Abstract
Temperature pervasively affects all cellular processes. In response to a rapid increase in temperature, all cells undergo a heat shock response, an ancient and highly conserved program of stress-inducible gene expression, to reestablish cellular homeostasis. In isolated cells, the heat shock response is initiated by the presence of misfolded proteins and therefore thought to be cell-autonomous. In contrast, we show that within the metazoan Caenorhabditis elegans, the heat shock response of somatic cells is not cell-autonomous but rather depends on the thermosensory neuron, AFD, which senses ambient temperature and regulates temperature-dependent behavior. We propose a model whereby this loss of cell autonomy serves to integrate behavioral, metabolic, and stress-related responses to establish an organismal response to environmental change.
The heat shock response counteracts the detrimental effects of protein misfolding and aggregation that result from biochemical and environmental stresses, including increases in temperature (1, 2). This response, orchestrated by the ubiquitously expressed heat shock factor–1 (HSF-1), involves the rapid transcription of a specific set of genes encoding the cytoprotective heat shock proteins (HSPs) (1, 2). The stress-induced appearance of non-native proteins imbalances cellular homeostasis, and the resulting shift in chaperone requirements is thought to trigger the heat shock response (1, 2). Because all these events occur at the cellular level, the heat shock response is thought to be cell-autonomous. Indeed, isolated cells in tissue culture, unicellular organisms (1, 2), and individual cells within a multicellular organism (3) can all produce a heat shock response when exposed directly to heat.
Although the heat shock response is essential for the survival of cells exposed to stress, the accumulation of large amounts of HSPs can be detrimental for cell growth and division (2, 4). Therefore, although cellular autonomy in initiating this response may be beneficial for unicellular organisms and isolated cells, the uncoordinated triggering of the heat shock response in individual cells within a multicellular organism could interfere with the complex interactions between differentiated cells and tissues.
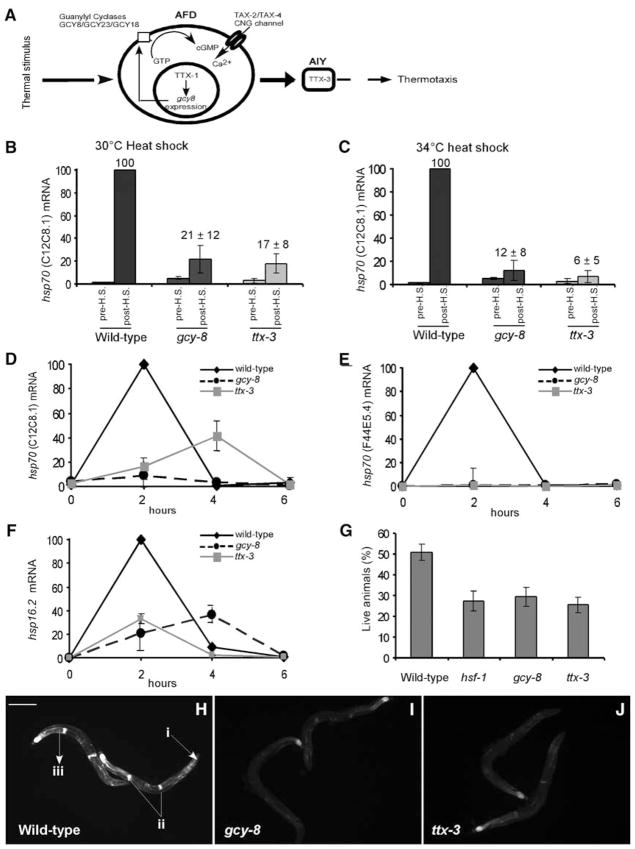
In Caenorhabditis elegans, a pair of thermosensory neurons, the AFDs, detect and respond to ambient temperature (5, 6). The AFDs, and their postsynaptic partner cells, the AIYs, regulate the temperature-dependent behavior of the organism and are required for finding the optimal temperature for growth and reproduction (6). We tested whether this thermosensory neuronal circuitry also regulates the heat shock response of somatic cells. For this, we exposed wild-type C. elegans or animals carrying loss-of-function mutations affecting the AFD or AIY neurons (Fig. 1A) to a transient increase in temperature and assayed their heat shock response. The mutations chosen {gcy-23, gcy-8 (7), tax-4, ttx-1, and ttx-3 [for details of these mutations see (8)]} exclusively affect neuronal function because the wild-type gene products are not expressed in other tissues, with gcy-8 and gcy-23 expressed solely in the AFDs (7). Wild-type and mutant adult animals, grown at low population densities at 20°C in the presence of abundant bacteria (8), were exposed to a transient increase in temperature (30°C or 34°C for 15 min), and their heat shock response was measured as the total amount of mRNA encoding the major heat-inducible cytoplasmic hsp70, C12C8.1 (9), 2 hours after heat shock. Mutations affecting the AFD or AIY neurons reduced heat shock–dependent accumulation of hsp70 (C12C8.1) mRNA at both temperatures (8) (table S1 and Fig. 1, B and C), whereas a mutation, ocr-2 (10), affecting four other sensory neurons of the animal had no effect (8) (table S1).
Fig. 1.
Role of AFD and AIY neurons in the organismal heat shock response. (A) Schematic depiction of genes affecting AFD and AIY function. AFD detects temperature by using the cyclic guanosine 5′-monophosphate (cGMP)–dependent TAX-4/TAX-2 cyclic-nucleotide–gated (CNG) channel. Guanylyl cyclases, gcy-8, gcy-18, and gcy-23, function upstream of tax-4. ODX transcription factor, TTX-1, regulates gcy-8 expression, and AIY function is specified by the LIM homeobox gene, ttx-3. Total hsp70 (C12C8.1) mRNA levels in gcy-8 and ttx-3 mutants relative to wild-type animals before heat shock (pre-H.S.) and 2 hours after heat shock (post-H.S.) at (B) 30°C and (C) 34°C for 15 min. Time course of total (D) hsp70 (C12C8.1), (E) hsp70 (F44E5.4), and (F) hsp16.2 mRNA accumulation after heat shock (34°C; 15 min) in gcy-8 and ttx-3 mutants relative to wild-type animals. mRNA amounts were measured by quantitative reverse transcription polymerase chain reaction (RT-PCR) and normalized to maximal wild-type values. (G) Survival of wild-type, gcy-8, ttx-3, and hsf-1 mutant animals. Error bars indicate ±SE [(B) and (C)] and ±SD [(D) and (E)]. hsp70 (C12C8.1) promoter-GFP reporter expression in (H) wild-type, (I) gcy-8, and (J) ttx-3 mutant animals 2 hours after heat shock (34°C; 15 min). (i) pharynx, (ii) spermatheca, and (iii) intestinal cell. Scale bar = 100 μm.
The decrease in hsp70 (C12C8.1) abundance was not merely due to a delay in the onset of heat shock response (Fig. 1D). The gcy-8 and ttx-3 mutants had consistently lower amounts of hsp70 (C12C8.1) mRNA compared with wild-type over a 6-hour period after heat shock, with some mRNA accumulation seen 4 hours after heat shock. Mutants with defective AFD or AIY neurons also had reduced heat shock–dependent accumulation of another cytoplasmic hsp70, F44E5.4 (9), and the small heat shock protein, hsp16.2, mRNAs (11) (Fig. 1, E and F).
The diminished expression of HSP genes in the gcy-8 and ttx-3 mutants might make them less viable than wild-type animals under conditions of heat stress. This was the case (Fig. 1G): The decrease in thermotolerance of the thermosensory mutants was similar to that of animals carrying a hsf-1 loss-of-function allele (12) (Fig. 1G), although hsf-1 mRNA levels were not diminished in gcy-8 and ttx-3 mutants (8) (fig. S3A) relative to that of wild type.
We examined whether the decreased accumulation of inducible hsp70 mRNA in the thermo-sensory mutants reflected selective reduction in neuronal tissue or corresponded to diminished expression in all cells throughout the animal. Heat shock promotes hsp mRNA expression in numerous somatic cells of wild-type C. elegans as monitored with a hsp70 (C12C8.1) promoter green fluorescent protein (GFP) reporter construct (13) (Fig. 1H). The expression of this hsp70 reporter in strains with mutant gcy-8 and ttx-3 genes was reduced in all somatic cells 2 hours after heat shock (Fig. 1, I and J) and continued to be impaired after 24 hours, although these somatic cells of the thermosensory mutants should have experienced the same heat shock temperature as the equivalent wild-type cells (8) (figs. S1 and S2). These results indicate that the heat shock response in C. elegans is not cell-autonomous. Instead, the AFD and AIY neurons appear to regulate both the magnitude and the time course of heat shock gene expression in nonneuronal cells, influencing organismal thermotolerance. Subsequent experiments were done at 34°C with use of the AFD-specific mutant gcy-8 that is the most upstream component known in the thermosensory neuronal circuitry.
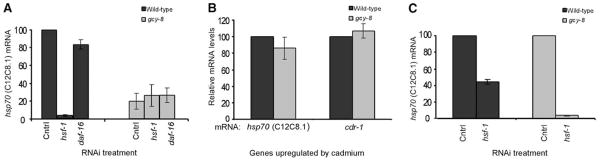
To control the heat shock response of non-neuronal cells, the AFD neurons must regulate the activities of cellular transcription factors. In eukaryotes, HSP expression after heat shock is HSF-1–dependent (2). Organismal thermotolerance also requires the FOXO transcription factor, DAF-16 (14). To test which transcription factor is required for AFD-dependent hsp70 (C12C8.1) mRNA expression after heat shock, we used RNA interference (RNAi). Depletion of hsf-1 mRNA but not daf-16 mRNA in wild-type C. elegans decreased hsp70 (C12C8.1) expression throughout the organism (Fig. 2A). Depletion of hsf-1 mRNA, however, had no effect on the already diminished amounts of hsp70 (C12C8.1) mRNA in the gcy-8 mutants (Fig. 2A). Thus, AFD appears to regulate HSP expression through HSF-1, although we cannot rule out the existence of a parallel transcriptional mechanism.
Fig. 2.
Impairment of HSF-1–dependent gene expression in gcy-8 mutants after temperature stress. (A) Total hsp70 (C12C8.1) mRNA, 2 hours after heat shock (34°C; 15 min), in wild-type and gcy-8 mutant animals subjected to RNAi-mediated knockdown of hsf-1 or daf-16. Relative mRNA levels were measured by quantitative RT-PCR and normalized to wild-type values on control RNAi. (B) Total hsp70 (C12C8.1) and cdr-1 mRNA levels in wild-type and gcy-8 mutants after cadmium stress (8). Relative mRNA levels were determined by quantitative RT-PCR and normalization to wild-type values. (C) Relative hsp70 (C12C8.1) mRNA levels in cadmium-treated wild-type animals and gcy-8 mutants subjected to RNAi-mediated knockdown of hsf-1. Relative mRNA levels were determined by quantitative RT-PCR and normalization to wild-type and gcy-8 values on control RNAi. Error bars indicate ±SE.
We tested whether the deficiency in HSF-1–dependent heat shock induction of HSPs in the gcy-8 mutants resulted from high constitutive expression of chaperones that negatively auto-regulate HSF-1 activity or other inhibitors of HSF-1 (2, 14). This appeared not to be the case: Both wild-type and mutant animals expressed similar amounts of constitutive hsp70 (hsp-1) (fig. S3B), the stress-inducible hsp70s [C12C8.1 and F44E5.4 (8) (table S1)], hsp90 (daf-21), and daf-16 (fig. S3B). To test whether the gcy-8 mutant animals were deficient in their ability to mount any stress-inducible transcriptional response, we exposed animals to another stress, the transition metal cadmium (15), and assayed their ability to activate transcription of two cadmium-responsive genes: hsp70 (C12C8.1) and cdr-1 (15). In contrast to what was observed upon heat shock, both hsp70 and cdr-1 mRNA were similarly increased in wild-type and gcy-8 mutant animals (Fig. 2B) after 3 hours of exposure. Moreover, as for wild-type animals, the cadmium-dependent induction of hsp70 (C12C8.1) was HSF-1–dependent in gcy-8 mutants (Fig. 2C). Thus, a deficiency in the AFD neuron does not compromise the molecular machinery required for the stress-dependent HSF-1 transcriptional response. The gcy-8 mutant animals are not pre-adapted to stress but instead are selectively impaired in their ability to induce HSF-1–dependent heat shock gene expression. Given the role of the AFD in sensing ambient temperature, this suggests that AFD signaling is required for heat shock–dependent gene expression. This was further confirmed by transiently and reversibly inhibiting neuronal activity in wild-type animals with the volatile anesthetics (VAs) halothane and isoflurane and observing a concomitant inhibition of hsp70 (C12C8.1) expression in somatic cells (8) (fig. S4).
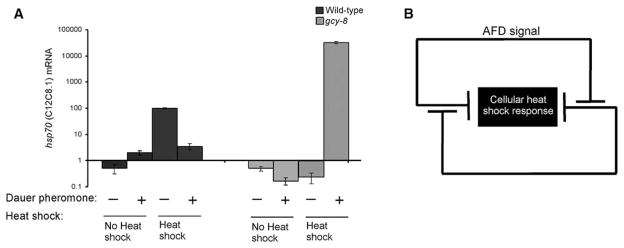
The AFD neurons and associated thermo-sensory circuitry in C. elegans also regulate thermotaxis behavior, integrating temperature information with environmental signals that modulate growth and metabolism (6). One such potent environmental signal is dauer pheromone: Low concentrations of dauer pheromone, when animals are at low population densities in the presence of food, promote continuous growth; high concentrations signal starvation and alter metabolism (16). AFD mutants show altered sensitivity to dauer pheromone (16, 17). Thus, dauer pheromone might also modulate the heat shock response in an AFD-dependent manner, integrating the organismal stress response with metabolism. C. elegans animals raised at low population density initiate transcription of hsp mRNA after heat shock (Figs. 1 and 3A). However, exposure of wild-type animals to high concentrations of dauer pheromone before or during heat shock decreased the amounts of hsp70 (C12C8.1) mRNA induced (Fig. 3A). Thus, the heat shock response in C. elegans is affected by the metabolic state and is dampened under conditions that do not support continuous growth and reproduction. In contrast, exposure of gcy-8 mutant animals to dauer pheromone had the opposite effect and induced even higher amounts of hsp70 (C12C8.1) mRNA after heat shock than is normally seen upon heat shock of wild-type animals raised under optimal growth conditions (Fig. 3A).
Fig. 3.
AFD-dependent regulation of the cellular heat shock response is modulated by metabolic signals. (A) Relative hsp70 (C12C8.1) mRNA levels before and 2 hours after heat shock (34°C; 15 min) in wild-type and gcy-8 mutant animals grown at low population densities or exposed to dauer pheromone 10 min before and during the 2 hours of recovery after heat shock. Note semi-logarithmic scale. Relative mRNA levels were determined by quantitative RT-PCR and normalized to maximal wild-type values. Error bars indicate ±SE. (B) Model depicting the regulation of the cellular heat shock response by AFD-dependent signaling of temperature and dauer pheromone–dependent signaling of growth conditions.
The opposing effects of dauer pheromone on wild-type and gcy-8 mutant animals can be explained by a model in which the heat shock response is regulated at the organismal level by two inputs: the AFD-dependent temperature input and a metabolic signal that responds to growth conditions (Fig. 3B). Each of these inputs negatively regulates the other and inhibits HSP expression. In wild-type animals under conditions that support growth, the growth-dependent inhibitory signal is active, and an increase in temperature activates the AFDs, which suppresses the inhibitory signal, thus allowing induction of the heat shock response. In the presence of positive growth signals but absence of AFD signaling during heat shock (as in gcy-8 mutants), the growth input is not inhibited, and the heat shock response is suppressed. The addition of dauer pheromone to wild-type animals suppresses the signal from the growth input, resulting in the inhibition of the cellular heat shock response by AFD signaling. In the absence of both AFD and growth signals, as occurs in the gcy-8 mutant exposed to dauer pheromone, the heat shock response is not inhibited.
The model we propose for the regulation of the heat shock response in C. elegans suggests that cells induce this response in the presence of AFD and growth signals, as in wild-type animals, but also in the complete absence of these regulatory signals, as observed for isolated cells in culture. Thus, neuronal control may allow C. elegans to coordinate the stress response of individual cells with the varying metabolic requirements of its different tissues and developmental stages. Indeed, neuronal signaling has been shown to modulate cellular homeostasis in C. elegans (18). Because the AFD neurons do not directly innervate any of the downstream tissues in which heat shock gene induction is affected, it is likely that this regulation is mediated through neuroendocrine signaling. The override of the cell-autonomous heat shock response by neuronal circuitry seen for C. elegans may be a common mechanism of regulation in other metazoans. Indeed, HSF-1 in rats can be activated by neuroendocrine signaling from the hypothalamic-pituitary-adrenal axis in the absence of external stress (19). Thus, the hierarchical organization of regulatory networks may allow organized tissues comprised of heterogenous cell types to establish a highly orchestrated stress response in the metazoan organism.
Supplementary Material
Acknowledgments
We thank the Morimoto lab members for discussions and comments; T. Stiernagle and the Caenorhabditis elegans Genetics Center, supported by a grant from National Institute of General Medical Sciences (NIGMS) and the International Consortium, for providing strains; and I. Mori at Nagoya University, Nagoya, Japan for the gcy-8(oy44) strain. These studies were supported by grants to R.I.M. from NIH (NIGMS and National Institute on Aging), the Huntington’s Disease Society of America Coalition for the Cure, and the Daniel F. and Ada L. Rice Foundation.
Footnotes
References and Notes
- 1.Lindquist S, Craig EA. Annu Rev Genet. 1988;22:631. doi: 10.1146/annurev.ge.22.120188.003215. [DOI] [PubMed] [Google Scholar]
- 2.Morimoto RI. Genes Dev. 1998;12:3788. doi: 10.1101/gad.12.24.3788. [DOI] [PubMed] [Google Scholar]
- 3.Stringham EG, Candido EP. J Exp Zool. 1993;266:227. doi: 10.1002/jez.1402660309. [DOI] [PubMed] [Google Scholar]
- 4.Feder JH, Rossi JM, Solomon J, Solomon N, Lindquist S. Genes Dev. 1992;6:1402. doi: 10.1101/gad.6.8.1402. [DOI] [PubMed] [Google Scholar]
- 5.Clark DA, Gabel CV, Gabel H, Samuel AD. J Neurosci. 2007;27:6083. doi: 10.1523/JNEUROSCI.1032-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mori I. Annu Rev Genet. 1999;33:399. doi: 10.1146/annurev.genet.33.1.399. [DOI] [PubMed] [Google Scholar]
- 7.Inada H, et al. Genetics. 2006;172:2239. doi: 10.1534/genetics.105.050013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Materials and methods are available on Science Online.
- 9.Snutch TP, Heschl MF, Baillie DL. Gene. 1988;64:241. doi: 10.1016/0378-1119(88)90339-3. [DOI] [PubMed] [Google Scholar]
- 10.Sokolchik I, Tanabe T, Baldi PF, Sze JY. J Neurosci. 2005;25:1015. doi: 10.1523/JNEUROSCI.3107-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Link CD, Cypser JR, Johnson CJ, Johnson TE. Cell Stress Chaperones. 1999;4:235. doi: 10.1379/1466-1268(1999)004<0235:doosri>2.3.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hajdu-Cronin YM, Chen WJ, Sternberg PW. Genetics. 2004;168:1937. doi: 10.1534/genetics.104.028423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morley JF, Morimoto RI. Mol Biol Cell. 2004;15:657. doi: 10.1091/mbc.E03-07-0532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hsu AL, Murphy CT, Kenyon C. Science. 2003;300:1142. doi: 10.1126/science.1083701. [DOI] [PubMed] [Google Scholar]
- 15.Cui Y, McBride SJ, Boyd WA, Alper S, Freedman JH. Genome Biol. 2007;8:R122. doi: 10.1186/gb-2007-8-6-r122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hu PJ, editor. The C. elegans. Vol. 1. Research Community; WormBook: 2007. http://www.wormbook.org. [DOI] [Google Scholar]
- 17.Golden JW, Riddle DL. Proc Natl Acad Sci USA. 1984;81:819. doi: 10.1073/pnas.81.3.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garcia SM, Casanueva MO, Silva MC, Amaral MD, Morimoto RI. Genes Dev. 2007;21:3006. doi: 10.1101/gad.1575307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fawcett TW, Sylvester SL, Sarge KD, Morimoto RI, Holbrook NJ. J Biol Chem. 1994;269:32272. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.