.4–28.3, Table 4) while the highest incidence rate was in the subgroup of Black patients (39.5 cases per 100,000 person-years, 95% CI: 9.3–108.5, Table 4). The incidence rate for Pacific Islanders was significantly lower than the incidence rate for non-Pacific Islanders, 35.3 per 100,000 person-years (p=0.007). There was no significant difference in incidence rates for any other racial subgroup.
Table 4.
Estimated herpes zoster ophthalmicus incidence by race
| Race | HZO Incidence1 2006/2007 |
|---|---|
| Overall | 30.9 (25.9–36.6) |
| Alaskan/Native American | 23.7 (4.4–100.5) |
| Asian | 35.5 (27.3–44.9) |
| Black | 39.5 (9.3–108.5) |
| Pacific Islander | 19.0 (12.4–28.3) |
| White | 38.6 (28.3–50.9) |
Herpes zoster ophthalmicus(HZO) incidence rates are reported per 100,000 person-years. Range in parentheses indicates 95% confidence interval
Twenty-eight HZO patients (20.9%) developed PHN. Eighteen of these patients (64.3%) were over the age of 65, and only one patient (3.6%) was under the age of 45 (Figure 1). HZO patients over the age of 65 had a disproportionately higher rate of PHN development compared to those younger than 65 (p=0.009). Thirty percent of patients who had some form of HZO beyond skin involvement (keratitis, conjunctivitis, and/or uveitis) developed PHN compared to sixteen percent of patients with only skin involvement (p=0.08). There was no significant difference in the rate of PHN development between genders (p=0.84) or by race (p=0.63).
Figure 1.
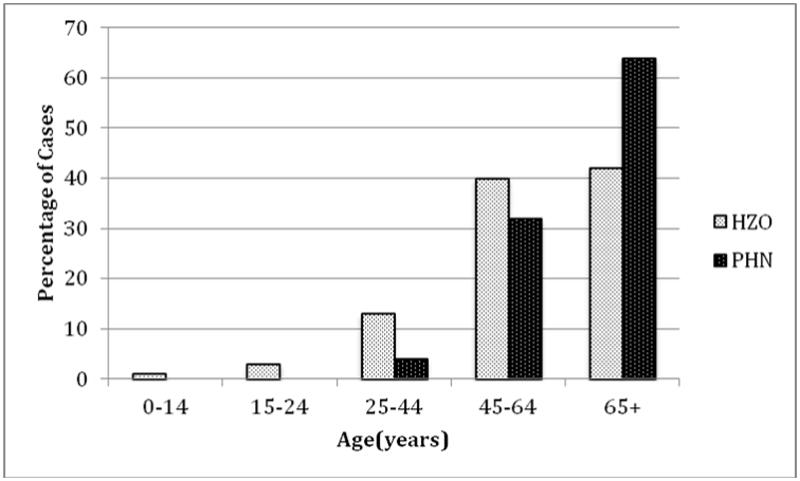
Age distribution of postherpetic neuralgia cases compared to herpes zoster ophthalmicus cases overall
Bar graph showing the age distributions of all 134 herpes zoster ophthalmicus (HZO) cases and the 28 patients who developed postherpetic neuralgia (PHN).
Discussion
Several studies have estimated the overall incidence of herpes zoster, with estimates ranging from 1.25 to 5.25 per 1,000 person-years.1–12 However, estimates of the incidence of HZO as well as data on specific ocular manifestations have been limited.2, 15–17 In this population-based study, we found that the overall incidence of HZO was 30.9 per 100,000 person-years. Older age was significantly associated with higher incidence. Pacific Islanders had the lowest incidence rate compared to all other racial groups. Dermatitis, keratitis, and conjunctivitis were the most common manifestations of HZO. No significant difference was found between the incidence rates for males and females. More than one-fifth of the patients in the population subsequently developed PHN, and older age as well as manifestations beyond skin involvement were found to be risk factors.
Prior studies have shown that ten to twenty percent of herpes zoster can be attributed to HZO; however, these studies did not provide actual incidence rates and were done more than forty years ago.2, 13, 14 Multiple studies estimating the incidence of herpes zoster have shown an increasing incidence rate over time.2, 8, 11 Specifically, the most recent estimates of herpes zoster range between 320 to 410 per 100,000 person-years.9–12 The overall incidence rate of HZO in our population was 30.9 per 100,000 person-years, which is approximately ten percent of these recent estimates of herpes zoster overall.
Our finding that the incidence of HZO increases significantly with age is consistent with several other studies estimating the incidence of herpes zoster overall.1–9, 11, 12 Incidence rates increased with age in our population. Most notably, the incidence rate for the subgroup of the population over 65 years old was approximately five times that of the rest of the population. Increased age has been associated with a decrease in cell-mediated immunity, which is necessary to avoid reactivation of the latent varicella zoster virus.1, 21–25
In our population, we did not find a significant difference in HZO incidence between males and females overall, and this held up in age-specific comparisons. Results from previous studies have been mixed regarding differences in incidence of herpes zoster by gender. While some have found significant differences between incidence rates for males and females within specific age groups, there has been limited evidence of a significant difference overall between incidence rates for men and women.2, 3, 5, 6, 9, 10, 12
Dermatitis was the most common manifestation overall of HZO, followed by keratitis and conjunctivitis. Notably, more than sixty percent of patients in our population presented with dermatitis without any other manifestations. Keratitis and conjunctivitis have been reported as common manifestations of HZO in multiple previous studies.2, 15–17 Some studies have also found uveitis to be one of the most common intraocular manifestations of HZO. 2, 16 However, we found a very low incidence of uveitis in our population.
In this Hawaiian population, more than one quarter of patients identified themselves as Pacific Islanders. The estimated incidence of HZO in this subgroup was 19.0 per 100,000 person-years, which was significantly lower than the incidence rate for non-Pacific Islanders. It is unknown if this racial difference exists for all cases of herpes zoster since prior incidence estimates have had limited information by racial subgroup.1–14, 18 Surveillance of varicella cases in Antelope Valley, California by the Centers for Disease Control and Prevention showed that the racial group consisting of Pacific Islanders and Native Americans had the lowest incidence of varicella compared to Hispanics, blacks, and whites.26 Since prior varicella infection is necessary for herpes zoster, it is possible that the lower incidence rate of HZO that we found in the Pacific Islander subgroup reflects this finding.27
Additionally, we found that 20.9 percent of the HZO cases in our population developed PHN and that older age was associated with a significantly higher rate of this complication. Prior estimates of the rate of developing PHN ranged between 5.4 and 19.5 percent after all cases of herpes zoster, and many of these studies also found an age-specific increase in the rate of this sequela.4, 11, 17, 28, 29 Ophthalmic location has been shown to be a risk factor for developing PHN, which may explain the slightly higher proportion of PHN found in this study.2, 4 Notably, we found that patients with keratitis, conjunctivitis, or uveitis had a much higher risk of developing PHN compared to patients who did not have these manifestations, suggesting that disease beyond just skin involvement may be a risk factor for this complication.
There are a few limitations to consider. Fisher’s exact test was used for initial demographic comparisons of the general Kaiser Hawaii membership to incident cases. Repeated used of this test could increase the chance of spurious associations; however, we used Poisson regression when making statistical comparisons of incidence rates. Although the Kaiser Hawaii membership comprises more than fifteen percent of the general Hawaiian membership, it is possible that differences in demographic characteristics may affect the generalizability of our results to the Hawaiian population. While the age and gender distribution of the Kaiser population is comparable to the general Hawaiian population based on the most recent census data, there was a higher percentage of Pacific Islanders in Kaiser Hawaii.30 Since Pacific Islanders had a lower incidence of HZO, it is possible that the overall incidence rate found in Kaiser Hawaii may be an underestimate of the true incidence in the Hawaiian population. Differences in factors such as racial demographics may also affect the applicability of our results to other populations. However, the racial diversity in this population allowed us to ascertain race-specific differences in incidence.
Additionally, race information was only available for a portion of the Kaiser population, as well as for the HZO cases specifically. As a result, our race-specific incidence estimates are based on the assumption that the race distribution within the reporting subgroup is similar to the remainder of the population. While it is very rare, some patients may seek specialty care outside of the Kaiser healthcare system resulting in some incident HZO cases being missed. However, only five percent of Kaiser Hawaii patients have dual insurance that would facilitate receiving outside care.
The introduction of the varicella and zoster vaccines in recent years may have an impact on future incidence rates.4–9, 19 To our knowledge, the zoster vaccine was not used widely in our population prior to or during the study period. The Centers for Disease Control and Prevention began recommending the zoster vaccine for patients 60 years of age and older in October 2006.31 This is when Kaiser Hawaii physicians began administering the vaccine; however, less than four percent of the Kaiser Hawaii population 60 years of age or older had received the vaccine by the end of the study period (personal communication, Dr. Cynthia Nakasato, Kaiser Permanente Hawaii).
There are also some potential diagnostic considerations. Diagnosis codes were used as an initial screen to identify cases of HZO that were subsequently adjudicated by chart review. A patient would be missed if the diagnosis was not initially captured by coding. Additionally, the diagnosis of PHN was made using ICD9 coding, which is also limited by the accuracy of diagnostic coding by physicians. One of the diagnosis codes for PHN can include a few other herpes zoster sequelae, including herpes zoster encephalitis and meningitis. Although these other sequelae have been reported to be extremely rare, it is possible that this may have led to an overestimation of the rate of PHN development.32, 33 While the diagnosis of HZO is usually evident in the presence of characteristic skin lesions, as was the case with most of our patients, there were some patients who did not present with dermatitis in the V1 dermatomal distribution. In the absence of these specific skin findings, the diagnosis could be supported by skin lesions elsewhere, as well as by ocular findings, including pseudodendrites, neurotrophic cornea, and uveitis with high intraocular pressure. There is some subjectivity when making a diagnosis of HZO in the absence of skin lesions, and this could lead to misdiagnosis. However, all HZO patients were evaluated by Kaiser ophthalmologists.
In summary, the incidence of HZO in this population-based study was 30.9 per 100,000 person-years. Age and race appear to be useful demographic characteristics in determining the risk of HZO, while specific ocular manifestations of HZO may predict development of PHN. This study provides a population-based estimate of HZO, which has been lacking in recent years, and highlights differences across various age and racial groups.
Acknowledgments
Financial Support: Dr. Acharya is supported by National Eye Institute grant K23EY017897, a Research to Prevent Blindness Career Development Award and a UCSF Research Evaluation and Allocation Committee Award. The UCSF Department of Ophthalmology is supported by National Eye Institute grant EY06190, That Man May See Foundation, and an unrestricted grant from the Research to Prevent Blindness Foundation. The sponsors or funding organizations had no role in the design or conduct of this research.
Footnotes
Conflict of Interest: No conflicting relationship exists for any author.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hope-Simpson RE. The nature of herpes zoster. Practitioner. 1964;193:217–9. [PubMed] [Google Scholar]
- 2.Ragozzino MW, Melton LJ, III, Kurland LT, et al. Population-based study of herpes zoster and its sequelae. Medicine (Baltimore) 1982;61:310–6. doi: 10.1097/00005792-198209000-00003. [DOI] [PubMed] [Google Scholar]
- 3.Donahue JG, Choo PW, Manson JE, Platt R. The incidence of herpes zoster. Arch Intern Med. 1995;155:1605–9. [PubMed] [Google Scholar]
- 4.Opstelten W, Mauritz JW, de Wit NJ, et al. Herpes zoster and postherpetic neuralgia: incidence and risk indicators using a general practice research database. Fam Pract. 2002;19:471–5. doi: 10.1093/fampra/19.5.471. [DOI] [PubMed] [Google Scholar]
- 5.Chapman RS, Cross KW, Fleming DM. The incidence of shingles and its implications for vaccination policy. Vaccine. 2003;21:2541–7. doi: 10.1016/s0264-410x(03)00034-3. [DOI] [PubMed] [Google Scholar]
- 6.Mullooly JP, Riedlinger K, Chun C, et al. Incidence of herpes zoster, 1997–2002. Epidemiol Infect. 2005;133:245–53. doi: 10.1017/s095026880400281x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jumaan AO, Yu O, Jackson LA, et al. Incidence of herpes zoster, before and after varicella-vaccination-associated decreases in the incidence of varicella, 1992–2002. J Infect Dis. 2005;191:2002–7. doi: 10.1086/430325. [DOI] [PubMed] [Google Scholar]
- 8.Yih WK, Brooks DR, Lett SM, et al. The incidence of varicella and herpes zoster in Massachusetts as measured by the Behavioral Risk Factor Surveillance System (BRFSS) during a period of increasing varicella vaccine coverage, 1998–2003. [Accessed August 29, 2012];BMC Public Health [serial online] 2005 5:68. doi: 10.1186/1471-2458-5-68. Available at: http://www.biomedcentral.com/1471-2458/5/68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Insinga RP, Itzler RF, Pellissier JM, et al. The incidence of herpes zoster in a United States administrative database. J Gen Intern Med. 2005;20:748–53. doi: 10.1111/j.1525-1497.2005.0150.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Melker H, Berbers G, Hahne S, et al. The epidemiology of varicella and herpes zoster in The Netherlands: implications for varicella zoster virus vaccination. Vaccine. 2006;24:3946–52. doi: 10.1016/j.vaccine.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 11.Yawn BP, Saddier P, Wollan PC, et al. A population-based study of the incidence and complication rates of herpes zoster before zoster vaccine introduction. Mayo Clin Proc. 2007;82:1341–9. doi: 10.4065/82.11.1341. [DOI] [PubMed] [Google Scholar]
- 12.Cebrian-Cuenca AM, Diez-Domingo J, Rodriguez MS, et al. Herpes Zoster Research Group of the Valencian Community. Epidemiology of herpes zoster infection among patients treated in primary care centres in the Valencian community (Spain). BMC Fam Pract [serial online]; 2010. [Accessed August 29, 2012]. p. 33. Available at: http://www.biomedcentral.com/1471-2296/11/33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burgoon CF, Jr, Burgoon JS, Baldridge GD. The natural history of herpes zoster. J Am Med Assoc. 1957;164:265–9. doi: 10.1001/jama.1957.02980030041010. [DOI] [PubMed] [Google Scholar]
- 14.Molin L. Aspects of the natural history of herpes zoster. A follow-up investigation of outpatient material. Acta Derm Venereol. 1969;49:569–83. [PubMed] [Google Scholar]
- 15.Cobo M, Foulks GN, Liesegang T, et al. Observations on the natural history of herpes zoster ophthalmicus. Curr Eye Res. 1987;6:195–9. doi: 10.3109/02713688709020090. [DOI] [PubMed] [Google Scholar]
- 16.Womack LW, Liesegang TJ. Complications of herpes zoster ophthalmicus. Arch Ophthalmol. 1983;101:42–5. doi: 10.1001/archopht.1983.01040010044004. [DOI] [PubMed] [Google Scholar]
- 17.Puri LR, Shrestha GB, Shah DN, et al. Ocular manifestations in herpes zoster ophthalmicus. Nepal J Ophthalmol. 2011;3:165–71. doi: 10.3126/nepjoph.v3i2.5271. [DOI] [PubMed] [Google Scholar]
- 18.Cooper M. The epidemiology of herpes zoster. Eye (Lond) 1987;1:413–21. doi: 10.1038/eye.1987.63. [DOI] [PubMed] [Google Scholar]
- 19.Seiff SR, Mehta AM. Herpes zoster ophthalmicus--the changing epidemiology and its implications for treatment. West J Med. 1990;153:445–6. [PMC free article] [PubMed] [Google Scholar]
- 20.Cameron AC, Trivedi PK. Regression Analysis of Count Data. Cambridge, UK: Cambridge University Press; 1998. pp. xx–xx. [Google Scholar]
- 21.Miller AE. Selective decline in cellular immune response to varicella-zoster in the elderly. Neurology. 1980;30:582–7. doi: 10.1212/wnl.30.6.582. [DOI] [PubMed] [Google Scholar]
- 22.Galil K, Choo PW, Donahue JG, Platt R. The sequelae of herpes zoster. Arch Intern Med. 1997;157:1209–13. [PubMed] [Google Scholar]
- 23.Burke BL, Steele RW, Beard OW, et al. Immune responses to varicella-zoster in the aged. Arch Intern Med. 1982;142:291–3. [PubMed] [Google Scholar]
- 24.Levin MJ, Murray M, Rotbart HA, et al. Immune response of elderly individuals to a live attenuated varicella vaccine. J Infect Dis. 1992;166:253–9. doi: 10.1093/infdis/166.2.253. [DOI] [PubMed] [Google Scholar]
- 25.Hayward AR, Herberger M. Lymphocyte responses to varicella zoster virus in the elderly. J Clin Immunol. 1987;7:174–8. doi: 10.1007/BF00916011. [DOI] [PubMed] [Google Scholar]
- 26.Acute Communicable Disease Control Program: Special Studies Report-2010. Los Angeles, CA: Los Angeles County Department of Public Health; 2010. [Accessed August 29, 2012]. pp. xx–xx. Available at: http://publichealth.lacounty.gov/acd/reports/SpecialStudiesReport2010.pdf. [Google Scholar]
- 27.Liesegang TJ. Herpes zoster ophthalmicus: natural history, risk factors, clinical presentation, and morbidity. Ophthalmology. 2008;115(suppl):S3–12. doi: 10.1016/j.ophtha.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 28.Klompas M, Kulldorff M, Vilk Y, et al. Herpes zoster and postherpetic neuralgia surveillance using structured electronic data. Mayo Clin Proc. 2011;86:1146–53. doi: 10.4065/mcp.2011.0305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gauthier A, Breuer J, Carrington D, et al. Epidemiology and cost of herpes zoster and post-herpetic neuralgia in the United Kingdom. Epidemiol Infect. 2009;137:38–47. doi: 10.1017/S0950268808000678. [DOI] [PubMed] [Google Scholar]
- 30.Humes KR, Jones NA, Ramirez RR. 2010 Census Briefs. Washington, D.C: United States Census Bureau; Mar, 2011. [Accessed August 29, 2012]. Overview of race and Hispanic origin: 2010; p. xx. Available at: http://www.census.gov/prod/cen2010/briefs/c2010br-02.pdf. [Google Scholar]
- 31.Harpaz R, Ortega-Sanchez IR, Seward JF. Prevention of herpes zoster: recommendations of the Advisory Committee on Immunization Practices (ACIP) [Accessed August 29, 2012];MMWR Recomm Rep. 2008 57(RR-5):1–30. Available at: http://www.cdc.gov/mmwr/preview/mmwrhtml/rr5705a1.htm. [PubMed] [Google Scholar]
- 32.Elliott KJ. Other neurological complications of herpes zoster and their management. Ann Neurol. 1994;35(suppl):S57–61. doi: 10.1002/ana.410350717. [DOI] [PubMed] [Google Scholar]
- 33.Mazur MH, Dolin R. Herpes zoster at the NIH: a 20 year experience. Am J Med. 1978;65:738–44. doi: 10.1016/0002-9343(78)90791-x. [DOI] [PubMed] [Google Scholar]


