Abstract
Introduction
We evaluated the ability of histopathologic response criteria to predict overall survival (OS) and disease-free survival (DFS) in patients with surgically resected non-small cell lung cancer (NSCLC) treated with or without neoadjuvant chemotherapy.
Methods
Tissue specimens from 358 patients with NSCLC were evaluated by pathologists blinded to the patient treatment and outcome. The surgical specimens were reviewed for various histopathologic features in the tumor including percentage of residual viable tumor cells, necrosis, and fibrosis. The relationship between the histopathologic findings and OS was assessed.
Results
The percentage of residual viable tumor cells and surgical pathologic stage were associated with OS and DFS in 192 patients with NSCLC receiving neoadjuvant chemotherapy in multivariate analysis (p = 0.005 and p = 0.01, respectively). There was no association of OS or DFS with percentage of viable tumor cells in 166 patients with NSCLC who did not receive neoadjuvant chemotherapy (p = 0.31 and p = 0.45, respectively). Long-term OS and DFS were significantly prolonged in patients who had ≤10% viable tumor compared with patients with >10% viable tumor cells (5 years OS, 85% versus 40%, p < 0.0001 and 5 years DFS, 78% versus 35%, p < 0.001).
Conclusion
The percentages of residual viable tumor cells predict OS and DFS in patients with resected NSCLC after neoadjuvant chemotherapy even when controlled for pathologic stage. Histopathologic assessment of resected specimens after neoadjuvant chemotherapy could potentially have a role in addition to pathologic stage in assessing prognosis, chemotherapy response, and the need for additional adjuvant therapies.
Keywords: Lung cancer, Neoadjuvant chemotherapy, Histopathology
Surgical resection is the treatment of choice in patients with localized non-small cell lung cancer (NSCLC).1 Neoadjuvant chemotherapy followed by resection has been used in patients with locally advanced NSCLC to address the high rate of local and systemic failure.2–5 Histopathologic features in the resected specimen of patients receiving neoadjuvant chemotherapy or chemoradiation have been reported in a small number of studies to be useful in the prediction of survival and assessment of tumor response after neoadjuvant treatment.6–17 The purpose of this study was to assess in a larger cohort of patients the ability of histopathologic criteria to predict survival and chemotherapy response in patients with NSCLC treated with neoadjuvant chemotherapy even when controlled for surgical pathologic stage.
PATIENTS AND METHODS
Patients and Tissue Samples
We examined 192 patients with NSCLC treated with neoadjuvant chemotherapy followed by complete surgical resection from 2001 to 2006. We also examined a control group of 166 patients with NSCLC from the same time period who did not receive neoadjuvant chemotherapy. Histologic slides from the files of the Department of Pathology, M. D. Anderson Cancer Center18 and all cases were reviewed. The study was approved by the University of Texas M. D. Anderson Institutional Review Board.
Histopathologic Evaluation
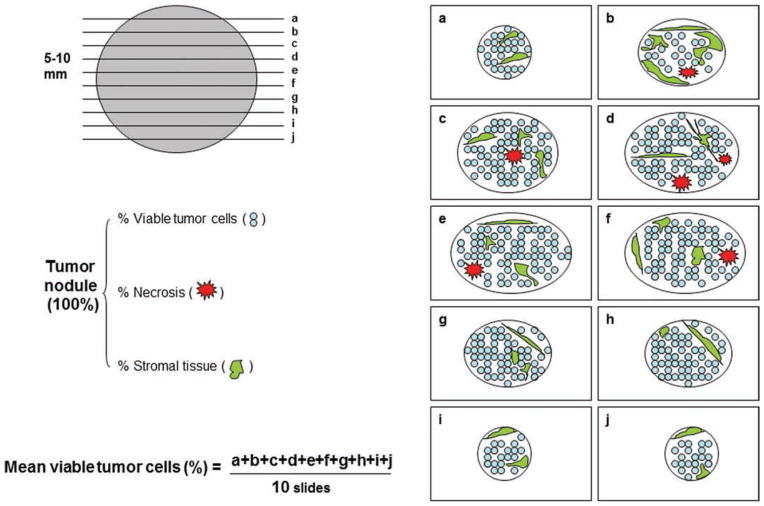
Hematoxylin and eosin-stained slides of sections of the gross residual tumor were assessed in a total of 358 patients by pathologists blinded to the patient treatment and outcome. In this study, at least 1 section per cm of tumor greatest diameter was obtained. The number of slides examined for each case ranged from 5 to 30. Figure 1 shows the schematic diagram for histopathologic evaluation of NSCLC. The percentage of residual tumor was estimated by comparing the estimated cross-sectional area of the viable tumor foci with estimated cross-sectional areas of necrosis, fibrosis, and inflammation on each slide. Histologic parameters were analyzed including necrosis, fibrosis, foamy macrophages, giant cell reaction, cholesterol cleft granuloma, and inflammation. The results for all slides were averaged together to determine the mean values for each patient. All histopathologic changes were then compared with patients who had not received neoadjuvant chemotherapy.
FIGURE 1.
Schematic diagram of histologic evaluation of lung cancer tissue resected from patients treated with neoadjuvant chemotherapy.
Statistical Analysis
Overall survival (OS) was defined as the time from date of the surgery until death from any cause. Disease-free survival (DFS) was defined as the time from surgery until time of the tumor recurrence or date of last follow-up. Survival probability as a function of time was computed by the Kaplan-Meier estimator. The log-rank test was used to compare patient survival times between groups. Univariable Cox proportional hazards regression model was used to examine the association between histopathologic features and various clinical factors with OS and DFS. The variables found significant on univariate analysis (p value < 0.25) were evaluated by multivariable analysis using the Cox proportional hazards model after backward stepwise Wald elimination. A p value of less than 0.05 on multivariate analysis was taken to be significant. The statistical analyses were performed using SPSS Software (version 15; SPSS, Inc., Chicago, IL).
RESULTS
Patient Demographics and Treatment Characteristics
Table 1 presents the patient demographics of the patients with NSCLC treated with and without neoadjuvant chemotherapy. Patients treated with neoadjuvant chemotherapy tended to have a higher clinical and pathologic stage. There was some evidence of clinical downstaging in the resected specimens of the neoadjuvant-treated patients (clinical stage IIIA/B 41%, pathologic stage IIIA/B 30%, p < 0.05), which was not seen in patients treated with surgery alone. Neoadjuvant-treated patients also tended to have more patients classified as “other” on histology (NSCLC-not otherwise specified, adenosquamous, and neuroendocrine carcinoma). No difference was noted between groups in the type or extent of surgery. The majority of patients with NSCLC treated with neoadjuvant chemotherapy received a platinum and taxane-based regimen (171 patients, 89%, Table 1). The median number of treatment cycles was three cycles (range: 2–7 cycles).
TABLE 1.
Patient Demographics and Treatment Characteristics
| Characteristics | Chemotherapy Followed by Surgery (N = 192) | Surgery Alone (N = 166) | p |
|---|---|---|---|
| Age mean (range) | 63 (40–85) | 66 (40–90) | 0.29 |
| Gender, n (%) | 0.31 | ||
| Male | 111 (58) | 79 (48) | |
| Female | 81 (42) | 87 (52) | |
| Histology, n (%) | <0.0001 | ||
| Adenocarcinoma | 89 (46) | 107 (65) | |
| Squamous cell carcinoma | 58 (30) | 55 (33) | |
| Others | 45 (24) | 4 (2) | |
| Tumor size (cm), n (%) | 0.38 | ||
| 0.0–2.0 | 47 (25) | 24 (15) | |
| 2.1–3.0 | 49 (25) | 46 (28) | |
| 3.1–4.0 | 32 (17) | 39 (23) | |
| 4.1–5.0 | 21 (11) | 28 (17) | |
| >5.1 | 43 (22) | 29 (17) | |
| Clinical stage, n (%) | <0.0001 | ||
| IA/IB | 60 (31) | 118 (71) | |
| IIA/IIB | 44 (23) | 30 (18) | |
| IIIA/IIIB | 79 (41) | 14 (9) | |
| IV | 9 (5) | 4 (2) | |
| Pathologic stage, n (%) | <0.0001 | ||
| 0/IA/IB | 78 (40) | 98 (59) | |
| IIA/IIB | 49 (26) | 45 (27) | |
| IIIA/IIIB | 57 (30) | 21 (13) | |
| IV | 8 (4) | 2 (1) | |
| Type of resection n (%) | 0.69 | ||
| Wedge or segmentectomy | 5 (2) | 7 (4) | |
| Bilobectomy or lobectomy | 174 (91) | 148 (89) | |
| Pneumonectomy | 13 (7) | 11 (7) | |
| Neoadjuvant chemotherapy, n (%) | |||
| T + C | 171 (89) | ||
| Carboplatin | 134 (70) | ||
| Cisplatin | 58 (30) | ||
| Taxol | 98 (51) | ||
| Taxotere | 75 (39) | ||
| Gemcitabine | 17 (9) | ||
| Etoposide | 3 (1) | ||
| Treatment cycle mean (range) | 3 (2–7) | ||
Others of chemotherapy group (39 patients with NSCLC-NOS, 5 with adenosquamous carcinoma, and 1 with neuroendocrine carcinoma) and surgery alone group (4 patients with NSCLC-not otherwise specified).
T, taxol or taxotere; C, carboplatin or cisplatin; AJCC7, American Joint Committee on Cancer 7.
Histopathologic Features in Patients Treated with and without Neoadjuvant Chemotherapy
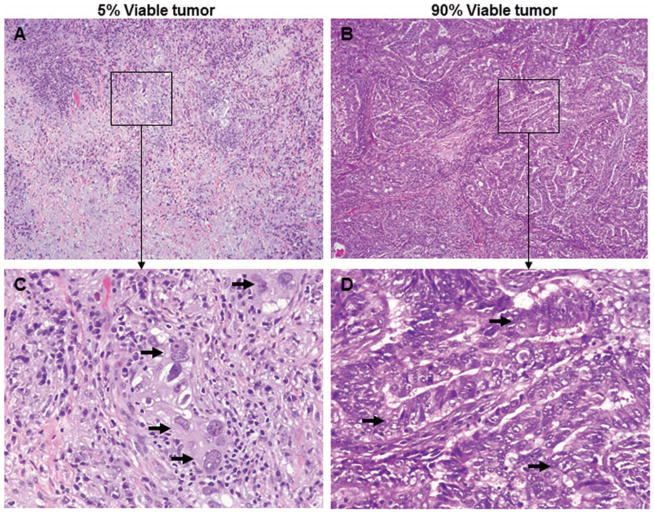
Histopathologic patterns observed with treatment-induced tumor regression included necrosis, fibrosis, foamy macrophages, cholesterol cleft granuloma, giant cell reaction, and inflammation. Figure 2 shows typical examples of the histopathologic features of tumors associated with extensive (A and C) or no (B and D) response to neoadjuvant chemotherapy.
FIGURE 2.
Pathologic response to neoadjuvant chemotherapy for lung cancer. Representative examples of the histopathology of tumors associated with extensive response to treatment (A, C) or no response to treatment (B, D). Arrows indicate viable tumor cells (C, D). Original magnification: ×40 (pictures) and ×200 (insets).
We compared the percentage of viable tumor cells in patients treated with or without neoadjuvant chemotherapy. In patients treated with neoadjuvant chemotherapy, 36 (19%) of 192 patients had ≤10% viable tumor cells (Table 2). All patients who underwent surgery alone had >10% viable tumor cells (Table 2). The percentage of viable tumor cells was a significant predictor of the survival only in the patients with NSCLC who received neoadjuvant chemotherapy (Table 2, p < 0.003). There was no relationship with survival in patients with NSCLC who did not receive neoadjuvant chemotherapy (Table 2). Compared with patients with ≤10% viable tumor cells, the hazard ratio for neoadjuvant-treated patients with NSCLC with >70% viable tumor cells was 4.78 with a 95% confidence interval of 2.06–11.11.
TABLE 2.
Association of Survival with Percentage of Viable Tumor Cells in Patients with NSCLC with or without Neoadjuvant Chemotherapy
| Percentage of Viable Tumor Cells | Neoadjuvant Treatment (N = 192) | Surgery Alone (N = 166) | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| No. of Patients (%) | HR (95% CI) | p | No. of Patients (%) | HR (95% CI) | p | |
| 0–10% | 36 (19) | 1.00 | 0.003 | 0 (0) | 0.51 | |
| 11–30% | 19 (10) | 2.51 (0.91–6.96) | 6 (4) | 1.00 | ||
| 31–50% | 35 (18) | 3.39 (1.40–8.22) | 27 (16) | 1.02 (0.31–3.33) | ||
| 51–70% | 56 (29) | 4.57 (1.98–10.52) | 64 (38) | 0.62 (0.19–1.96) | ||
| 71–100% | 46 (24) | 4.78 (2.06–11.11) | 69 (42) | 0.76 (0.25–2.41) | ||
HR, hazard ratio; CI, confidence interval.
Histopathologic Criteria of Chemotherapy Response and Pathologic Stage are Associated with Long-Term Survival
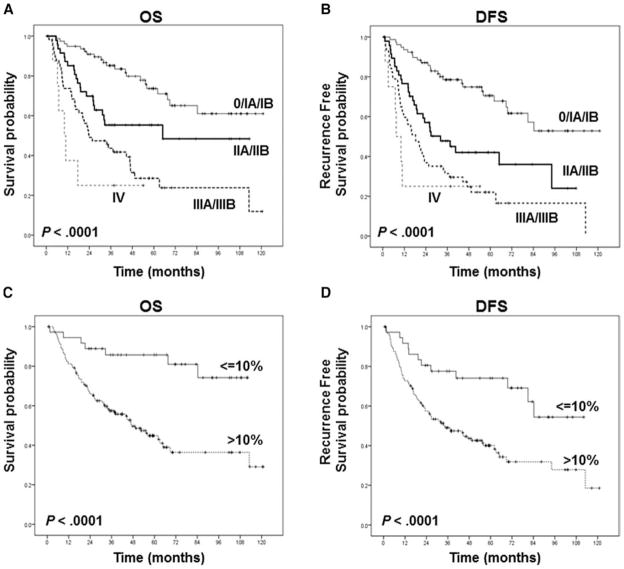
We analyzed the relationship between pathologic stage and survival in patients with neoadjuvant-treated NSCLC and found that even after chemotherapy the pathologic stage was a significant predictor of long-term survival (Figure 3). The percentage of viable tumor cells in the resected specimens was also a significant predictor of long-term survival after neoadjuvant chemotherapy when assessed in a categorical (Figure 3) or continuous fashion (Table 3). Multivariable analysis (Table 3) suggests that the significant predictors of OS and DFS after neoadjuvant chemotherapy include pathologic stage and percentage of viable tumor cells. In multivariable analysis, for every 1% increase in viable tumor, hazard ratio increased by 0.01.
FIGURE 3.
Kaplan-Meier estimates of overall survival (A, C) and disease-free survival (B, D) based on pathologic stages (A, B) and percentage of viable tumor cells (C, D). A, The overall survival was significantly longer in patients with stages 0, IA, and IB than in patients with pathologic stage II, III, or IV. B, The disease-free survival was significantly longer in patients with stages 0, IA, and IB than in patients with pathologic stage II, III, or IV. C, The overall survival was significantly longer in patients with ≤10% viable tumor cells than in patients with >10% viable tumor cells. D, The disease-free survival was significantly longer in patients with ≤10% viable tumor cells than in patients with >10% viable tumor cells.
TABLE 3.
Univariate and Multivariate Analyses for Survival on Patients with NSCLC Treated with Neoadjuvant Chemotherapy
| Characteristics | No. of Patients | OS
|
DFS
|
||
|---|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | p | ||
| Univariate Cox model | |||||
| Age (continuous) | 192 | 1.01 (0.98–1.03) | 0.59 | 1.00 (0.98–1.03) | 0.8 |
| Gender | 0.45 | 0.11 | |||
| Female (reference) | 81 | 1.00 | 1.00 | ||
| Male | 111 | 0.85 (0.56–1.3) | 0.71 (0.49–1.04) | ||
| Histology | 0.26 | 0.29 | |||
| Adenocarcinoma (reference) | 89 | 1.00 | 1.00 | ||
| Squamous cell carcinoma | 58 | 0.64 (0.38–1.09) | 0.71 (0.45–1.14) | ||
| Other | 45 | 0.89 (0.54–1.47) | 0.76 (0.47–1.22) | ||
| Pathologic stage | <0.001 | <0.001 | |||
| 0/IA/IB (reference) | 78 | 1.00 | 1.00 | ||
| IIA/IIB | 49 | 2.24 (1.22–4.11) | 2.66 (1.55–4.58) | ||
| IIA/IIB | 57 | 4.23 (2.48–7.22) | 4.54 (2.76–7.47) | ||
| IV | 8 | 7.69 (3.07–19.33) | 6.73 (2.73–16.61) | ||
| Viable tumor cells (continuous) | 192 | 1.02 (1.01–1.03) | <0.001 | 1.01 (1.01–1.02) | <0.001 |
| Multivariate Cox model | |||||
| Pathologic stage | <0.001 | <0.001 | |||
| 0/IA/IB (reference) | 78 | 1.00 | 1.00 | ||
| IIA/IIB | 49 | 1.85 (1.00–3.43) | 2.36 (1.36–4.09) | ||
| IIA/IIB | 57 | 3.16 (1.80–5.52) | 3.72 (2.19–6.31) | ||
| IV | 8 | 7.25 (2.88–18.29) | 6.60 (2.66–16.33) | ||
| Viable tumor cells (continuous) | 192 | 1.01 (1.00–1.02) | 0.005 | 1.01 (1.00–1.02) | 0.01 |
OS, overall survival; DFS, disease-free survival; HR, hazard ratio; CI, confidence interval; AJCC7, American Joint Committee on Cancer 7.
DISCUSSION
Although the survival benefits of neoadjuvant chemotherapy remain controversial,2–7 it has been observed that pathologic response after neoadjuvant therapy in patients with resected stage IIIA NSCLC is associated with improved OS.8 In a multicenter, phase II trial evaluating pN2 patients treated with three cycles of neoadjuvant docetaxelcisplatin, Betticher et al.8 noted that the 60% of patients who downstaged from pN2 at mediastinoscopy to pN0–N1 at surgery had improved 3 years OS (60% versus 10%, p < 0.0001). Several authors have also noted that histopathologic response criteria may be a prognostic factor in clinical N2 (cN2) patients treated with neoadjuvant chemotherapy or chemoradiotherapy.9,10 Because of these preliminary observations, we wanted to see whether reproducible histopathologic response criteria could be developed that would predict long-term survival in a larger cohort of patients with stages I to III NSCLC treated with neoadjuvant chemotherapy even when controlled for pathologic stage. We also wanted to see whether these criteria might provide a surrogate end point for long-term survival and chemotherapy response in biomarker-driven translational clinical trials.
Neoadjuvant chemotherapy is a therapeutic option that is used in patients with locally advanced resectable NSCLC. The response to neoadjuvant chemotherapy in these patients is typically assessed by radiologic measurements of tumor size before and after therapy. Unfortunately, this change in tumor size is not always reliable in the prediction of long-term survival because of the difficulty in differentiating fibrosis from viable tumor radiographically. Attempts to improve the prediction of chemotherapy response with positron emission tomography/computed tomography findings have also been confounded by false-positive F-fluorodeoxyglucose avidity due to macrophage infiltration.11 Several small studies have suggested that the degree of tumor regression after neoadjuvant therapy as determined by histopathologic findings in the resected tumor may be a more objective criterion of chemotherapy response (Table 4).12–15 Our data on 192 patients with NSCLC treated with neoadjuvant chemotherapy suggest that the percentage of viable tumor cells does indeed predict OS even when controlled for pathologic stage. Importantly, in patients who are not treated with neoadjuvant chemotherapy (Table 2), the percentage of viable tumor cells is not predictive of OS. The prognostic effect of the percentage of viable tumor cells is significant when looked at in a continuous (p < 0.003) or categorical (>10% versus ≤10% viable tumor, p < 0.001) fashion and when controlled for pathologic stage (Table 3). Several other authors have observed a relationship with histopathologic response and survival in patients with NSCLC, but these studies have been limited by small numbers, variable types of induction therapy (chemotherapy and chemoradiation), and have not controlled for pathologic stage or included a control group of patients with NSCLC treated with surgery alone (Table 4).12–15 These studies evaluated only one slide for each tumor. Nevertheless, we evaluated multiple slides for each tumor on a large number of patients with NSCLC who only received neoadjuvant chemotherapy. Assessment of histopathologic response in the tumor was performed in a continuous (i.e., percent viable tumor) and categorical fashion (10%, 20%, 30%, 40%, or 50%, data not shown) with a modification of the regression grading system introduced by Junker et al.,15 nonresponder = morphologic evidence of therapy-induced changes but >10% viable tumor cells and responder = extensive response with ≤10% viable tumor cells. Our study clearly demonstrates that the percentage of viable tumor cells is a significant predictor of OS and DFS in patients with neoadjuvant-treated NSCLC but not in those patients who undergo surgery alone. Although necrosis was present in patients with resected NSCLC who did not receive neoadjuvant therapy (Table 2), it was not predictive of OS or DFS. Although not statistically significant, there is a suggestion in the surgery-only patients (Table 2) that increased tumor necrosis is associated with reduced OS perhaps because larger tumors outgrow their native blood supply and are associated with a worse prognosis and less viable tumor.
TABLE 4.
Summary of Previous Histology Analysis on Patients with NSCLC Treated with Neoadjuvant therapy
| Authors | No. of Patients | Stages | Treatment | Histologic Criteria | Prognostic Data |
|---|---|---|---|---|---|
| Junker et al.12 | 40 | IIIA and IIIB | Combined chemotherapy and radiotherapy | % Viable tumor (≤10% vs. >10%) | p = 0.02 |
| Liu-Jarin et al.14 | 30 | IIB, IIIA, IIIB, and IV | Combined chemotherapy and radiotherapy | % Viable tumor | None |
| Yamane et al.15 | 53 | I–IV | 40 chemotherapy, 11 chemoradiotherapy, and 1 radiotherapy | Area of residual tumor (≤400 vs. >400 mm2) | p = 0.01 |
Numerous histopathologic criteria were reviewed, and the only significant factors when controlled for pathologic stage were the percentage of viable tumor and stromal tissue noted on the resected specimens. The percentage of necrosis did not correlate with OS or DFS (data not shown). This may have been due to the fact that a certain amount of necrosis is present in all tumors even those which are not treated with neoadjuvant chemotherapy. Several other histopathologic features such as cholesterin clefts, foreign body reactive giant cells, stromal hyalinosis, granulation tissue, and peripheral scar formation were associated with receiving neoadjuvant chemotherapy. Nevertheless, these histologic features had no significant correlation with clinical response and prognosis. Additionally, several other histologic features, such as coagulation necrosis, foam cell infiltration, and inflammatory cell infiltration, were present in the resected specimens from both those patients who received neoadjuvant chemotherapy and those who underwent surgical resection alone. Similar to the histologic features related to neoadjuvant chemotherapy, there was no significant correlation of these unrelated histopathologic features to response and prognosis.
A potential limitation in our study is that variations of histologic features can occur in any grading system. In an attempt to decrease interobserver variability, all surgical specimens were histologically evaluated by two pathologists. It is important to note that histopathologic criteria depend on complete sampling of the resected specimen, especially when no gross residual tumor is appreciable. As incomplete evaluation of the treated tumor site in cases with only rare microscopic foci of viable tumor could result in misclassification, examination of multiple tissue slices obtained from the tumor site is important for accurate and reproducible classification of histopathologic features. The variation between slides was as much as 5 to 10% in the same specimen. Because of this variability, we believe that it is important to assess numerous slides and take the mean of all the slides characterized (minimum of 1 slide per cm of resected tumor).
Chemotherapy resistance may be a significant contributor to treatment failure in some patients with NSCLC who receive neoadjuvant chemotherapy. A personalized approach to treatment selection could potentially improve survival in patients with NSCLC who receive neoadjuvant therapy. In this regard, chemotherapeutic agents selected on the basis of molecular determinants of the tumor may augment response rates and survival. Clinical studies suggest that epidermal growth factor receptor mutations (particularly exon 19 deletions) have increased sensitivity to some chemotherapeutic agents.16–17 It has also been reported that high expression levels of excision repair cross complementation group 1 protein and ribonucleotide reductase predict resistance to platinum or gemcitabine chemotherapy.18,19 The histopathologic response reported in this article may form a surrogate end point for survival in phase II clinical trials. Such a surrogate end point would help accelerate biomarker-driven questions of response in translational clinical trials. The ability to separate biomarkers of response from biomarkers of prognosis may also be helped by assessment of pathologic response. The surrogate end point of pathologic response may ultimately be a better and faster correlate for chemotherapy response than OS or DFS.
In summary, our results indicate that the percentage of viable tumor cells in the resected specimen correlates with OS and DFS in patients with NSCLC treated with neoadjuvant chemotherapy even when controlled for pathologic stage. The routine histologic assessment of the resected specimen could potentially have a role in the subsequent therapeutic management of patients who undergo surgery after neoadjuvant therapy. The percentage of viable tumor cells in the resected specimen may also serve as a surrogate end point for survival and may provide a more accurate and rapid comparison between different neoadjuvant treatment regimens, shortening the period needed to evaluate novel chemotherapeutic and biologic therapies in clinical trials.
Acknowledgments
Supported, in part, by Department of Defense W81XWH-07-1-0306 and the National Institutes of Health through M. D. Anderson’s Cancer Center Support Grant CA 016672—Lung Program and by the Homer Flower Research Fund, the Charles Rogers Gene Therapy Fund, the Margaret Wiess Elkins Endowed Research Fund, the Flora and Stuart Mason Lung Cancer Research Fund, and the Phalan Thoracic Gene Therapy Fund.
The authors thank Lakshimi Kakarala for her technical assistance.
Footnotes
Members of the M.D. Anderson Lung Cancer Collaborative Research Group: John Heymach, Lauren Byers, Joseph Chang, George Blumenschein, James D. Cox, Wayne Hofstetter, Bingliang Fang, Frank Fossella, Bonnie Glisson, Waun Ki Hong, Kathryn Gold, Faye Johnson, Merrill S. Kies, Zhongxing Liao, Steven Lin, Scott Lippmann, Ritsuko Komaki, Michael O’ Reilly, Vali Papadimitrakopoulou, Katherine Pisters, David Rice, Pierre Saintigny, Anne Tsao, Garrett L. Walsh, James Welsh, and William N. William Jr.
Disclosure: The authors declare no conflicts of interest.
References
- 1.American Cancer Society. Cancer Facts and Figures. Atlanta, GA: American Cancer Society; 2008. [Accessed April 1, 2011.]. Available at: http://www.cancer.org/downloads/STT/2008CAFFfinalsecured.pdf. [Google Scholar]
- 2.Roth JA, Atkinson EN, Fossella F, et al. Long-term follow-up of patients enrolled in a randomized trial comparing perioperative chemotherapy and surgery with surgery alone in resectable stage IIIA non-small-cell lung cancer. Lung Cancer. 1998;21:1–6. doi: 10.1016/s0169-5002(98)00046-4. [DOI] [PubMed] [Google Scholar]
- 3.Rosell R, Gomez-Codina J, Camps C, et al. Preresectional chemotherapy in stage IIIA non-small-cell lung cancer: a 7-year assessment of a randomized clinical trial. Lung Cancer. 1999;26:7–14. doi: 10.1016/s0169-5002(99)00045-8. [DOI] [PubMed] [Google Scholar]
- 4.Pisters KM, Ginsberg RJ, Giroux DJ, et al. Induction chemotherapy before surgery for early-stage lung cancer: a novel approach. Bimodality Lung Oncology Team. J Thorac Cardiovasc Surg. 2000;119:429–439. doi: 10.1016/s0022-5223(00)70120-6. [DOI] [PubMed] [Google Scholar]
- 5.Depierre A, Milleron B, Moro-Sibilot D, et al. Preoperative chemotherapy followed by surgery compared with primary surgery in resectable stage I (except T1N0), II, and IIIa non-small-cell lung cancer. J Clin Oncol. 2002;20:247–253. doi: 10.1200/JCO.2002.20.1.247. [DOI] [PubMed] [Google Scholar]
- 6.Detterbeck FC, Boffa DJ, Tanoue LT. The new lung cancer staging system. Chest. 2009;36:260–271. doi: 10.1378/chest.08-0978. [DOI] [PubMed] [Google Scholar]
- 7.Song W-A, Zhou N-K, Wang W, et al. Survival benefit of neoadjuvant chemotherapy in non-small cell lung cancer. An updated meta-analysis of 13 randomized control trials. J Thorac Oncol. 2010;5:510–516. doi: 10.1097/JTO.0b013e3181cd3345. [DOI] [PubMed] [Google Scholar]
- 8.Betticher DC, Schmitz S-F, Totsch M, et al. Mediastinal lymph node clearance after docetaxel-cisplatin neoadjuvant chemotherapy is prognostic of survival in patients with stage IIIA pN2 non-small cell lung cancer: a multicenter phase II trial. J Clin Oncol. 2003;21:1752–1759. doi: 10.1200/JCO.2003.11.040. [DOI] [PubMed] [Google Scholar]
- 9.Stefani A, Alifano M, Bobbio A, et al. Which patients should be operated on after induction chemotherapy for N2 non-small cell lung cancer? Analysis of a 7-year experience in 175 patients. J Thorac Cardiovasc Surg. 2010;55:1–8. doi: 10.1016/j.jtcvs.2010.02.018. [DOI] [PubMed] [Google Scholar]
- 10.Betticher DC, Schmitz S-F, Totsch M, et al. Prognostic factors affecting long-term outcomes in patients with resected stage IIIA p N2 non-small-cell lung cancer: 5 year follow-up of a phase II study. Br J Cancer. 2006;94:1099–1106. doi: 10.1038/sj.bjc.6603075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Poetten C, Theegarten D, Eberhardt W, et al. Correlation of PET/CT findings and histopathology after neoadjuvant therapy in non-small cell lung cancer. Oncology. 2007;73:316–323. doi: 10.1159/000134474. [DOI] [PubMed] [Google Scholar]
- 12.Junker K, Thomas M, Schulmann K, et al. Tumour regression in non-small-cell lung cancer following neoadjuvant therapy. Histological assessment. J Cancer Res Clin Oncol. 1997;123:469–477. doi: 10.1007/BF01192200. [DOI] [PubMed] [Google Scholar]
- 13.Junker K, Langner K, Klinke F, et al. Grading of tumor regression in non-small cell lung cancer: morphology and prognosis. Chest. 2001;120:1584–1591. doi: 10.1378/chest.120.5.1584. [DOI] [PubMed] [Google Scholar]
- 14.Liu-Jarin X, Stoopler MB, Raftopoulos H, et al. Histologic assessment of non-small cell lung carcinoma after neoadjuvant therapy. Mod Pathol. 2003;16:1102–1108. doi: 10.1097/01.MP.0000096041.13859.AB. [DOI] [PubMed] [Google Scholar]
- 15.Yamane Y, Ishii G, Goto K, et al. A novel histopathological evaluation method predicting the outcome of non-small cell lung cancer treated by neoadjuvant therapy: the prognostic importance of the area of residual tumor. J Thorac Oncol. 2010;5:49–55. doi: 10.1097/JTO.0b013e3181c0a1f8. [DOI] [PubMed] [Google Scholar]
- 16.Herbst RS, Giaccone G, Schiller JH, et al. Gefitinib in combination with paclitaxel and carboplatin in advanced non-small-cell lung cancer: a phase III trial-INTACT 2. J Clin Oncol. 2004;22:785–794. doi: 10.1200/JCO.2004.07.215. [DOI] [PubMed] [Google Scholar]
- 17.Gatzemeier U, Pluzanska A, Szczesna A, et al. Phase III study of erlotinib in combination with cisplatin and gemcitabine in advanced non-small-cell lung cancer: the Tarceva Lung Cancer Investigation Trial. J Clin Oncol. 2007;25:1545–1552. doi: 10.1200/JCO.2005.05.1474. [DOI] [PubMed] [Google Scholar]
- 18.Bepler G, Kusmartseva I, Sharma S, et al. RRM1 modulated in vitro and in vivo efficacy of gemcitabine and platinum in non-small-cell lung cancer. J Clin Oncol. 2006;24:4731–4737. doi: 10.1200/JCO.2006.06.1101. [DOI] [PubMed] [Google Scholar]
- 19.Ceppi P, Volante M, Novello S, et al. ERCC1 and RRM1 gene expressions but not EGFR are predictive of shorter survival in advanced non-small-cell lung cancer treated with cisplatin and gemcitabine. Ann Oncol. 2006;17:1818–1825. doi: 10.1093/annonc/mdl300. [DOI] [PubMed] [Google Scholar]