Abstract
Summary: Several enteric protozoa cause severe morbidity and mortality in both humans and animals worldwide. In developed settings, enteric protozoa are often ignored as a cause of diarrheal illness due to better hygiene conditions, and as such, very little effort is used toward laboratory diagnosis. Although these protozoa contribute to the high burden of infectious diseases, estimates of their true prevalence are sometimes affected by the lack of sensitive diagnostic techniques to detect them in clinical and environmental specimens. Despite recent advances in the epidemiology, molecular biology, and treatment of protozoan illnesses, gaps in knowledge still exist, requiring further research. There is evidence that climate-related changes will contribute to their burden due to displacement of ecosystems and human and animal populations, increases in atmospheric temperature, flooding and other environmental conditions suitable for transmission, and the need for the reuse of alternative water sources to meet growing population needs. This review discusses the common enteric protozoa from a public health perspective, highlighting their epidemiology, modes of transmission, prevention, and control. It also discusses the potential impact of climate changes on their epidemiology and the issues surrounding waterborne transmission and suggests a multidisciplinary approach to their prevention and control.
INTRODUCTION
Parasitic diseases contribute significantly to the burden of infectious diseases worldwide. While most infections and death from parasitic diseases affect people in developing countries, they also cause significant illness in developed countries (305). In 2004, the WHO reported that diarrheal disease affected far more individuals than any other illness, even in regions that include high-income countries (468). Several species of enteric protozoa are associated with diarrheal illnesses in humans, with some causing severe debilitating illness, especially in immunosuppressed populations (228, 382, 390, 406, 410). Protozoan-related morbidity and mortality in both humans and animals worldwide are well documented (89, 94, 181, 222). Other protozoa have caused significant amounts of disease in livestock, often associated with losses in production, resulting in millions of dollars of losses in the food and livestock industry (288, 383, 431, 437, 438). Other impacts of parasitic infections include reduced worker productivity, reduced commodity yields, effects on income, and impacts on food security (70, 288). Several enteric protozoa cause zoonotic (transmitted from animals to humans) illnesses associated with livestock and domestic pets, and more recently, the prominence of open farms and petting zoos has featured in several zoonotic outbreaks and transmission to humans (55, 66, 146, 290, 340). Human-to-animal transmission of parasites is also becoming an emerging issue of public health and veterinary significance (89, 432). As humans and their livestock move further into wildlife domains, parasitic disease might represent a serious threat to wildlife, which in turn may act as reservoirs and/or amplifiers of emerging and exotic diseases for humans and their livestock (429, 432).
Much attention has been paid to enteric protozoa in human infections in developing countries, where poor sanitary conditions and the unavailability of effective water treatment have sustained conditions for their transmission (21, 110, 178, 180, 278, 349, 410). Climate change is predicted to influence changes in precipitation quantity, intensity, frequency, and duration and subsequently affect environmental conditions that predispose developing countries to the transmission of waterborne disease (181). Less focus has been placed on the impact of these changes in more industrialized settings, presumably because of better health standards. Therefore, estimation of the disease burden is often complicated by a lack of reliable data as a result of underdiagnosis and the lack of monitoring programs (288). However, despite the lower prevalence of parasitic diseases in industrialized countries, they may potentially result in a greater economic burden due to higher income, medical, and treatment costs (438).
In many developed countries, only a few or no parasitic protozoa are included in operational surveillance systems, as the major focus is on bacterial and viral infections. Where these systems exist, they are used mainly as indicators for identifying outbreaks of food-borne and waterborne diseases and in institutional settings (72, 73, 231, 463, 476–478). However, evidence suggests that while some enteric protozoa, such as Entamoeba spp., Cryptosporidium, and Giardia, are isolated frequently from diarrheal patients in developing regions such as Asia and sub-Saharan Africa (126, 284, 363), others, such as Blastocystis spp. and Dientamoeba fragilis, are isolated mainly in developed countries (390; S. M. Fletcher, Y. Li, D. Stark, and J. Ellis, presented at the Communicable Disease Control Conference, Canberra, ACT, Australia, 2011). In developed settings, however, enteric protozoa are often ignored as a cause of diarrhea due to better hygiene conditions. In many cases, the sick person may not seek medical attention, and even if he or she does, a stool specimen is not routinely requested from persons with diarrheal illnesses (170, 357, 427, 446, 476). For example, during an outbreak of gastroenteritis linked to a water supply in Austria in 2006, there was no identification of pathogenic microorganisms in stool samples from affected patients, and no parasite etiology was considered in this large outbreak (269). This could have been due to the fact that test requests by general practitioners may not have complied with existing knowledge of the gastroenteritis etiology at that time (427, 446).
In industrialized settings, the prevalence of protozoan illnesses is frequently captured by several surveillance systems, including outbreak surveillance, passive and active surveillance of notifiable diseases, and laboratory-based surveillance (258, 260, 298). Estimates of parasite prevalence are sometimes affected by the lack of sensitive diagnostic techniques to detect them in clinical specimens, while carrier stages and subclinical infections are often not diagnosed (236, 460). The development of technologies that can simultaneously detect several protozoa in stool is desirable in industrialized settings (386, 427). This includes the development of molecular markers for the detection of outbreaks, for source attribution, and to estimate their contribution to the overall burden of infectious diseases (236, 318, 426, 463). Current opinion suggests that molecular techniques are the most promising methods for the sensitive, accurate, and simultaneous detection of protozoan parasites in comparison to conventional staining and microscopy methods (386, 387), with much benefit to the water industry and public health (7, 40, 47, 48, 375). Unfortunately, molecular methods can be quite costly and labor-intensive and thus are not used routinely for the detection of parasitic protozoa, even in resource-rich settings (341). Much effort must now be placed on developing inexpensive molecular tools for routine laboratory applications in industrialized settings.
This review seeks to discuss the public health impact of common enteric protozoa associated with diarrheal illnesses in industrialized settings. The role that protozoa play in human and animal infections is discussed, along with the implications of climate change and the importance of water quality management to their prevention and control. The main enteric protozoa considered of public health significance and covered here are Cryptosporidium spp., Giardia intestinalis, Entamoeba histolytica, Dientamoeba fragilis, Cyclospora cayetanensis, Blastocystis spp., Cystoisospora belli, and Balantidium coli. Two microsporidian species, Enterocytozoon bieneusi and Encephalitozoon intestinalis, are also included.
Distribution in Developed Countries
One report (Fletcher et al., presented at the Communicable Disease Control Conference, Canberra, ACT, Australia, 2011) estimated that an enteric pathogen is isolated in an average of 40.9% (95% confidence interval [CI], 33.4 to 48.8%) of diarrheal cases in developed countries, among which enteric parasites represent less than 1% (95% CI, 1.1 to 3.5%) of cases. However, enteric parasites—mainly protozoa—are isolated from 1% to 65% of patients with diarrhea in various settings. The relative prevalences of enteric protozoa reported in several developed countries in outbreak and nonoutbreak settings among humans are reported in Table 1 (1, 11, 12, 24, 60, 85, 90, 96, 128, 144, 184, 192, 193, 204, 215, 225, 247, 248, 255, 264, 265, 301, 335, 355, 370, 395, 416, 417, 425, 442, 444, 452, 467). Giardia intestinalis (0.2% to 29.2% of cases), Cryptosporidium spp. (0.1% to 9.1% of cases), Entamoeba spp. (0.2% to 12.5% of cases), and Cyclospora cayetanensis (0.2% to 4.3% of cases) were the most common protozoa reported in developed settings. The common protozoa, however, were rivalled in prevalence by Blastocystis spp. (0.4% to 18.1%) and D. fragilis (0.4% to 6.3%). Both have the potential to cause illness but are more frequently associated with asymptomatic infection. Nevertheless, evidence from the literature suggests that Blastocystis spp. and D. fragilis have relatively high prevalences in developed settings (395; Fletcher et al., presented at the Communicable Disease Control Conference, Canberra, ACT, Australia, 2011). Infections are associated with recent travel to developing regions (394), with immigrants and refugees (164, 273), and with domestic transmission (387). The prevalence rates of D. fragilis vary widely, from 0.4% to 42%, and the incidence of this parasite was found to be second only to that of Blastocystis spp. and of similar or greater magnitude to those of the more commonly diagnosed parasites Giardia, Cryptosporidium spp., and Entamoeba spp. in many developed regions when diagnostic methods were implemented for these species. Several reports have also identified D. fragilis as the most common pathogenic protozoan found in stool when appropriate diagnostic methods are used (389). It is therefore recommended that both Blastocystis spp. and D. fragilis be considered in the differential diagnosis of gastrointestinal infections in developed settings (26).
Table 1.
Prevalence of protozoa as described by various studies from developed countries
| Reference | Study location; period | Cases and samples | Lab method(s) | Overall pathogen isolation rate (%) | No. of samples | Parasites detected (%)a,b |
|---|---|---|---|---|---|---|
| 184 | Melbourne, Australia; 1997 | Fecal specimens from community-based asymptomatic individuals | Modified iron-hemotoxylin stain to detect Giardia, Cryptosporidium, Blastocystis, Entamoeba, and other protozoa | 11.8 | 1,091 | Protozoa (8.3), Giardia spp. (1.6), Blastocystis spp. (6.0), Cryptosporidium (0.4), D. fragilis (0.4) |
| 335 | Helsinki, Finland; 1985-1986 | Diarrhea in adult out- and inpatients attending three health centers and a municipal hospital | Formalin-ether concentration method and modified Ziehl-Neelsen method for Cryptosporidium | 35.2 | 253 | G. intestinalis (2.0), Cryptosporidium sp. (2.8), E. histolytica/E. dispar (0.4) (majority of isolates were imported from other countries) |
| 452 | Madrid, Spain; 1980–1983 | Fecal samples from infected persons (50% children) in hospital | Microscopic examination for parasitesc | 46.1 | 5,022 cases | Giardia intestinalis (2.7), Entamoeba histolytica/E. dispar (<1) |
| 247 | Ljubljana, Slovenia; 1992-1993 | Patients with diarrhea | Permanent staining, Gomori's trichrome modification for Blastocystis sp., and safranin-methylene blue stain for Cryptosporidium, with bright-field microscopy | 3.7 (39/1,066 patients) | 1,066 cases, 150 controls | Blastocystis spp. (3.7 [cases] and <1 [controls]) |
| 11 | Population of England; 1993-1996 | Fecal samples from patients with diarrhea and controls | Novel real-time PCR for Giardia sp., real-time nested PCR for Cryptosporidium sp. | 20 (population), 51 (pathogen isolated) | 2,422 cases, 2,205 controls | Cases: Giardia (2), Cryptosporidium spp. (2); controls: Giardia (1), Cryptosporidium spp. (0.5) |
| 396 | Sydney, Australia; 2003–2006 | HIV-positive and HIV-negative MSM with diarrhea, presenting at general medical practice (GP) | Molecular methods, modified iron-hematoxylin stain, and a carbol-fuschin staining step to detect coccidian parasites | 23.98 (448 pathogens isolated) | 1,868 total, 628 from HIV-negative patients, 618 from HIV-positive patients, 622 from non-MSM | Entamoeba histolytica/E. dispar complex (2.9), Cryptosporidium species (1), Giardia intestinalis (2.9), Dientamoeba fragilis (0.8), Blastocystis spp.(16.9) |
| 425 | Northeast region of England; 2003–2005 | Humans | Cryptosporidium screen using auramine staining, microscopic examination for OCP | NA | 279 outbreaks, 2,889 cases tested | Cryptosporidium (3.0) |
| 90 | Ankara, Turkey; 2005–2007 | Hospital patients presenting with IBS, inflammatory bowel disease (5), and chronic diarrhea | Native Lugol's, trichrome, and Kinyoun's acid-fast staining; genomic DNA preparation; subtyping by PCR with STS primers | 18.1 (cases), 16.7% (controls) | 105 cases, 96 controls | Blastocystis (18 [cases]) and 16.7 [controls]) |
| 442 | Uppsala, Sweden; 1981 | Hospital inpatients and outpatient Swedish children of <15 years of age with acute gastroenteritis | Parasitic studies, formalin-ether concentration method; Cryptosporidium detected by light microscopy and Kinyoun's acid-fast stainingd | 68 | 416 cases, 200 controls | Giardia intestinalis (1 [cases] and 1 [controls]) |
| 355 | Brisbane, QLD, Australia; circa 1992 | 260 nonhospital patients with diarrhea sent to a private laboratory in Brisbane | Fecal culture and microscopy (trichrome staining for identification by oil immersion microscopy) | 15 | 260 specimens | G. intestinalis (1.5), Blastocystis spp. (10.8), G. intestinalis (1.5) |
| 416 | Stockholm, Sweden; 1996-1997 | Consecutive adult patients of >15 years of age with diarrhea and healthy control subjects | OCP by direct microscopy and Cryptosporidium oocysts by a modified Ziehl-Nielsen technique | 56 (cases), 16 (controls), 8 (protozoa) | 851 cases, 203 controls | Cases: E. histolytica (1), G. intestinalis (2), Blastocystis spp. (4), Cryptosporidium (2), microsporidia (<1); controls: E. histolytica (<1), Blastocystis spp. (9) |
| 417 | 42 specialized travel or tropical medicine sites located on six continents; 1996–2005 | Returned travelers diagnosed with an “infectious gastrointestinal disease” | Best available reference diagnostics; there was considerable heterogeneity between centers in terms of availability and sophistication of diagnostic (e.g., molecular) testing | 39 (65% of isolates were parasitic) | 7,442; 2,902 tested for parasites | Giardia (27.9), E. histolytica (12.5), Dientamoeba fragilis (4.0), Cryptosporidium spp. (1.1), Cyclospora (1.1), Cystoisospora belli (0.1) |
| 12 | 26 U.S. states and two Canadian provinces; 1996 | Fecal samples from infected and noninfected persons | Formalin-ethyl acetate concentration sedimentation procedure and confirmatory permanent smears when necessary | 5,250 cases | C. cayetanensis (4.3); note that 91% of infected patients had gastroenteritis symptoms | |
| 144 | Noumea, New Caledonia; 1990-1991 | Patients of all ages with diarrhea | Formalin-ether concentrates/smears stained with merthiolate-iodine-formaldehyde solution and examined microscopicallye | 40.4 | 2,088 cases | E. histolytica/dispar (3.5), Giardia intestinalis (7.8) |
| 60 | Republic of Korea; 2004–2006 | Diarrheal patients hospitalized in 96 hospitals | Enzyme immunoassay kit | 36.8 | 76,652 cases | Protozoa (2.3), Cryptosporidium spp. (0.8), Entamoeba histolytica/E. disparf (0.6), Giardia (1.7) |
| 81 | Netherlands; 1996–1999 | Stool samples from patients who consulted with a GP regarding gastroenteritis | Microscopic examination of SAF-fixated samplesg | 37.5 (cases), 9.8 (controls) | 857 cases, 574 controls | Cases: G. intestinalis (5.4), Cryptosporidium spp. (2.1), Cyclospora (0.2), Entamoeba spp. (0.9), D. fragilis (10.3); controls: G. intestinalis (3.3), Cryptosporidium (0.2), Cyclospora (0.2), Entamoeba spp. (0.7), D. fragilis (14.6) |
| 192 | Gyeonggi-do, South Korea; 2004–2006 | Gastroenteritis patients in hospital | ELISA | 3.4 | 6,071 cases | Protozoa (3.4), G. intestinalis (2.5), E. histolytica/E. dispar (0.4), Cryptosporidium parvum (0.4) |
| 301 | 16 Danish counties, Denmark; 2000-2001 | Stool samples from children of less than 5 years of age from hospitals and general practice offices | Microscopyh plus Ziehl-Neelsen acid-fast staining for Cryptosporidium and Cyclospora sp. | 54 (cases), 22 (controls) | 424 cases, 866 controls | Cases: G. intestinalis (<1), Cryptosporidium (1.7), Blastocystis spp. (<1); controls: G. intestinalis (<1), Blastocystis spp. (1.3) |
| 24 | Melbourne, Victoria, Australia; 1980–1993 | Hospitalized children (0 to 14 years old) | Microscopyi for OCP, modified acid-fast smears for cryptosporidia | 56.6 | 3,785 | Cryptosporidium (0.5), G. intestinalis (0.3) |
| 264 | Sydney, Australia; 2001 | 412 children under 6 years of age with diarrhea, either hospitalized or outpatients at The Sydney Children's Hospital | Giardia-specific enzyme immunoassay and microscopy with modified Ziehl-Neelsen stain for cryptosporidia | 33 | 412 | Cryptosporidium spp. (2.4), G. intestinalis (2.7) |
| 370 | Melbourne, Australia; 1997–1999 | Fecal samples from family units (community) of at least two children (≤15 years old) and two adults each | A modified iron-hematoxylin stain was used to detect Giardia and Cryptosporidium in concentrated specimens | 25 | 791 | Cryptosporidium spp. (1.6), Giardia intestinalis (2.5) |
| 193 | Neusiedl am See, rural eastern Austria; January to December 2007 | Patients who consulted general practitioners for gastroenteritis | Giardia intestinalis plus Cryptosporidium parvum (RIDA Quick Cryptosporidium/Giardia Combi test) | 23.2 | 306 | Protozoa (1.3), Cryptosporidium spp. (1.6), Giardia intestinalis (2.5) |
| 128 | Canadian hospital morbidity database (HMDB); 1995–2004 | Population hospitalized in Canada for gastorintestinal illness (acute, chronic, and rehabilitation care) | Not given | 21.7 | 927,645 hospitalizations | Entamoeba spp. (0.05), Giardia intestinalis (0.15), Cryptosporidium spp. (0.08) |
| 467 | England; 1993–1996 | Patients with diarrhea in the community and seen by GP | Microscopyj (12) | 24 | 1,262 | Protozoa (1.6), Cryptosporidium spp. (1.3), Giardia intestinalis (0.32) |
| 204 | Berlin, Germany; 2005–2007 | Patients of ≥18 years of age hospitalized with community-acquired gastroenteritis | SAF fixation-concentration and microscopy for OCP; direct immunofluorescence antibody test for G. intestinalis and Cryptosporidium; Kinyoun's staining method for Cryptosporidium, C. cayetanensis, and I. belli | 59.8 | 132 | Blastocystis spp. (7.6), Giardia intestinalis (7.6) |
| 255 | Parma, Italy; 1983-1984 | Hospitalized patients with acute enteritis | Microscopy | 26.2 | 797 | Protozoa (3.1), Giardia intestinalis (1.4) |
| 225 | Seattle, WA, USA; 1998–2001 | Children with diarrhea who presented to a pediatric emergency department | Trichrome stains and formalin-ethyl acetate sedimentation for OCPD and fluorescence antibody testing for Giardia and Cryptosporidium sp. | 19.5 | 656 (from 1,626 patients) | Protozoa (2.13), Blastocystis spp. (1.1), Cryptosporidium spp. (0.15), Giardia spp. (0.46), E. histolytica/E. dispar (0.15) |
| 265 | United States; 1996-1997 | National surveillance data for food-borne illness cases | Not given | Not given | Estimated 38,629,641 cases | Parasites (6.6), Cryptosporidium parvum (0.8), Cyclospora cayetanensis (0.4), Giardia intestinalis (5.2) |
| 215 | Helsinki, Finland; 1982-1983 | Fecal samples sent by GPs for routine parasitological examination | Ritchie's formalin ether concentration method, used along with a modified Ziehl-Neelsen method | Not given | 154 | Cryptosporidium spp. (9.1), Giardia (29.2), Blastocystis spp. (13), E. histolytica/E. dispar (2) |
| 1 | Tenerife, Canary Islands, Spain; ∼2004 | Clinical samples (156 stools) | Light microscopy, staining with Weber's chromotrope and PCR-hybridization for the identification of Enterocytozoon bieneusi | 11.54 | 156 | Protozoa (11.54), E. bieneusi (11.54) |
| 96 | United States (10 cities); 1998-1999 | Diarrheal stool specimens from HIV-infected patients in 3 U.S. hospitals and a database | Moura's quick-hot Gram chromotrope technique, modified trichrome blue stain (confirmed by chromotrope 2R staining and oil immersion microscopy) | 1.5 | 737 | Microsporidian species (1.5) |
| 248 | Vigo, Spain; ∼2001 | Elderly HIV-negative patients; 47 of 60 had diarrhea | Light microscopy with Weber's chromotrope-based stain and PCR-hybridization | 17.02 | 60 | E. bieneusi (17.02) |
| 448 | Central area of Brussels, Belgium; 2002-2003 | Patients suspected of suffering from a parasitic gastrointestinal illness | Bright-field microscopy and PCR | 37 | 1,207 stool samples from 448 outpatients | D. fragilis (6.3), G. intestinalis (7.1), C. parvum (1.6%), E. hystolytica (0.2%), Blastocystis sp. (9.8%), Entamoeba coli (5.4%) |
In some reports, some patients had multiple pathogens isolated; hence, the percentages may not add up to 100%.
The designation E. histolytica is reported as E. histolytica/E. dispar for consistency, unless the study's methodology indicated clearly that the test was specific for E. histolytica.
Incomplete techniques and no permanent stained smears used. The methods were described as follows. “Wet mounts of 5,022 freshly passed stools were examined for leukocytes or parasites or both. The samples which were not immediately processed were stored at 4degrees Celcius for a maximum of 36 h.”
Permanent stains were not performed for routine investigations of protozoa.
No permanent stains performed. Although the authors indicate the presence of E. histolytica, there is nothing in the article to indicate that the organisms were confirmed as E. histolytica versus E. histolytica/E. dispar.
A Ridascreen immunoassay kit was used, and this test detects E. dispar trophozoites. However, the authors reported that the organisms were E. histolytica.
Microscopic examination included a combination of (i) a wet film (iodine stained or unstained); (ii) Ridley concentration (iodine staining); (iii) modified Ziehl-Neelsen staining of Ridley concentrate; and (iv) permanent staining by hematoxylin.
Microscopy of concentration sediment wet mount for protozoa only, other than special stains for coccidia.
Microscopy for wet mount examination only, other than special stains for coccidia.
Microscopy not defined.
Asymptomatic carriage of protozoan parasites is also common in developed countries, as several types have been isolated from healthy individuals without diarrhea (0.5% to 16.5%) (90, 301). For example, about 90% of individuals infected with Entamoeba are colonized by the nonpathogenic species Entamoeba dispar, and as such, they are asymptomatic; this is true even for immunosuppressed populations (14, 385). Table 2 presents an epidemiological summary of several protozoa that have been implicated in human illnesses in developed countries, and examples of stained enteric protozoa are shown in Fig. 1. Recommended treatment regimens for enteric protozoa are presented in Table 3, based on international standards (218, 390).
Table 2.
Summary of the epidemiology of pathogenic protozoa associated with human illness
| Parasite | Disease symptom(s) | Primary host(s) | Mode(s) of transmission | Susceptible individuals |
|---|---|---|---|---|
| Cryptosporidium spp. | Diarrhea | Humans, other mammals, and birds | Oocysts in water and on uncooked or undercooked food; person to person; zoonotic | Animal handlers, travelers, MSM, caterers, day care staff |
| Cyclospora cayetanensis | Diarrhea | Humans and other mammals | Oocysts in water and on uncooked or undercooked food; person to person | Travelers to nonindustrialized countries (South America); major food and water outbreak risk |
| Giardia intestinalis | Diarrhea, malabsorption | Humans, other mammals, and birds | Cysts in water and on uncooked or undercooked food; person to person; zoonotic | Young adults, MSM, day care staff |
| Entamoeba histolytica | Dysentery, liver abscess | Humans and other mammals | Cysts in water and on uncooked or undercooked food; person to person; zoonotic | Immigrants/travelers to areas of endemicity, MSM, HIV patients, and institutionalized persons |
| Blastocystis sp. | Abdominal pain and diarrhea | Humans and other mammals | Cysts in untreated or minimally treated water and on uncooked or undercooked food; person to person; zoonotic | Anyone, especially in child care centers or other institutional settings |
| Dientamoeba fragilis | Diarrhea | Humans | Fecal-oral; uncertain | Children and adults, both immunocompetent and immunosuppressed populations |
| Cystoisospora belli | Diarrhea | Humans | Oocysts in contaminated water or food; person to person | Travelers to nonindustrialized countries, AIDS patients, and indigenous populations (United States) |
| Balantidium coli | Diarrhea, dysentery | Humans, pigs, nonhuman primates, cats, rodents | Cysts in untreated or minimally treated water and on uncooked or undercooked food; person to person; zoonotic | People living in close proximity to pigs, travelers to nonindustrialized countries (Southeast Asia, Western Pacific Islands, rural South America) |
| Microsporidia | Persistent diarrhea | Humans and other mammals | Ingestion of spores; person to person; zoonotic | Immunosuppressed and HIV/AIDS patients, immunocompetent patients, travelers |
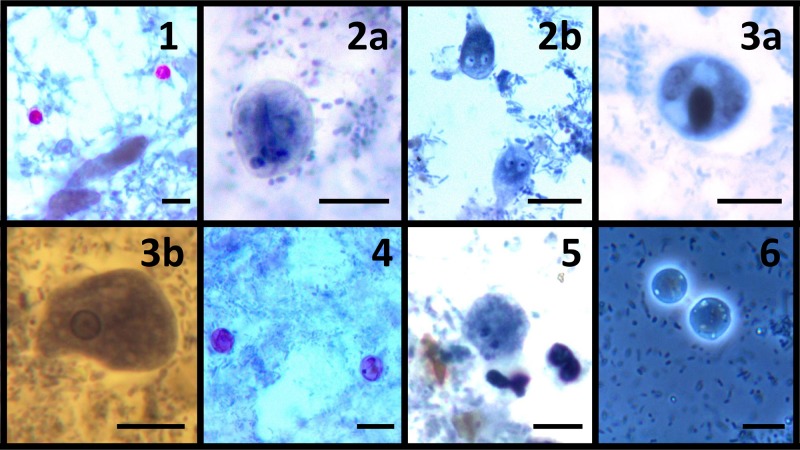
Fig 1.
Photomicrographs of six enteric protozoa. Plates 1 to 5 were stained with a modified iron-hematoxylin stain (incorporating a carbol fuschin staining step). Plate 6 was a wet preparation. (1) Cryptosporidium oocysts; (2a) Giardia intestinalis cysts; (2b) Giardia intestinalis trophozoite; (3a) Entamoeba histolytica cyst; (3b) Entamoeba histolytica trophozoite; (4) Cyclospora cayetanensis oocysts; (5) Dientamoeba fragilis binucleated trophozoite; (6) Blastocystis oocysts. Bars, 10 μm. (All graphics by Damien Stark.)
Table 3.
Treatment options for infections with enteric protozoaa
| Intestinal parasite or disease | Antimicrobial therapy (dosing) |
|---|---|
| Blastocystis spp. | Nitazoxanide (500 mg twice a day for 3 days)b |
| Cryptosporidium spp. | Nitazoxanide (500 mg twice a day for 3–14 days); for AIDS-associated infections, include HAART |
| Cyclospora cayetanensis | Cotrimoxazole (TMP-SMX; 160 mg trimethoprim plus 800 mg sulfamethoxazole, twice a day for 7 days), pyrimethamine (50–75 mg daily) and leucovorin (5–10 mg daily), or ciprofloxacin (500 mg twice a day); for non-AIDS patients receiving TMP-SMX, use 1 double-strength tablet orally twice daily for 7–10 days; for AIDS patients receiving TMP-SMX, use 1 double-strength tablet orally four times daily for 10 days, followed by twice a day for 3 weeks |
| Dientamoeba fragilis | Iodoquinol (650 mg three times a day for 20 days), metronidazole (500–750 mg three times a day for 10 days), or paromomycin (25–35 mg/kg of body weight/day for 7 days) (271); for treatment failures, tetracycline (500 mg orally four times daily for 10 days) plus iodoquinol (650 mg orally three times daily for 10 days) |
| Entamoeba histolytica invasive disease (amoebic colitis, amoebic liver abscess, or disseminated disease) | Luminal agent, i.e. paromomycin (8–12 mg/kg or 500 mg orally three times a day for 7 days) or iodoquinol (650 mg orally three times a day for 20 days) |
| Entamoeba histolytica/E. dispar complex intestinal disease | Paromomycin (8–12 mg/kg or 500 mg three times a day for 7 days) or iodoquinol (650 mg orally three times a day for 20 days) |
| Giardia intestinalis | Metronidazole (250 mg daily for 3 days), tinidazole (2-g single dose), or albendazole (200–400 mg twice a day for 5 days) |
| Cystoisospora belli | Cotrimoxazole (160 mg trimethoprim plus 800 mg sulfamethoxazole, four times a day for 10 days) or ciprofloxacin (500 mg twice a day for 7 days); for non-AIDS patients receiving TMP-SMX, use 1 double-strength tablet orally twice daily for 10 days; for AIDS patients receiving TMP-SMX, use 1 double-strength tablet orally four times daily for 10 days, followed by twice a day for 3 weeks |
| Microsporidia | |
| E. bieneusic | Fumagillin (20 mg three times a day for 14 days) |
| E. intestinalis | Albendazole (400 mg twice a day for 28 days) |
EPIDEMIOLOGY, DIAGNOSIS, AND TREATMENT
Cryptosporidium Species
Cryptosporidium was first recognized as an important cause of infection in AIDS patients (410). It is now well recognized and accounts for about 20% of diarrheal episodes in children in developing countries and up to 9% of episodes in developed settings and causes a considerable amount of diarrheal illness in young farm animals worldwide (340, 473). The environmentally resistant oocysts are fully sporulated when they are excreted in feces, and therefore they are immediately infectious (116, 441, 477). Infections are usually characterized by self-limiting diarrhea associated with severe abdominal pain in both immunosuppressed and immunocompetent persons, especially HIV-infected persons and children worldwide (16, 116, 390). The duration of clinical symptoms is highly dependent on the person's immunological competence (235). It is still an important cause of potentially life-threatening diarrhea in HIV-infected patients with limited access or poor compliance with highly active antiretroviral therapy (HAART) (228, 326). Cryptosporidium spp. are of importance to transplant patients, especially in regions where the organisms are endemic, where they can cause life-threatening prolonged diarrhea, dehydration, and malabsorption in transplant recipients (100). Species of Cryptosporidium and subtype families of Cryptosporidium hominis have been shown to induce different clinical manifestations and have different potentials to cause outbreaks (473). In developed countries, transmission occurs from person to person, especially in day care settings and between men who have sex with men (MSM), as well as through waterborne and zoonotic infections (473). Cryptosporidium spp. have been identified as a common cause of diarrhea in persons from developed countries visiting less developed areas (343, 473, 474, 476). Animal models have demonstrated the role of Cryptosporidium parvum in the formation of polyps and adenocarcinoma lesions in the guts of dexamethasone (Dex)-treated severe combined immunodeficiency (SCID) mice, suggesting the need to investigate whether a similar C. parvum-induced gastrointestinal cancer occurs in humans (50–52, 74, 459).
In developed countries, sporadic outbreaks due to fecal-oral transmission have been reported among children attending playgroups and day care centers and by ingestion of contaminated salads, contaminated water supply, or recreational water, contact with sick animals, swimming in public pools, and person-to-person transmission (55, 66, 325, 340). Epidemiological variations have been observed in the geographical, seasonal, and socioeconomic effects of the distribution of Cryptosporidium spp. in humans that may influence the sources and routes of transmission (473). Cryptosporidium spp. have a wide host range and cause infections in humans, livestock, domestic pets, and wildlife, among all four classes of vertebrates, and, most likely, in all mammalian species (55, 89, 290, 345, 441). Most animals are not infected with human-pathogenic species and thus play no role in zoonotic transmission of cryptosporidiosis. However, zoonotic transmission from direct contact with infected animals or their feces (55, 89, 345, 476) or indirectly through the consumption of contaminated water (63, 66, 327, 476) can occur.
The small size and subtle staining characteristics of Cryptosporidium spp. have contributed to the difficulties of identifying these parasites in routine stool preparations (116, 375). The diagnosis of cryptosporidiosis is generally undertaken by identification of oocysts in stool of the host/patient. Traditionally, the identification of Cryptosporidium oocysts was based on microscopic examination (300, 330). However, the identification of morphological characters of Cryptosporidium is unreliable and relatively time-consuming, even with light microscopy (209). Staining and preservation methods have been used to enhance the sensitivity of tests. Unfortunately, these do not identify species, and their analytical sensitivity can be poor, especially for samples containing small numbers of oocysts (138, 253, 329). The modified Ziehl-Neelsen technique (413) is used widely; however, one recent study found that it was less sensitive than PCR (75.7%; 95% CI = 68.3 to 81.8%) but was highly specific (100%; 95% CI = 96.5 to 100%) (56). Kinyoun's acid-fast staining technique (102), modified Sheather's flotation technique (76, 294, 296), and the iron-hematoxylin staining technique (119) have also been described.
Antigen detection by immunoassays has been used widely in the diagnosis of cryptosporidiosis, as these assays are thought to be more sensitive than conventional staining and more effective in cases where oocyst numbers are low (139, 141, 329). An evaluation of the Meridian Premier Cryptosporidium test and the Alexon ProSpecT Cryptosporidium microplate assay found that both systems performed according to the manufacturers' values for sensitivity and specificity (for the Meridian test, 91 and 99%, respectively; and for the Alexon test, 97 and 100%, respectively) (139). Highly rapid immunoassays have also been developed that can be used with fresh, frozen, or unfixed human fecal specimens. For example, the Biosite Diagnostics (San Diego, CA) Triage rapid qualitative enzyme immunoassay (EIA) for the detection of Giardia, E. histolytica/E. dispar, and Cryptosporidium antigens has been demonstrated to have a 98.3% sensitivity and 99.7% sensitivity in the detection of Cryptosporidium spp. from stool specimens (140). A multicenter French study evaluated the sensitivity and specificity of four immunochromatographic (ICT) assays and found that RIDAQuick (mean sensitivity, 73.3%), Remel Xpect (mean sensitivity, 74.1%), and ImmunoCard STAT (mean sensitivity, 73.3%) were fairly sensitive for detecting C. parvum and C. hominis but were very limited in detecting other Cryptosporidium spp. (3). Fluorescence microscopy and direct fluorescent-antibody (DFA) assay have been used with relatively high specificities (96 to 100%) and sensitivities (98.5 to 100%) for the detection of Cryptosporidium oocysts in clinical and environmental samples (139, 158, 209). However, the sensitivity and specificity of monoclonal antibody (MAb)-based DFA assays have been affected by different factors, such as the purity of the Cryptosporidium antigen originally used to raise the MAb (139, 158, 209, 214, 329).
Molecular methods have been developed for the detection and differentiation of Cryptosporidium spp. at the species/genotype and subtype levels (9, 168, 290, 372, 386). These methods, including a nested PCR (348), real-time PCR, multiplex real-time PCR, reverse transcription–quantitative real-time PCR (372, 455), and multiplex tandem real-time PCR (386), have been used for identification of species in diarrheal stools in relatively short time frames. More recently, an automated multiplex tandem PCR using a robotic platform to simultaneously detect Cryptosporidium spp. and coinfecting diarrheal pathogens from human fecal genomic samples was described. The assay was rapid (taking <2 h, with ∼5 to 10 min of technical work following the extraction of genomic DNA), and due to automation, data analysis required little molecular biological expertise, making it well suited to various diagnostic facilities and settings (e.g., hospitals or quarantine facilities) (210). Other tools, based on the Cryptosporidium oocyst wall protein (COWP) gene, have been used to amplify DNAs of C. parvum, C. hominis, Cryptosporidium meleagridis, and species and genotypes closely related to C. parvum (9, 242). However, it has been suggested that these methods have limited usefulness in genotyping Cryptosporidium spp. in animals because of their narrow specificity (342, 473). The disadvantage of some PCR tools is that they are designed to detect the dominant Cryptosporidium genotype in the specimen and require a substantial amount of PCR product to be visible on an agarose gel, and when specific genotyping and subtyping tools are used, they fail to detect concurrent infections with mixed Cryptosporidium species/genotypes or fail to detect other divergent species/genotypes (168). New methods such as reverse line blot (RLB) hybridization, isothermal methods such as the loop-mediated isothermal amplification method (319, 372), and nucleic acid sequence-based amplification (NASBA) methods that amplify RNA from either RNA or DNA templates also provide additional diagnostic platforms for the detection of Cryptosporidium spp. (154, 372).
Cryptosporidiosis is usually self-limiting in immunocompetent persons, requiring little or no treatment, but it is especially challenging to treat among high-risk immunosuppressed groups (39, 83). Nitazoxanide has proven effective in the treatment of immunocompetent patients (133); however, a higher dosage and longer duration of treatment have been indicated for immunosuppressed patients (39, 129). Rehydration fluids and nutritional management may be required for immunosuppressed persons with dehydration (57, 83).
Dientamoeba fragilis
There has been much debate about the pathogenicity of Dientamoeba fragilis (26, 390). Unlike the case for other protozoa, a cyst stage has not been demonstrated, and trophozoites degenerate within hours of being passed in stool (26, 391–393). Although the mode of transmission remains unknown, based on high rates of coinfection with Enterobius vermicularis, it was suggested previously that infection occurs via the pinworm vector (25, 153, 191). Recent studies have discounted this idea (229). However, high rates of coinfection with other enteric pathogens and protozoa suggest that transmission occurs directly via the fecal-oral route (26, 191). Infection may be acute or chronic and has been reported in both children and adults and both immunocompetent and immunosuppressed populations (26, 127, 361, 389, 392, 444). The most common clinical symptoms include abdominal pain, persistent diarrhea, loss of appetite, weight loss, and flatulence (22, 355, 389, 444). These symptoms are akin to those of irritable bowel syndrome (IBS), so D. fragilis should be considered in the differential diagnosis of IBS (399). Dientamoeba fragilis infection also presents with similar symptoms to those of Giardia infection and is estimated to occur with a similar or greater prevalence to that of Giardia infection in Belgium (444) and Australia (127, 355, 394, 395, 401). Dientamoeba fragilis trophozoites have been reported in nonhuman primates, but the limited host range suggests that human infection may not involve transmission from other animal species (396).
Traditional diagnosis relies on the microscopic detection of trophozoites in fresh or fixed stool specimens. The demonstration of the characteristic nuclear structure of D. fragilis needed for a definitive diagnosis cannot be achieved with unstained fecal material (392); as such, prompt fixation and permanent staining are necessary for definitive diagnosis (355, 388, 391). Microscopy of fixed smears with permanent staining (modified iron-hematoxylin or trichrome staining) (127, 393, 403) is considered the gold standard for diagnosis of D. fragilis infection, but this method is time-consuming and relatively insensitive compared to molecular methods (391). More recently, diagnostic tests based on various conventional and real-time PCRs to detect the small-subunit (SSU) rRNA gene of D. fragilis have been developed to facilitate rapid, sensitive, and specific diagnosis in fresh stools (386, 388, 401, 455), and the successful culture and cryopreservation of viable D. fragilis trophozoites from clinical specimens have been achieved (27, 355). It was demonstrated that when microscopy is combined with other methods, the success of detection of D. fragilis is increased significantly (26, 386, 388). However, while molecular methods are more sensitive than microscopy and staining, many of the necessary products are not commercially available and the methods are not employed routinely, even in developed countries.
Treatment is recommended in symptomatic patients and asymptomatic family members to prevent reinfection (153, 444). Various antimicrobial therapies have resulted in the successful clearance of D. fragilis and total resolution of gastrointestinal symptoms in infected patients (152, 387, 445). Paromomycin, secnidazole, iodoquinol, tetracycline, ornidazole, and metronidazole have been used successfully to treat D. fragilis infections (22, 152, 305, 445). However, there is emerging evidence of treatment failures of metronidazole among D. fragilis isolates, suggesting the increased need for combination therapy for these protozoa (22, 389). Combination therapy has been effective in the complete eradication of the parasite and in resolution of symptoms (387, 389).
Entamoeba Species
Six species of the genus Entamoeba have been described in humans, including Entamoeba histolytica, Entamoeba dispar, Entamoeba moshkovskii, Entamoeba poleki (also called Entamoeba chattoni), Entamoeba coli, and Entamoeba hartmanii. Among these, E. histolytica is the only pathogenic species (201, 236, 351). Improvements in the understanding of the biochemical, immunological, and genetic differences of the members of this genus have resulted in the confirmation of three species, E. histolytica, E. dispar, and E. moshkovskii, that are morphologically identical in both their cyst and trophozoite stages (41, 169). The vast majority (about 90%) of individuals infected with Entamoeba spp. are colonized by the nonpathogenic strain E. dispar (324, 385). In developed countries, infections of E. histolytica (a true pathogen) are largely confined to immigrants from or travelers to areas of endemicity, MSM, HIV-infected patients, and institutionalized populations (164, 174, 315). The WHO reports that approximately 500 million people worldwide are infected annually with E. histolytica, resulting in symptomatic illnesses and death in about 50 million and 100,000 persons, respectively (470). However, it is believed that since 90% (450 million) of infections are due to E. dispar, while 10% (or 50 million) are infections with E. histolytica, the worldwide incidence of invasive disease is more likely to be 5 million cases annually, with global mortality still at 100,000 per annum (201). Approximately 4 to 10% of carriers infected with E. histolytica develop clinical disease within a year, and amoebic dysentery is considered the third leading cause of death from parasitic disease worldwide (178, 280, 385). Entamoeba histolytica causes a range of disease manifestations, including (i) dysentery or dysenteric syndrome, characterized by small volumes of bloody, mucoid stools without fecal leukocytes; (ii) amoebic colitis, characterized by ulcerations of the colonic mucosa with typical flask-shaped abscesses; (iii) amoeboma, the formation of a fibrotic mass in the intestinal wall; and (iv) invasive disease, resulting in amoebic abscesses in the brain, lung, or liver (84, 315, 324, 449). Amoebic brain abscess occurs when Entamoeba histolytica trophozoites travel to extraintestinal tissues through the bloodstream, invading the central nervous system and producing amoebic brain abscesses that are frequently lethal (257). Other rare forms of amoebiasis have been reported, including cutaneous amoebiasis (amoebiasis cutis), arising as a complication of amoebic dysentery (232, 323). This usually results from contamination of damaged skin or continuous contact with exudates containing virulent trophozoites that stick to the traumatized skin, such as in the perianal or perigenital area, resulting in lysis of the skin and subcutaneous tissue and, subsequently, ulceration and necrosis (36, 323, 454).
While asymptomatic E. histolytica infections are equally distributed between the genders, invasive disease is more common in men (which may be due to a male-related susceptibility to invasive disease). Higher rates of carriage have been observed in HIV-infected MSM in the Asia Pacific region (194, 203, 280, 400). Patients with amoebic colitis present with a gradual onset of bloody or profuse watery diarrhea and abdominal pain and tenderness (324, 385). Symptoms may last for several weeks, and fulminant necrotizing colitis, the most severe form of intestinal disease, is often fatal (174, 324, 379). In children, <40% of patients present with fever and rectal bleeding without diarrhea. Some patients develop fulminant amoebic colitis, with profuse bloody diarrhea, fever, pronounced leucocytosis, and widespread abdominal pain, often with peritoneal signs (280, 399).
The virulence of pathogenic E. histolytica is based on its ability to secrete enzymes and proteases that contribute to the invasion of the epithelial cells penetrating the intestinal mucosa and to degradation of the extracellular matrix proteins and subsequently to interfere with the host's humoral immune response (14, 315). More recently, the emergence of clear roles for human and parasite genetics and environmental factors in the virulence of E. histolytica has increased our understanding of infections. It is thought that some persons are genetically resistant to infection, while malnourished children are more susceptible, and a polymorphism in the leptin receptor increases susceptibility to amoebiasis in both adults and children (14, 277, 332). The evidence suggests that not all strains are capable of causing liver abscess, and the observed higher incidence in men may be a result of gender-based differences in the complement system (379). For example, higher gamma interferon and functional natural killer T cell levels in females might underlie their resistance to liver abscess (280, 332). Based on the many presentations of amoebiasis, a high level of clinical suspicion is necessary for the early diagnosis of invasive and extraintestinal amoebiasis (221, 454). The specific diagnosis of E. histolytica infection is important in order to minimize undue treatment of individuals infected with nonpathogenic species of Entamoeba (131).
Diagnosis is difficult based on the fact that the pathogenic species E. histolytica is morphologically identical to the nonpathogenic species E. dispar and E. moshkovskii; hence, microscopy is generally considered insufficient for differentiation of these species (397). Various methods are employed in the diagnosis of Entamoeba. In many countries, microscopy is widely used for protozoan identification (131, 204, 249). Microscopic techniques utilized in the clinical laboratory include the screening of wet preparations, concentrated samples, and permanently stained smears for the identification of E. histolytica/E. dispar/E. moshkovskii in feces (131). The CDC recommends that in order to maximize the recovery of cysts, stool samples in formalin or other fixatives should be concentrated prior to microscopic examination (49). Wet mount preparations and trichrome-stained smears of stool specimens are routinely used for identification of E. histolytica/E. dispar (49). Various fixatives utilized for the preservation of the morphology of the parasites can be used in conjunction with various stains. The pathogenic species E. histolytica can sometimes be differentiated from the nonpathogenic, morphologically identical species by microscopy on the basis of ingested red blood cells within the cytoplasm of the trophozoites; however, this phenomenon does not occur commonly and is rarely seen in clinical samples (49, 131). Therefore, when a definitive diagnosis is not possible via microscopy, the presence of the E. histolytica/E. dispar/E. moshkovskii complex should be reported (49). Microscopy is less reliable at identifying Entamoeba species than culture or antigen-based and molecular tests (179, 410).
While cultivation is more sensitive than microscopy and isoenzyme analysis can effectively distinguish between E. dispar and E. histolytica, these methods are time-consuming, are not cost-effective, and are not routinely utilized by most diagnostic laboratories (132, 177). EIA kits are commercially available that detect E. histolytica only, while others detect both E. histolytica and E. dispar (49, 131, 460). A rapid ICT assay is available that detects antigens of E. histolytica and E. dispar in stool; however, this assay does not distinguish between E. histolytica and E. dispar (49). This assay also detects antigens of Giardia and Cryptosporidium. Borderline positive results and questionable negative results obtained by this technique should be confirmed further by additional testing. This assay is quick and easy to perform, and no special equipment is needed (49). EIAs and rapid ICT tests require the use of fresh or frozen stool specimens and cannot be used with the majority of preserved specimens because antigens are lost during the concentration procedure (49, 397). However, some newer single-vial collection systems that utilize nontraditional fixatives can be used in these assays. Many stool antigen assays have been shown to be as sensitive and specific as culture with isoenzyme analysis and outperform microscopy for the detection of E. histolytica in areas of endemicity (177, 179, 316). One study found that in comparison to PCR, some stool antigen tests lacked sensitivity but were highly specific in diagnosing E. histolytica/E. dispar in areas where these parasites are not endemic (460), while another study found that antigen detection tests can be both rapid and technically simple to perform (177).
A number of molecular assays have been described in the scientific literature for detection and/or differentiation of Entamoeba species and are now considered the gold standard for diagnosis (177, 397). Both conventional and real-time PCRs have proven to be more sensitive and specific than microscopic examination for the detection of E. histolytica and E. dispar in single stool samples (455, 460). In developed settings, PCR is more useful for detection of E. histolytica in stools than antigen detection tests due to the higher sensitivities observed for PCR and the reduced chance of cross-reactivity with other Entamoeba species (167, 397, 460). However, PCR has yet to become the mainstream for the detection of parasites in the clinical laboratory, even in developed countries, due to the need for specialized equipment, dedicated molecular areas, specified workflow, and associated costs. It must be emphasized that when species differentiation is not possible, the E. histolytica/E. dispar complex should be reported.
Serological methods such as indirect hemagglutination (IHA), latex agglutination, immunoelectrophoresis, immunofluorescence assay (IFA), and enzyme-linked immunosorbent assay (ELISA) are highly sensitive at detecting E. histolytica antibodies in human serum and are useful for detecting invasive disease (41, 131, 236). However, serology is of limited use in areas of endemicity because of the difficulty in distinguishing between past and present infections (167, 236); hence, it is of more value in nonendemic settings in establishing the diagnosis of E. histolytica infection if antibodies are present (460). A combination of microscopy, culture, and serology should be complemented with a PCR assay or with abdominal imaging (when PCR is unavailable) for detection of invasive disease (41, 131). Diagnosis of amoebic brain abscesses usually requires computerized axial tomography scans of the brain (257). Identification of E. histolytica antigens in tissue aspirates or PCR analysis of tissue aspirates is also effective (390).
All infections with E. histolytica should be treated because of its potential for causing invasive disease and the risks to public health (110, 324, 385). Asymptomatic carriers should be treated with a luminal agent to minimize the spread of disease. The treatment of choice differs for intestinal and invasive disease; hence, diagnosis is important before treatment begins. Metronidazole is a highly effective tissue amoebicide and is used in the treatment of invasive amoebic disease. However, a luminal agent should be used in combination, as treatment does not eliminate intestinal colonization in up to 50% of patients with invasive amoebiasis, resulting in relapse of invasive infection months later (174, 222, 236). Other nitroimidazole derivatives, such as tinidazole and ornidazole, are equally effective for treatment of invasive disease (218, 324). The luminal agents paromomycin, iodoquinol, and diloxanide furoate are strictly recommended for treatment of patients with intestinal and asymptomatic infections, as they are effective in eliminating cysts from the intestinal tract (174, 218).
Giardia intestinalis
Giardia intestinalis (syn. Giardia duodenalis and Giardia lamblia) is a common cause of parasitic diarrhea, with prevalences ranging from 2 to 7% in developed countries to 20 to 30% in most developing countries worldwide (134, 207). Because of the burden of illness from Cryptosporidium spp. and Giardia, their ability to impair development and socioeconomic improvements, and their associations with poverty, they were included in the WHO Neglected Diseases Initiative in 2004 (354). Giardia intestinalis consists of seven genetically distinct genotypes, designated A to G, but a novel lineage designated assemblage H has been identified in marine vertebrates (233). Assemblages A and B infect mammalian species and are the only two assemblages known to infect humans; hence, they are considered to be zoonotic (118, 134, 146, 196, 236, 262, 433). Recent developments in the study of protein coding capacities have found genomic differences between strains WB (assemblage A) and GS (assemblage B), which may explain some of the observed biological and clinical differences between the two isolates. These observations led to the suggestion that Giardia assemblages A and B may be two different species (134, 207, 285).
It has been suggested that Giardia trophozoites may remain in the small intestine for weeks to years (315). However, this is unsupported, since many long-term infections are suspected to represent either persisting abdominal symptoms elicited post-Giardia infection or reinfections (176, 196, 484). The fecal-oral route still remains the most important mode of infection (33), and various studies have found evidence of zoonotic transmission (89, 118, 237). The symptoms of giardiasis can be variable, but it presents mainly as acute or chronic diarrhea associated with abdominal pain, nausea, malabsorption, and weight loss. In malnourished children, infection can lead to growth retardation, and asymptomatic illness is also possible (37, 40). There have been a few reports suggesting that giardiasis may be a risk factor for zinc deficiency in school-aged children (80, 328). High rates of asymptomatic carriage of Giardia have been reported for humans and animals in developing settings (213).
In developed settings, Giardia intestinalis has also been isolated from various animals, including livestock (17, 31, 65), fish (475), and nonhuman primates (262). Giardia infestation is common in dogs and cats, with prevalences of approximately 8% to 16% and 4% to 11%, respectively (44, 302). While there is limited evidence for direct transmission from companion animals to people, rare infections and the isolation of zoonotic genotypes from cats and dogs suggest that they are a potential source of human infection that may be acquired through handling, sleeping together, licking, and kissing (61, 308, 346, 424, 480). Giardiasis is frequently associated with waterborne and day care center disease outbreaks and is related to travel-associated diarrhea (471). A study of travel- and migration-associated illnesses in Europe revealed that G. intestinalis was the second most common pathogen and the most common parasite as a cause of gastrointestinal illnesses (122). Giardia is frequently isolated from diarrheal stools from MSM, with or without HIV/AIDS, in whom transmission is most likely via the fecal-oral route (33, 324). In the United States, it is suspected that seasonal peaks are related to increased use of recreational water venues, such as lakes, rivers, swimming pools, and water parks, in summer months (188).
Diagnosis is usually based on the microscopic detection of Giardia cysts or trophozoites in a stool specimen. Stools may be examined either directly as fresh smears, preserved in formalin or polyvinyl alcohol and stained with iodine, trichrome, or hematoxylin, or after formalin-ethyl acetate concentration (222, 236, 262, 289, 305, 457, 465). Several antigen detection assays are available for G. intestinalis, including EIAs, ELISAs, and monoclonal antibody and direct fluorescent-antibody tests, which are widely used (130, 140, 141, 145, 234, 431). Many have proven to be cost- and labor-effective, and many of them are designed for the sensitive and specific testing of several protozoa simultaneously (140, 141, 465). These antigen detection tests are useful in screening settings and are more sensitive than routine microscopic examinations for detection of ova, cysts, and parasites (OCP) (137). For example, one rapid qualitative EIA has been demonstrated to have a 95.9% sensitivity and 97.4% sensitivity for the detection of Giardia in stool specimens (140). The use of real-time PCR as a tool for the detection of Giardia is increasing in developed settings. Conventional single, nested, and multiplex PCRs have also been developed but are utilized more often in specialized centers involved in molecular studies of Giardia (40, 236, 475). These molecular methods have proven to provide higher sensitivity than conventional methods; however, many of them are still not commercially available (236). The use of PCR in molecular epidemiology studies has advanced the knowledge of the population structure of Giardia, which has improved the understanding of zoonotic transmission and the molecular epidemiology of giardiasis (430).
Treatment is usually the same for both immunosuppressed and immunocompetent patients (83). Metronidazole or tinidazole has been used as the therapy of choice against giardiasis; however, treatment failures and clinical relapses have been known to occur (37) and could be due to the emergence of resistant isolates of Giardia (37). Furazolidone, albendazole, nitazoxanide, and paromomycin are suitable alternatives when available (83). One vaccine (GiardiaVax) has been licensed for use in the United States to prevent clinical disease in dogs and to significantly reduce the incidence, severity, and duration of cyst shedding in cats (267). However, more recent data have shown limited vaccine efficacy (15), and no human vaccines are currently available (434).
Cyclospora cayetanensis
Cyclospora cayetanensis has emerged as an important cause of endemic or epidemic diarrheal illness in children and adults worldwide (53). Cyclospora cayetanensis is the only species of this genus found in humans and is host specific (79). An important feature of the biology of C. cayetanensis is that oocysts excreted in feces require days to weeks outside the host to sporulate and to become infectious; hence, direct fecal-oral transmission from relatively fresh stool does not occur (189, 259). Clinical illness is characterized by persistent diarrhea, bloating, flatulence, abdominal cramps, constipation, and fatigue (305). Illness associated with travel to nonindustrialized countries has been reported in the United Kingdom (291) and Netherlands (456) and is a common cause of illness among returned international travelers (417). However, non-travel-related and waterborne cases of cyclosporiasis have been reported in several developed countries (12, 450). Food-borne outbreaks have been reported in North America (189, 291, 450) and Germany (92). Infections in immunosuppressed patients have been reported in Turkey (350), and although symptoms may be similar to those in immunocompetent individuals, they may be prolonged (410). Of the 1,110 laboratory-confirmed cases of sporadic cyclosporiasis captured by U.S. disease surveillance over the 1997-2008 period, approximately one-third of cases occurred in persons with a known history of international travel, the vast majority of whom traveled to countries in Southern and Central America (173). Domestically acquired cases were concentrated in time (late spring and summer) and were probably linked to an undetected outbreak (173).
Standard laboratory procedures for ova and parasites do not identify Cyclospora; therefore, the laboratory must be notified when Cyclospora is being considered (368, 461).
Diagnosis is made by the demonstration of Cyclospora oocysts by examination of their autofluorescence and staining characteristics. Oocysts appear as acid-fast round or ovoid structures and autofluoresce white-blue under an epifluorescence microscope, using a 330- to 380-nm dichromatic (DM) excitation filter, or blue or green with a 450- to 490-nm DM filter (53, 137, 305, 306, 330, 456). However, detection is often difficult because the pathogen is very small, measuring 8 to 10 μm in diameter; hence, oocyst measurement is important to differentiate the oocysts of this species from smaller Cryptosporidium oocysts, which measure 4 to 6 μm (137, 259, 330). The oocysts can be seen in concentrated or nonconcentrated feces by light microscopy using various stains, but the disadvantage is that C. cayetanensis oocysts stain only variably with Gram, Giemsa, and hematoxylin-eosin stains and results are variable with modified Ziehl-Neelsen staining (259, 368, 456). A safranin-based stain has been described that uniformly stains oocysts of Cyclospora a brilliant reddish orange when the smears are heated in a microwave oven prior to staining (305, 461). Other routine procedures in use include concentration by the formalin-ethyl acetate technique followed by either (i) UV epifluorescence and bright-field microscopy, (ii) examination of a modified acid-fast-stained stool slide, or (iii) examination using a modified safranin-based technique (151, 307, 368, 377, 461). Samples can be stored in 2.5% potassium dichromate for 14 days at temperatures ranging from 22 to 37°C for sporulation or molecular detection (306, 377). In comparison to UV detection, the sensitivity of the acid-fast technique is about 78% (93).
Various molecular techniques have been developed for the identification of Cyclospora. These include spectrophotometry-based detection with an oligonucleotide ligation assay. Various PCR tools have been used that target the internal transcribed spacer region, using primers for the 18S rRNA gene (93, 306). These include conventional PCR, reverse transcriptase PCR in combination with agarose gel electrophoresis, and nested PCR (281, 304, 306, 314, 450). Some of these techniques for the identification of C. cayetanensis are time-consuming, labor-intensive, and subject to problems of contamination; hence, further studies are needed for the development of highly sensitive rapid tests for this protozoan. On a cautionary note, it was reported that PCRs for Cyclospora cross-amplify DNAs from other coccidia, especially those belonging to the genus Eimeria. This cross-reactivity with Eimeria is problematic mainly in outbreak settings and with environmental specimens, since human infections by Eimeria are currently unknown (259, 314). A restriction fragment length polymorphism (RFLP) protocol is required to distinguish between these species in environmental samples (259).
Laboratory diagnosis is important before empirical treatment commences, since the organism requires different treatment from that for some of the other protozoa of similar presentation (368). Trimethoprim-sulfamethoxazole (TMP-SMZ; also known as cotrimoxazole) is the drug of choice for managing cyclosporiasis. Infection can be treated effectively with cotrimoxazole for 10 days, with results occurring in a few days (218, 222, 306). Oral or intravenous rehydration may be appropriate, based on the degree of dehydration (368).
Blastocystis Species
Blastocystis spp. are commonly found in stools from humans and numerous animal hosts (341, 418). There is considerable genetic heterogeneity within Blastocystis organisms, and currently, human, mammalian, avian, and reptilian isolates have been assigned to 1 of 13 subtypes (312, 341, 384, 402). While it is unclear whether any of these subtypes are specific to human disease, Blastocystis sp. subtype 3 is most commonly associated with illness in human prevalence studies (90, 341, 418, 479). The name Blastocystis hominis refers to approximately 10 different genetically diverse populations that are all morphologically identical and cannot be differentiated on the basis of microscopy alone (406, 435). In many reports, B. hominis refers to the parasite isolated from humans and Blastocystis spp. represent those isolated from other animal hosts (293). Based on the discrepancies in the use of these terms, several experts in the field have come to a consensus that based on published small-subunit rRNA gene analyses, all mammalian and avian isolates should be designated Blastocystis spp. and assigned to one of nine subtypes (408). Knowledge about the life cycle of Blastocystis spp. remains elusive, and various morphological forms have been described (82, 320). These are the vacuolar, granular, amoeboid, and cyst forms, and other, less frequently encountered forms are the avacuolar and multivacuolar cells and cells containing filament-like inclusions (191, 274, 371, 409, 419).
The role that Blastocystis spp. play in eliciting gastrointestinal pathology and symptoms remains uncertain and controversial (191, 421). Boorom et al. argue that there may be some pathogenic variants of Blastocystis spp. and that the limitations in their detection by existing diagnostic methods may have added to the controversy about their pathogenicity (32). Another thought is that the amoeboid form of Blastocystis spp. could be either an indicator of or contributor to the pathogenicity of these protozoa in patients exhibiting symptoms (422), but this suggestion remains unsupported (341, 420). However, more recent in vivo and in vitro studies suggest that some of the “strains” or potentially different species of this organism may be pathogenic (32, 399, 418, 479). The genetic and antigenic diversity among Blastocystis spp. complicates the epidemiological understanding of these parasites; however, emerging evidence—despite still being controversial—suggests that pathogenicity may be subtype dependent (419, 421). It is thought that the cyst is probably the infectious form and is transmitted by the fecal-oral route: when the parasite passes down the intestinal tract, small vesicles within its cytoplasm coalesce into a vacuole, eventually forming a cyst which is passed into the environment in the stool of the host (191, 274, 371).
Clinical features of illness which have been attributed to Blastocystis spp. include nausea, anorexia, abdominal pain, flatulence, and acute or chronic diarrhea (216, 381). In several instances, Blastocystis spp. have been the most common enteric organisms isolated from diarrheal patients but have been reported as noninfectious pathogens (422, 438, 444). Blastocystis spp. are the most common enteric protozoa isolated from diarrheal patients in most developed countries (247, 309, 395, 416). For example, they are commonly isolated from immigrants in Italy (164), associated with chronic gastrointestinal illness of unknown etiology (216), and often associated with IBS-like symptoms (32, 90, 91, 211).
However, numerous studies have found no link between Blastocystis spp. and active disease (90, 91, 404, 420). It has also been argued that identification of Blastocystis from patient samples is not clinically significant but should be used as a marker of potential exposure to other pathogenic protozoa (315). Another study suggested that immunosuppression plays an important role in the display of clinical symptoms (418). More recently, clinical and molecular investigations demonstrated an association with Blastocystis spp. in IBS patients that supports a pathogenic role of this parasite in Latin America (211). However, other studies have failed to establish an association between Blastocystis spp. and IBS (334, 414, 440). It has been suggested that since IBS is a functional disorder that can be caused by various microbiological, genetic, and environmental factors, these factors could also explain the discrepancy in the literature (321). Cysts may be waterborne, food borne, or passed from person to person, especially in child care centers or other institutional settings. It has also been suggested that since many zoonotic subtypes exist, there is increased potential for zoonotic transmission (191, 420, 438).
The diagnosis of infection with Blastocystis spp. is usually based on the detection of the vacuolar, granular, amoebic, or cystic form in stool samples, using wet mount smears, iodine staining, trichrome staining, or iron-hematoxylin staining (309, 341, 390, 402, 404, 416, 481). However, identification by direct wet mounts is difficult and has resulted in false-negative results due to the polymorphic nature of Blastocystis spp. (404, 419). It has been suggested that trichrome-stained fecal smears or xenic in vitro culture systems offer the best sensitivity (90, 418). However, slower-growing subtypes (e.g., subtype 7) may be missed with this procedure (418). The formol ether concentration technique (FECT), which is commonly used for laboratory identification of OCP, is not recommended for Blastocystis due to its inability to isolate Blastocystis spp. (309, 404, 419).
Several molecular techniques with increased sensitivity have been developed (341). Advancements in the sequence analysis of Blastocystis-specific PCR products and subtype-specific PCR primers have led to progress in identifying several subtypes (268, 312, 341, 402, 418, 451). PCR using the SSU rRNA gene is being used increasingly for detection of Blastocystis spp. Despite being more costly, PCRs have demonstrated much higher sensitivities than more commonly used methods such as permanent staining (48%) (211, 341), direct light microscopy (29%), and xenic in vitro stool culture analysis (52%) (320). Owing to the wide genetic diversity among Blastocystis spp., the choice of primers is crucial from a diagnostic perspective (341).
Although disagreement about the pathogenicity of Blastocystis spp. still exists, treatment is indicated when there is no alternative explanation for symptoms (109, 218). Metronidazole is the suggested drug of choice, although failures of this drug in eradicating the organism are common (275, 407, 409). Cotrimoxazole, nitazoxanide, and a combination of paromomycin and metronidazole have also been used (222, 485). A recent study found extensive differences in drug sensitivities among two clinically important zoonotic subtypes (subtypes 4 and 7) and identified four new potential therapeutic options against Blastocystis spp., namely, mefloquine, cotrimoxazole (trimethoprim-sulfamethoxazole) (1:2), ornidazole, and furazolidone; the study also confirmed the antiprotozoal activities of 10 compounds already reported to be effective against Blastocystis spp. (272). It is clear that antimicrobial eradication of Blastocystis spp. is possible; however, because of the difficulty of the problem, a reevaluation of current treatment regimens is required, with a view to defining clear new treatments. Consideration should also be given to the fact that improvements in symptoms after treatment with metronidazole or trimethoprim-sulfamethoxazole may be due to clinical effectiveness of these drugs against coinfecting pathogens (71). There is therefore a need for further investigations to better understand drug efficacy, resistance, and reinfection issues (407).
Cystoisospora belli (formerly Isospora belli)
Cystoisospora belli causes intestinal disease in several mammalian hosts (42). Infections are thought to be acquired through the ingestion of mature environmentally resistant sporulated oocysts in contaminated food or water, although good evidence for the source of infection in most infected patients is limited (109, 219). Infection is almost indistinguishable from cryptosporidiosis (208) and is usually self-limiting and characterized by watery diarrhea, abdominal cramps, anorexia, and weight loss (175, 311). Cystoisospora belli is often implicated in traveler's diarrhea in travelers to developing countries with high levels of endemicity (2, 156, 311, 313, 427). It is more common in AIDS patients (165, 230, 453), other immunocompromised patients (163, 227, 266, 336, 344), and indigenous populations in the United States (291, 315). In HIV-infected patients, infection may be characterized by chronic diarrhea, acalculous cholecystitis cholangiopathy, and extraintestinal infection (337, 453). Other Cystoisospora species are important causes of diarrhea in domestic animals (108), and Cystoisospora suis is an economically important parasite causing severe diarrheal illness in pigs (8, 212, 282).
Diagnosis is made by direct microscopic observation of the oocyst in feces, with acid-fast staining, since the oocysts are large (20 to 23 μm by 10 to 19 μm) and morphologically distinctive. Diagnosis has also been done from mucosal biopsy specimens (227, 453). Molecular techniques such as conventional and real-time PCR can be used to augment diagnosis where possible, since these methods can be more sensitive in detecting infection (283). However, while PCRs have shown excellent sensitivity and specificity for the detection of C. belli in fecal samples, these are neither widely nor commercially available (283, 337, 347, 390, 427, 453). Infection usually responds to treatment with oral cotrimoxazole, and where resistance or intolerance exists, ciprofloxacin is a good alternative (109).
Balantidium coli
Balantidium coli is a ciliate and is the largest protozoan that infects humans (114, 383). Pigs are thought to be the natural host of this parasite, despite showing no clinical disease (111, 359). Infections in humans are acquired via the fecal-oral route from the ingestion of cysts present in untreated or minimally treated water and on uncooked or undercooked food (114, 374). Human infection is common mainly in communities that live in close proximity to pigs (114, 359). The vast majority of infections are asymptomatic, but mild diarrhea and abdominal discomfort have been reported in symptomatic patients. A few patients develop fulminating acute balantidiasis, with intestinal perforation leading to a case fatality rate of about 30%, or fulminating dysentery associated with hemorrhage and shock, resembling amoebic dysentery (120, 359). Balantidiasis is uncommon in developed countries, but infections have been reported as far north as Sweden, Finland, and Northern Russia, with the highest prevalence rates in tropical and subtropical regions (383). In developed countries, clinicians and laboratory technologists should consider balantidiasis as an alternate diagnosis for patients presenting with watery diarrhea who have a recent travel history to developing countries, especially those in Southeast Asia, the Western Pacific islands, and rural South America (111, 120, 359, 360).
Because of its large size (cysts are 50 to 70 μm; trophozoites are 30 to 200 μm by 40 to 70 μm) and spiraling motility, Balantidium is easily recognized in wet mount slide preparations. Trophozoites are visible with a hand lens, and sometimes with the naked eye, in freshly collected diarrheic stools (114, 359) as well as in bronchoalveolar wash fluid; cysts are more common in formed stools. Collection of stool samples over several days is recommended because excretion of parasites can be erratic (359). Treatment is done with tetracycline or metronidazole for 5 or 10 days, respectively (109).
Intestinal Microsporidiosis (Enterocytozoon bieneusi and Encephalitozoon intestinalis)
Enterocytozoon bieneusi and Encephalitozoon intestinalis are species of microsporidia that cause enteric disease. These parasites, historically considered spore-forming protozoa, are now classified as fungi (69, 150, 157). However, based on their importance, especially in immunocompromised populations, they are included in this review. Intestinal microsporidiosis was first identified in AIDS patients, with E. bieneusi being the more common of the two causative species (81, 398). Many identical genotypes of E. bieneusi from humans and animals are known (81), raising concerns over waterborne, food-borne, and zoonotic transmission. The importance of this parasite in human infections increased as it became a cause of opportunistic infections in HIV/AIDS and other immunosuppressed patients during the 1980s (6, 29, 114, 248, 315). However, with increasing access to antiretroviral therapy, infection rates in HIV/AIDS patients have reduced significantly (390, 448), with prevalences ranging from 0 to 42% (13). Infections have also been found in non-HIV/AIDS and immunocompetent patients (1, 248) and in persons with corneal infections (263), and there are also many infections thought to be associated with protracted traveler's diarrhea, during or after travel (156). Infections with Encephalitozoon spp. are believed to occur through ingestion or inhalation of spores (86), with the primary infection developing in the epithelium of the small intestinal or respiratory tract (86). Severe ulceration of the small bowel is associated with mucosal atrophy and acute and chronic inflammation (13). In the case of E. bieneusi, infection occurs mainly through ingestion of spores, which subsequently develop within the epithelial cells lining the duodenum and jejunum of the small intestine (86). Gastrointestinal symptoms include persistent diarrhea, abdominal pain, and weight loss, especially in immunosuppressed individuals (86). In otherwise healthy individuals such as travelers, E. bieneusi infection has resulted in self-limiting diarrhea of approximately 1 month (86). Fecal-oral transmission is implicated most often as the means of infection (96).
The diagnosis of intestinal microsporidiosis is difficult and is usually based on microscopic detection of the spores in stool samples, requiring special fluorescent or trichrome stains to detect the small spores (114, 200, 222). However, the detection of the spores and species determination can be difficult due to their small size (222), requiring reliable immunological diagnostics to supplement PCR or histochemistry when sporadic spore shedding is suspected (88). One study found that microscopy and staining demonstrated low sensitivity for the detection of microsporidian species compared with the calcofluor white technique and staining with DAPI (4′,6-diamidino-2-phenylindole), which demonstrated a higher sensitivity and specificity (97.12% and 98.55%, respectively) (439). However, the disadvantage of calcofluor white staining is that it requires a fluorescence microscope and is a nonspecific stain leading to the fluorescence of other organisms and artifacts which can be mistaken for microsporidia (78, 423). PCR-based methods have now been used successfully for detection of microsporidian infections, exhibiting excellent sensitivity and specificity (186, 248, 349).
Where treatment is indicated, albendazole, administered at 400 mg every 12 h for 1 month, is effective against Encephalitozoon species (13, 114, 222). Enterocytozoon bieneusi is less responsive to albendazole, and while symptoms may improve, microsporidian spore shedding is likely to continue (136). Fumagillin is the only treatment that has demonstrated consistent efficacy against E. bieneusi infection; however, neutropenia and thrombocytopenia have been reported as major side effects in both HIV-infected patients and transplant recipients, requiring monitoring of tacrolimus levels (58, 276). Supportive fluid therapy in immunocompromised patients and early initiation of HAART in HIV-infected patients are crucial to help restore the immune status of the host (87, 114, 117). Significant reductions have been seen in the incidence of intestinal microsporidiosis in HIV-infected patients since the introduction of HAART (398, 448).
MODES OF TRANSMISSION
Any discussion about the importance of enteric protozoa in public health cannot be complete without the inclusion of their modes of transmission, as these routes play a significant role in their widespread dissemination and affect people from all walks of life (Fig. 2). Interventions aimed at prevention and control of these routes of transmission have proven effective in various settings in reducing the incidence and prevalence of diarrheal illnesses. Food- and waterborne transmission is the main focus of this section.
Fig 2.
Complex interactions in the transmission and control of enteric protozoal infections. Infectious parasites are transmitted to humans through several routes, including contaminated food and water, inadequately treated sewage/sewage products, and livestock and domestic pet handling. Prevention and control strategies can be implemented at different levels of food production, liquid waste management, water quality control, and livestock and pet handling processes.
Food-Borne Transmission
The majority of the enteric protozoa discussed herein can be transmitted by food. Although the risk of obtaining a food-borne protozoan infection is lower in developed settings, the significance and impact of these infections cannot be ignored (125, 183, 222, 288). In developed countries, large-scale food production, distribution, retailing, and importation of raw food ingredients increase the risk for the spread of food-borne infectious disease and often result in costly recalls (250). Intestinal protozoa can contaminate food through a variety of routes (42). Food can become contaminated with parasites during the production stage, from contaminated irrigation water, soil, untreated manure, or biosolids used as fertilizers (54). Food may also become contaminated during the harvesting, handling, and preparation processes, from cross-contamination with soiled implements, animal manure, or contaminated water used for preparation or by the hands of the food handlers themselves (79, 157, 174). The risk of food-borne transmission is increased when food is consumed raw, undercooked, or in a semicooked form (198, 322). Food becomes contaminated directly from feces, soil, irrigation water, sewage, and human handling during various phases of the food production chain (115, 288, 383).
Food-borne outbreaks cost individuals and families thousands of dollars in medical costs and lost wages. The food industry suffers financially even more due to recalls of food products, litigation, and, sometimes, closure (38, 184, 197). In recognizing the public health and trade implications of pathogens in fresh fruit and vegetables, the FAO and WHO have provided scientific advice to the Codex Committee on Food Hygiene (CCFH) on the fresh produce commodities of greatest concern from a global perspective (113). The submission looked at microbial hazards in produce that is marketed fresh, and often ready-to-eat, throughout the production-to-consumption continuum, with the development of specific management guidance (113).
Cryptosporidium, C. cayetanensis, and Giardia are the main enteric protozoa associated with food-borne infections in developed countries (291). A U.S. report for the years 2000 to 2008 indicated that a significant proportion of laboratory-confirmed cases of food-borne parasitic diseases were due to G. intestinalis (356). In addition, of a total of 53 cases of seafood-associated outbreaks of parasitic infection reported in the United States from 1973 to 2006, Giardia was responsible for 43%; more than half (55%) of these were associated with the consumption of fish (199). It was reported that from 1990 to 2000, there were 11 food-borne outbreaks of cyclosporiasis in North America that affected at least 3,600 people (259). Almost all cases of C. cayetanensis infection in the United States are food borne (173, 356). Several cases of food-borne cyclosporiasis related to the consumption of salads have also been reported in European countries (92, 198, 350). According to one U.S. report, source attribution is often affected by insufficient numbers of cases for definitive case-control studies and a lack of tools for molecular epidemiology (173). Approximately 10% of Cryptosporidium infections are ascribed to food-borne transmission (171), and outbreaks have been associated with eating raw produce or salads (66, 187, 322, 476). Food-borne outbreaks associated with imported foods have been reported in several industrialized countries (92, 189, 271, 291, 322, 450). It is therefore important to have adequate food safety standards in place to govern locally produced and imported foods, which is essential to the safety of the food supply chain (220). In addition, industrialized countries should give priority to the institution of sensitive disease surveillance systems and the development of molecular methods for linking cases of protozoan infections (162, 173, 258).
Waterborne Transmission
The role of water resources in the transmission of enteric protozoa cannot be overemphasized, especially where there is the potential for water supply contamination. Despite ongoing investment in better sanitation infrastructure, water quality, and environmental protection legislations and the subsequent reduction in pathogen loads in public water supplies, waterborne disease outbreaks still pose significant risks to human health in developed countries (34). Small water supplies in rural communities in developed countries may be more vulnerable to contamination than the larger public water supplies, resulting in outbreaks of infectious disease (195, 317). In the United States, it is suspected that seasonal peaks of cryptosporidiosis are related to increased use of recreational water venues such as lakes, rivers, swimming pools, and water parks in summer months (188, 463). Cryptosporidium hominis subtype IbA10G2 was the causative agent identified in the largest waterborne outbreak of cryptosporidiosis reported in Australia to date and was associated with public swimming pools in densely populated coastal cities (463). Surveillance-based data indicate a significant increase in onset of cryptosporidiosis and giardiasis during summer through early fall in the United States (476, 477). These are likely associated with increased outdoor activities and increased exposures, such as camping and use of communal swimming venues (e.g., lakes, rivers, swimming pools, and water parks) by young children, during the summer recreational water season (476, 477). Cryptosporidium spp. remain the leading cause of diarrheal illness outbreaks in treated recreational water venues in the United States (476). One study determined that acute diarrheal illness associated with small community water supplies could cost an estimated $4.671 billion (95% CI, $1.721 to $9.592 billion), with the capital costs of intervention amounting to $13.703 billion (95% CI, $6.670 to $20.735 billion) and those of postinfectious IBS resulting from these acute gastroenteritis outbreaks amounting to $11.896 billion (95% CI, $3.118 to $22.657 billion) (195).
The oocysts of several protozoa are highly resistant to chlorination, a conventional water treatment method (43). It is suspected that when water becomes contaminated, oocysts can become trapped by chlorine and UV-resistant biofilms in water pipelines and constantly shed into the water supply (68, 123, 466). While availability of potable water and water treatment standards are generally better in developed countries, remote and isolated areas often have deficient water systems, resulting in waterborne outbreaks, for example, in South Bass Island, OH (303), and in France (135). Giardia intestinalis and Cryptosporidium spp. have been implicated in several large waterborne outbreaks in the United States (72, 252, 303, 352), Norway (297), Australia (295, 463), and elsewhere (360).
Many countries worldwide have legislation or follow standard guidelines to monitor microbial indicator organisms to determine the microbiological quality of water (68, 317). Traditionally, fecal indicator bacteria (FIB)—total coliforms, fecal coliforms, and enterococci—are used as microbial indicators, and there are very few reports outlining the use of protozoa as indicators of fecal pollution (190, 269, 310, 353). Unfortunately, many waterborne pathogens are still difficult to detect, and despite advances in molecular diagnostics, such methods are not widely available or used even in developed countries (123). Increased water shortages in some developed countries have caused authorities to resort to the use of recycled water from highly contaminated sources such as sewage (67, 239). The increased use of biosolids from sewage as soil conditioners as a means of sustainable disposal has raised issues about the potential for the transmission of infectious pathogens, including the protozoa G. intestinalis, Cryptosporidium spp., Balantidium, Entamoeba spp., Blastocystis spp., and Dientamoeba fragilis (23, 104, 217). These issues have been the subject of extensive debate in many countries, such as Australia, New Zealand, the United States, and countries in the European Union, highlighting an extensive gap in knowledge on the spread of enteric protozoa through recycled sewage and biosolids (67, 243).
Potable water.
A water supply can become contaminated by sewage and runoff from farms, fecal matter from animal activity, or decomposing animal carcasses washed into water bodies (154). In properly operated conventional water treatment plants, most protozoa are excluded from drinking water by their size (<30 μm). However, due to their smaller size (range, 1 to 17 μm), G. intestinalis cysts, Cryptosporidium oocysts, and the spores of microsporidia have been able to penetrate water treatment systems and have been detected in aquatic environments (415). While waterborne infections are not as widespread in many developed countries, climatic events that can tax treatment plant operations or inadequate, interrupted, and intermittent treatment can occur, resulting in large outbreaks in the community (297, 338). In a Spanish study, Cryptosporidium oocysts were found in 15.4% to 63.5% of various raw surface water samples, 30.8% of treated water from small treatment facilities, and 26.8% of chlorinated tap water. Giardia cysts were found in 26.9% to 92.3% of various raw surface water samples, 19.2% of treated water from small treatment facilities, and 26.8% of chlorinated tap water. The presence of Cryptosporidium and G. intestinalis was significantly associated with the turbidity levels of the samples and with increased count levels for total coliforms and Escherichia coli (P < 0.01) (46).
A review of documented waterborne disease outbreaks in the United States from 1971 to 2006 found that parasites accounted for about 18% of outbreaks (72). Giardia intestinalis was the sole pathogen identified in 86.0% (123) of the 143 drinking water outbreaks of known parasitic etiology, affecting 28,127 individuals (6.3% of the 449,959 cases) (72). On the other hand, Cryptosporidium was responsible for only 13 outbreaks (9.1%), but these outbreaks were responsible for the vast majority (421,301 cases [93.6%]) of the total cases. The majority of cryptosporidiosis (403,000 cases) cases were attributed to a single outbreak of C. hominis in Milwaukee, WI (72, 252). Entamoeba spp. and C. cayetanensis were responsible for two and one outbreak, respectively, with fewer cases, while coinfections were also identified (Cryptosporidium-Giardia and Giardia-Entamoeba spp.) as the cause of two and one outbreak, respectively (72).
More recently, concern has been raised about the role of biofilms in drinking water distribution networks and the role they play in becoming transient or long-term habitats for hygienically relevant microorganisms, including parasitic protozoa (e.g., Cryptosporidium). These organisms can attach to preexisting biofilms, where they become integrated and survive for days to weeks or even longer, depending on the biology and ecology of the organism and the environmental conditions, remaining undetected by conventional detection methods (472). It has been suggested that protozoa may act as protective environments for pathogenic bacteria, protecting them from disinfection and promoting extended survival under these conditions (378). Further study is therefore important to determine the role of biofilms in drinking water systems and their potential for long-term harborage of enteric protozoa and other pathogenic organisms and subsequent health risks to humans (378, 472).
Rain/roof water has been used worldwide, especially where water scarcity is a problem, as an alternative water source for drinking and various nonpotable uses (4, 239). This generally involves installing rainwater tanks to collect roof water from residential dwellings for uses such as drinking, cooking, irrigation, showering, clothes laundering, and toilet flushing (4, 103, 458). Despite the obvious benefits from using rainwater, there are public health risks, including the transmission of zoonotic bacterial and protozoan pathogens from bird and animal droppings via individual and communal rainwater systems (4, 458). The Australian guidelines indicate that roof water should be harvested in a way that minimizes health and environmental risks or, at a minimum, reduces these risks to acceptable levels (103). In countries where the microbiological quality of rainwater is assessed, it is based mainly on the three bacterial indicators (coliforms, E. coli, and enterococci) that are commonly detected in harvested rainwater. Their presence may be an indication of the potential for contamination with environmentally resistant cysts or oocysts of protozoa (46, 240, 246). A report from South East Queensland, Australia, found Giardia in 10% of roof-harvested rainwater samples (5). Based on the assessment of the risk of infection associated with various types of exposure to rainwater, it was reported that although the risk of infection from the use of rainwater for showering and garden hosing was well below the threshold value of one extra infection per 10,000 persons per year, the risk of infection from ingesting G. intestinalis via drinking exceeded this threshold value and indicated that if undisinfected rainwater was ingested by drinking, then the incidence of giardiasis would be expected to range from 1.0 × 101 to 6.5 × 101 cases (with a mean of 1.6 × 101 cases based on Monte Carlo analysis) per 10,000 persons per year (5). These findings indicate the need for disinfection of rainwater for drinking purposes (5). Giardiasis is a frequently diagnosed waterborne disease and is a major public health concern for water utilities in developed nations (196, 238, 430).
Evidence for contamination of groundwater with protozoa was found in France. Cryptosporidium oocysts were detected in 78% of both surface water and groundwater samples, while Giardia cysts were found in 22% and 8% of surface water and groundwater (sinkhole, spring, and well bore) samples, respectively (223). Cryptosporidium oocysts were transported from the sinkhole to the spring and the well bore, suggesting that oocysts are subject to storage and remobilization in karst conduits (223, 269).
Wastewater and biosolids.
Increased urban populations, industrialization, and urbanization have resulted in the overwhelming production of sewage sludge (23). Wastewater treatment processes should reduce the number of pathogens in the wastewater by concentrating them with the solids in the sludge. Although some treatment processes are designed specifically to inactivate pathogens, many are not, hence the potential risk for pathogen survival in sewage sludge and its by-products (155). The most widely available and recommended option is land application of sewage sludge to cropping land as a soil conditioner and fertilizer (23). However, by virtue of its origin, sewage sludge is a reservoir for enteric pathogens. One major risk is that biosolids may contain ova and cysts of parasites and enteric bacteria and viruses (23, 67). Health risks may result from the consumption of food and water contaminated with sewage or biosolids (369) or crops grown on biosolid-enriched soil (286, 287). In managing the beneficial reuse of biosolids on land, a balance is achieved between treating contaminants in the biosolids and retaining the biosolids' nutrient value. The U.S. EPA legislation for the safe use of biosolids (known as the Part 503 Rule) was promulgated in 1993 and used operational standards intended to reduce pathogens to levels that are not expected to cause adverse health effects (105). Similar guidelines have been established in Europe, the United Kingdom, and Australia (104, 369). The proponents of the use of biosolids in this way suggest that for it to be considered a public health risk, a plausible transmission route should exist between the source (biosolids) and the susceptible community. The pathogenic protozoa would need to be present in a sufficient infectious dose, survive the sewage/biosolids treatment processes, survive through storage and land application, enter into the water supply system, and bypass drinking water treatment (67, 159, 369). Gerba et al. concluded that the probability of emerging parasites such as microsporidia and Cyclospora surviving various biosolids treatments was low, since they were unlikely to survive the temperatures achieved in anaerobic digestion and do not survive well under low-moisture conditions (142). However, another study detected Cryptosporidium oocysts in 10% and Giardia cysts in 35% of the samples of sludge sanitized by quicklime or peat and after 30 weeks of composting (339). In addition, many practitioners and researchers do not encourage the reuse of recycled sewage as potable water because pathogens such as Giardia are not always effectively removed and because of the possibility of malfunctioning of reverse osmosis (RO) systems—deemed the most effective in removing viruses—as often as 5 days a year (67).
Biosolids generated at wastewater treatment plants (WWTP), along with food waste, pet excrement (i.e., dog and cat feces), and human excrement in absorbent products (e.g., disposable baby napkins for children and adults and feminine hygiene products), are considered a potential source of infectious microorganisms in municipal solid waste (MSW) (143). The majority of protozoan parasites (97%) are expected to come from pet feces; however, the evidence suggests that enteric protozoa would be expected to be inactivated by temperatures above 45°C and that most landfills achieve temperatures of 38°C to 78°C (143). There is still much uncertainty about the survival of enteric protozoa in leachate from unlined landfills and the resultant contamination of underground aquifers and the water supply. One of the most recent studies to evaluate this found the presence of viable Giardia in large concentrations in leachate (143).
IMPLICATIONS OF CLIMATE-RELATED CHANGES
According to the Stern review, climate change is a serious global threat and demands an urgent global response (411). Climatologists and health experts have postulated that climate change will impact human health, and this is best understood by the identification of existing vulnerabilities of the ecosystem to climate variability (59, 97). Vulnerabilities include highly complex interactions between changing weather, ecosystem changes, microbial and parasitic evolution, and technological and societal adaptations (59, 98). The main impact to health may arise from changes in weather patterns associated with extreme weather events such as excessive rainfall, flooding, and droughts (172, 206). These events have the ability to significantly increase the risk of infectious diseases due to the abundance and distribution of disease agents in the environment (97, 279, 333). Some experts have projected that the following water-related risks are likely to increase in both developed and developing countries as a direct result of climate change: (i) excess precipitation and floods may result in increased runoff and turbidity and decreased effectiveness of water treatment (59); (ii) heavy rain, snow, or ice melt may flush animal manure, human sewage, and wildlife and pet droppings into surface water or groundwater reservoirs, leading to contamination of drinking water sources (59, 77); (iii) droughts may lead to reduced water availability, lower water pressure, compaction contributing to increased runoff when rain eventually does fall (59), and increased use of alternative water sources for domestic purposes and irrigation, increasing the likelihood of contamination of water and food (435); (iv) a decreased food supply associated with extended dry spells and drought in some countries may lead to increased importation of substandard foods, resulting in the introduction of food safety hazards such as enteric protozoa at various stages of the food chain, from primary production through to consumption (435); and (v) there may be increased risk for the contamination of recreational waters and exacerbation of the presence of biological contaminants in marine environments, leading to seafood contamination (261).
The greatest impact of climate change is expected to be on less developed countries, and small island states are particularly vulnerable to the effects of extreme weather, sea level rise, and increased temperatures (411, 413). In the context of infectious diseases, the smallest and poorest populations could be affected most seriously due to already vulnerable economies and public health infrastructure, inadequate fresh water resources, and poor sanitation and hygiene (99, 182). Health consequences such as diarrheal diseases, malnutrition, and malaria are projected to pose the largest risks to future populations, especially young children (97). Projections suggest that although low-income countries will be most vulnerable to adverse effects, requiring additional human and financial resources, high-income countries will also be affected adversely, with the severe impact on the local economy (97, 411). Generally speaking, the risk of diarrheal disease in developed countries may be mediated by more stringent public health measures for sewage disposal, water treatment, and hygiene. However, indigenous populations, institutionalized persons, and the immunosuppressed will remain at extremely high risk (206). There is therefore need for more research into the role of this phenomenon in the spread of enteric infections in developed settings.
The challenges of food-borne, waterborne, and zoonotic protozoan diseases associated with climate change are expected to increase, with a need for active surveillance systems, some of which have already been initiated by several developed countries (172, 206). Studies conducted in the United States (77), Taiwan (62), New Zealand (35), Australia (172), Bangladesh (182), Canada (428), and China (483) have found increased diarrhea-associated morbidity related to increased temperature and extreme rainfall days, although in Australia there were no differences in climate-associated increases in Salmonella infection rates between subtropical and tropical regions (482). Several authors have suggested that the seasonal incidence of infection with some enteric protozoa may be affected by increased rainfall, increased pollution from farm waste, or animal husbandry practices (172, 206). Exposure to floodwaters and swimming in lakes and ponds with elevated pathogen levels are other risk factors to be noted (99). Management of diarrheal illnesses in this complex setting will require dialogue and collaboration between public health, water treatment, veterinary, and food safety experts worldwide (288). The rate of climate change, the degree of its effect, and its impact on infectious diseases, including parasitic diseases, may differ from country to country (224, 362). These conclusions are therefore drawn based on scientific plausibility, and countries should seek to establish proper surveillance networks to connect epidemic intelligence and infectious disease surveillance with meteorological, entomological, water quality, remote sensing, and other data for multivariate analyses and predictions (362).
PREVENTION AND CONTROL OF PROTOZOAN INFECTIONS
The ubiquitous nature, small size, and abundance in the environment of protozoan parasites make them difficult to control. Health care providers and public health personnel should provide adequate instructions to infected patients and carriers on how to avoid spreading the infection to others and should employ infection control precautions for hospitalized and institutionalized patients. The following are suggested controls based on proven intervention measures that can be applied in various situations.
General Measures
In domestic settings, simple prevention and control measures are effective against most enteric parasites. These include personal hygiene; hand washing after using or handling the toilet, changing diapers, or caring for a person with diarrhea and before preparing or handling food (366); proper disposal of excreta; and washing of soiled materials such as clothing or bedding. The use of adequately treated water, for example, by boiling, for drinking, food preparation, and washing of fruits and vegetables is essential (57). In the food industry, some protozoa get into food through fecal contamination of raw materials and inadequate treatment of the food before consumption or through posttreatment contamination. This is true for the oocysts or sporocysts of Cryptosporidium spp. and C. cayetanensis (291, 374). Control measures must therefore be aimed at all aspects of the food chain, including minimizing dissemination of cysts and oocysts in the farming environment and via human waste management (373). This may include abstinence from using untreated human and animal manure in farming.
Food hygiene and management practices in food service and catering industries should include restricting the purchase of raw materials to suppliers with good agricultural practices (95) and having adequate facilities for washing and cleaning of raw fruits and vegetables before storage and before preparation (220). A system for identifying and controlling microbial hazards, such as the Hazard Analysis Critical Control Point (HACCP), should be employed by food and water production companies, in industries or sectors that use fresh produce, and in operations in which contaminated water or ingredients could end up in the product (79, 172, 202).
Persons working in high-risk environments such as schools, day care centers, food-handling facilities, and aged-care institutions should be educated properly on their risk of infection (297), and where infection occurs, persons should be excluded for up to 48 h after the last diarrhea episode (10). Infected persons such as travelers, campers, and hikers should refrain from passing of feces into rivers and streams and should not use recreational waters such as swimming pools and public baths for up to 2 weeks after symptoms cease (57). Cryptosporidium spp. and Giardia intestinalis in swimming pools are difficult to control (325). Open farms and petting zoos should have guidelines in place for the handling of animals and should ensure that adequate hand-washing facilities are in place for visitors and staff. Wilderness backpackers and travelers to developing countries must exercise precautions with food and drinking water safety (331). Basic hygiene, including hand washing and cleaning of cookware, is essential in the prevention of diarrhea (10). Long-term groups, including military, missionary, aid worker, volunteer, and tourist groups, should seek travel advice. They should also take with them adequate supplies to treat water for domestic use (e.g., filtration and boiling), as chlorination by itself cannot remove the oocysts of protozoa such as Cryptosporidium spp. and C. cayetanensis from water (12, 19). Routine boiling should be used to augment chlorination in less developed settings (331). Since domestic house flies can play a role in the efficient transmission of human protozoan parasites, care must be taken with food exposed to flies (160). In the case of cyclosporiasis, it is important that it be considered by microbiologists and physicians as a possible cause of illnesses in cases of prolonged diarrhea that are not associated with travel, as well as in food-borne outbreaks associated with imported foods (306).
Water and Wastewater Quality Control
Drinking water supply.
The benefits of supplying safe water cannot be overemphasized (256, 365). Hunter and colleagues estimated that the cost benefit of annual maintenance coupled with the benefits of reducing the impact of postgastroenteritis IBS from waterborne infections was nearly 10 times (9.87; 95% CI, 3.34 to 20.49), suggesting that the health benefits of improving the management of water supply systems outweigh the costs (195). Conventional water treatment involves a series of steps, including coagulation, flocculation, clarification, sedimentation, medium or membrane filtration, and disinfection (using chlorine/chloromination, ozone, or UV), aimed at improving the microbiological quality of water (30, 75). The challenge for the physical removal of cysts and oocysts in the water treatment process is their small size, and the general rule is that the smaller the cysts and oocysts, the more difficult it is to remove them using conventional water treatment technology. E. histolytica cysts range in size from 10 μm to 20 μm, G. intestinalis cysts are 8 to 12 μm by 7 to 10 μm, and C. parvum oocysts range in size from 4 μm to 6 μm, making C. parvum oocysts the most difficult of these parasites to physically filter from water (131, 236). The efficacy of the coagulation, sedimentation, and filtration processes for the removal of protozoan cysts and oocysts in water treatment systems is particularly important and ideally should be preceded by pretreatment methods such as those aimed at the reduction of disinfectant and disinfection by-product (DBP) levels (30). Unfortunately, conventional treatment processes are prone to vulnerabilities that affect their efficacy in the removal of protozoan cysts and oocysts (30, 358, 364; S. Li, Z. Ran, C. Cui, and Y. Yuan, presented at the International Conference on Computer Distributed Control and Intelligent Environmental Monitoring [CDCIEM], 2011). However, there is evidence that significant improvement in conventional water treatment standards has occurred in the past 20 years, with full conventional treatment effective at reducing the prevalence of oocysts by a factor of up to 114 (95% CI = 80.64 to 160.78) (64). A study in Spain found that approximately 40% of samples of the effluents from drinking water treatment plants contained Cryptosporidium oocysts and Giardia cysts after undergoing coagulation, flocculation, and clarification through sedimentation, filtration, and disinfection (chlorination) processes and that about 90% of the organisms in the drinking water remained viable after treatment (48). A U.S. study found that Cryptosporidium oocyst and Giardia cyst removal across conventional treatment was influenced by the initial pathogen concentrations and level of turbidity. The study found that the higher the initial amount of contamination, the larger was the amount of pathogen removal observed with coagulation by alum, and higher raw water turbidity appeared to result in a higher log removal level for both Cryptosporidium oocysts and Giardia cysts (20).
The U.S. Army recommends that improving flocculation mixing intensities and flow distribution throughout the water treatment plant will assist in the prevention of outbreaks of cryptosporidiosis in keeping with U.S. EPA standards (63). A study identified that polyaluminum chloride (PACl) is an alternative coagulant that improves floc formation and sedimentation, producing significantly less sludge (63). A pilot-scale test of the electrofiltration process in a drinking water treatment plant was demonstrated to be effective in the removal of waterborne particles of <4 μm and can be pursued further for large-scale use, with the added benefit of reducing or eliminating the need for coagulants and the subsequent residual/scum formation (245). Hence, careful selection of methods for the inactivation of protozoa in water treatments is necessary, as demonstrated by several studies (30, 358, 364; Li et al., presented at CDCIEM, 2011). However, significant removal of protozoan cysts or oocysts has been achieved during filtration processes as water passes through a porous medium or membrane, resulting in up to 1.7 to 3.6 log10 reductions of oocysts, depending on the filter medium used (75). It is therefore recommended that combinations of treatments and multiple-barrier approaches to optimize water treatment through a synergistic effect be employed (107, 226, 358, 364).
On a small scale, point-of-use (POU) water purification technologies (such as chlorination with safe storage, solar UV treatment, ceramic filtration, or biosand filtration [BSF]) (338, 380), employed in small communities or at the household level, are recommended for reducing risks of enteric infectious agents transmitted by drinking water, especially for pregnant women, institutionalized toddlers and elderly, and immunosuppressed populations (338, 436). On a larger scale, a combination of physical methods that affect survival or removal of protozoan parasites should be employed (67, 243). These include freezing, heating, filtration, sedimentation, UV light irradiation (67, 239), high pressure, and ultrasound (23). Some experts suggest that ozone is a more effective chemical disinfectant than chlorine or chlorine dioxide for inactivation of protozoan parasites in drinking and recreational waters (161, 469; Li et al., presented at CDCIEM, 2011). Biosand filtration has been used widely, with good effect at removing the common protozoa in developed countries and at low cost in developing countries (68, 358, 410, 436). HACCP principles have been applied in some countries as a means of providing potable water free of microbiological health hazards (166). Slow sand filtration, solar technology, and membrane technology are effective methods for reducing pollution from rainwater to be used as a safe drinking water supply (185).
Sewage and wastewater by-products.
The first step in the control of microbial risks from sewage and wastewater is the institution of proper legislation and guidelines for those involved. These should apply even at the household level, where sludge, including livestock wastes, may be added to compost (217), as well as to storage and treatment of such wastes (79). Studies have shown high concentrations of both Cryptosporidium oocysts and Giardia cysts detected in effluents from WWTPs after going through secondary and tertiary treatment (48). Due to the potential risks to public health, recycled wastewater should be approved for use only under circumstances where other options are technically or economically infeasible and only for industrial purposes using separated pipelines (67, 239).
Wastewater treatment reduces the number of pathogens in the wastewater by concentrating them with the solids in the sludge. Although some treatment processes are designed specifically to inactivate pathogens, many are not, and the actual mechanisms of microbial inactivation are not fully understood for all processes (155). One study evaluated six sewage sludge hygienization processes, including closed reactor and open windrow composting, and sludge sanitation by quicklime or peat addition. The study found that while these processes were effective in removing indicator bacteria (for example, fecal coliforms), cysts or oocysts of pathogenic protozoa survived the majority of these processes (339). The lime stabilization method has also been used successfully to destabilize fecal coliforms, Salmonella, adenovirus type 5, and rotavirus after only 2 h of treatment in biosolids. However, in this model, Cryptosporidium oocysts and Ascaris lumbricoides ova remained viable following 72 h of liming (28).
Several emerging methods for wastewater treatment involve membrane and filtration technologies. Pressure-driven membrane processes (microfiltration, ultrafiltration, nanofiltration, and reverse osmosis) are now commonly used (367, 447). Among these methods, ultrafiltration is quite efficient in the removal of suspended particles and colloids, turbidity, algae, bacteria, parasites, and viruses for clarification and disinfection purposes and, as such, can be used to replace several of these steps in the conventional treatment process (447). Another method is the use of membrane bioreactors (MBRs) that combine suspended biomass, similar to that in the conventional activated sludge process, with immersed microfiltration or ultrafiltration membranes that replace gravity sedimentation and clarify the wastewater effluent, producing high-quality effluent suitable for unrestricted irrigation and other industrial applications (364). Membrane technologies are prone to fouling caused by microbe-generated extracellular polymeric substances such as proteins and natural organic matter. Therefore, next-generation nonfouling membranes with much narrower pore size distributions than those derived from immersion precipitation, in addition to fouling-resistant surfaces, are needed for improved contaminant retention without intensive chemical treatment and while reducing the need for subsequent decontamination (364, 367).
Protozoan parasites form part of the microbial ecology in wastewater that is responsible for purification processes and improving the quality of the effluent, by maintaining the density of dispersed bacterial populations by predation (254). The persistence of these pathogenic parasites in biosolids emphasizes the need for guidelines and regulations to govern the use of biosolids (23). Bean et al. suggested that Cryptosporidium oocysts should be considered an indicator for evaluating biosolids intended for land application (28). Australia, the United States, and the European Union have developed regulations to control pathogen risk arising from land application of biosolids, based on the concept of multiple barriers to the prevention of transmission. The barriers are aimed at protecting human health, food quality, and the environment from potential microbial and chemical contaminants and include (i) treatment to reduce pathogen content and vector attraction, (ii) restrictions on crops grown on land to which biosolids have been applied, and (iii) minimum intervals following application and grazing or harvesting (104, 105, 112, 155, 464). The availability of multiple barriers, such as constructed barriers, catchment barriers, and dilution in the catchment area, reduces the risk of waterborne transmission of protozoa from sewage effluent (443).
Detection of Protozoa in Water Samples
Detection of protozoa in water samples is important for the monitoring of contaminants and for the prevention and control of parasitic infections from water. Their small size and dispersion in water and the difficulty in efficiently concentrating oocysts from environmental samples while excluding extraneous materials make it difficult to detect protozoa in water by traditional methods, with detection requiring well-trained and experienced personnel (45, 47). The biggest problem with identifying protozoa in water supplies is that cultivation from cysts or oocysts is difficult and it is almost impossible to concentrate oocysts from environmental samples while limiting the presence of extraneous materials. As a result, enteric bacteria are traditionally used as surrogate markers for fecal contamination of water sources (47). However, the development of new assays and methods for the purification and concentration of cysts or oocysts by the use of immunomagnetic separation (IMS) has addressed this problem (375). Currently, the U.S. EPA and other such bodies commonly use detection methods for isolation of protozoa, from either large (up to 1,000 liters) or small (10 to 50 liters) samples, involving four sequential steps: (i) the filtration of water, resulting in the cysts or oocysts and extraneous materials being retained on the filter; (ii) the elution and separation process, involving purification and concentration of cysts or oocysts by IMS and discarding of the extraneous material; (iii) staining with specific fluorescent antibodies (FA); and (iv) enumeration using fluorescence and differential interference contrast microscopy (45, 48, 106, 375). Other techniques that can also be used for cyst or oocyst purification purposes include density gradient, saturated-salt flotation, and continuous flow centrifugation, continuous flow filtration, and flow cytometry with cell sorting (45, 251, 299). Additional molecular methods are currently available for the identification of protozoa in water samples by detection of pathogen-specific genes or SSU rRNA (292, 375). For example, nested PCR-RFLP assays are used to target different loci (1 and 2) of the hypervariable region of the 18S rRNA gene for identification of different Cryptosporidium spp. in water (292). Other PCRs have been developed to amplify the COWP gene (9). Species of microsporidia have been identified in various water sources by use of Weber's chromotrope-based stain, with positive samples analyzed by PCR (200). Several authors suggest that molecular techniques are the most promising methods for the sensitive, accurate, and simultaneous detection of waterborne protozoa, with much benefit to the water industry and public health (47, 101), compared with conventional staining and microscopy (205, 386, 387).
Another approach is the use of alternative bioindicators together with conventional fecal markers to identify the source of fecal pollution and associated pathogens in water (353). Water can become contaminated by point sources (raw sewage, storm water, wastewater effluent, and industrial sources) and non-point-source discharges (agriculture, forestry, wildlife, and urban runoff). The identification of the source of fecal contamination and pathogens could aid in the management and remediation of water resources when other control strategies are not feasible (353). However, some scientists suggest that fecal indicators do not adequately indicate the presence of all fecal pathogens in natural waters because their presence is affected by different environmental conditions (147, 241, 310). Since the cysts and oocysts of enteric protozoa are hardy and chlorine resistant and can survive for longer periods in water, these could be evaluated for use as alternative fecal indicators (310). The WHO suggests that the spores of the anaerobic bacteria Clostridium perfringens and Bacillus spp. can be used to evaluate the effectiveness of protozoan cyst or oocyst removal by sewage/water treatment, since they are highly resistant in the environment and their vegetative cells do not reproduce in aquatic sediments (18).
Evaluating the presence of fecal indicator organisms in water or the bioaccumulation of encysted protozoa in shellfish has value for identifying source attribution for surveillance and in outbreak settings (115). Bivalve shellfish have been used as bioindicators of fecal protozoan contamination in water bodies. For example, Cryptosporidium and Giardia in clams (Corbicula fluminea) have been detected by PCR and DFA assays (270) or IFA (148). When it is not possible or practical to test for individual protozoan parasites, it may be useful to use a combination of fecal indicator source tracking methods to enhance identification of contaminants of public health interest in surface waters (121, 270, 353).
CONCLUSIONS
Enteric protozoa continue to contribute to the burden from preventable infectious diseases affecting humans and animal health in industrialized settings. Giardia intestinalis, Cryptosporidium spp., and Entamoeba spp. are the most commonly reported protozoa associated with enteric infections and are associated mainly with food- and waterborne outbreaks. Others, such as Cyclospora cayetanensis, Dientamoeba fragilis, Balantidium coli, Cystoisospora belli, and Blastocystis spp., are emerging as important causes of illness, with serious implications for travelers to developing regions, immunocompromised populations, and young children. Although public health measures in most developed countries are more stringent than those in developing settings, minority groups, institutionalized persons, and the immunocompromised remain at extremely high risk, which can be extended to the rest of the population, and hence they should be considered a public health priority. Furthermore, the challenges of protozoan diseases transmitted by food, water, and animals are expected to increase as a result of complex interactions between human and animal hosts, fueled by the emerging effects of climate change and urbanization and the need to increase food production, reuse of gray water, and biosolids. Advancements in molecular diagnostics and the use of molecular epidemiology have the potential to identify previously undetected species and zoonotic serotypes that cause illnesses in both animals and humans and are useful for surveillance and outbreak control. Although there is much evidence for some protozoa as a cause of illness in humans and/or animals, there is still need for the development of better diagnostic methods for the rapid and sensitive detection of others. There is therefore a need for much more research into these issues to better understand the potential impact of climate change on the incidence and prevalence of parasitic diseases and how governments can act now to mitigate their effects. The management of protozoan infections in this complex environment, coupled with the projected effects of climate change, will require an increase in the allocation of research and development funding and a multidisciplinary approach.
Biographies

Stephanie M. Fletcher has been a Ph.D. student at the University of Technology, Sydney, Australia, since 2009. She obtained a master's degree in public health from the University of the West Indies in 2007, with undergraduate qualifications in Environmental Health and Meat and Food Hygiene. She has worked extensively in environmental health in Jamaica and the Caribbean, with special interest in the burden of infectious diseases, including gastrointestinal illnesses. She is a Fellow of the Royal Society for Tropical Medicine and Hygiene and a member of the Public Health Association Australia and the National Environmental Health Association (United States).

Damien Stark is a Senior Hospital Scientist in the Microbiology Department at St. Vincent's Hospital, Sydney, Australia, and is also a Research Associate of the School of Medical and Molecular Biosciences, University of Technology, Sydney, Australia. His research interests include clinical, diagnostic, and molecular parasitology, with a special interest in all aspects of D. fragilis research.

John L. Harkness graduated from Monash University Medical School in Melbourne, Australia, in 1967. He trained as a junior medical officer at the Alfred Hospital in Melbourne, followed by training in pathology at the Alfred Hospital, Hammersmith Hospital, London, United Kingdom, and the Mayo Clinic, Rochester, NY. He was appointed Director of Microbiology at St. Vincent's Hospital, Sydney, Australia, in 1975. He is an Associate Professor in the Medical Faculty, University of NSW, and Adjunct Professor at the University of Technology in Sydney. He is actively involved in teaching medical undergraduates and postgraduates. He is interested in infections in the immunocompromised host, antimicrobial therapy, and parasitology. His particular research interests in the laboratory are in parasitology and mycology.

John T. Ellis completed a Ph.D. thesis on leishmaniasis with J. M. Crampton at the Liverpool School of Tropical Medicine and subsequently did postdoctoral research on Eimeria vaccines at the Houghton Poultry Research Station and on parasite phylogeny at Flinders University of South Australia. Over the last 20 years, he has continued to study parasitic protozoa of both veterinary and medical importance. Dr. Ellis is currently a Professor of Molecular Biology, and his present research interests include development of vaccines and diagnostics for protozoal diseases of economic importance. He was awarded a D.Sc. degree by Liverpool University in 2006 for his pioneering research on the biology of cyst-forming coccidia. He is a Fellow of the Royal Society for Tropical Medicine and Hygiene and a member of the Australian, British, and American Societies for Parasitology.
REFERENCES
- 1. Abreu-Acosta N, et al. 2005. Enterocytozoon bieneusi (microsporidia) in clinical samples from immunocompetent individuals in Tenerife, Canary Islands, Spain. Trans. R. Soc. Trop. Med. Hyg. 99:848–855 [DOI] [PubMed] [Google Scholar]
- 2. Agnamey P, Djeddi D, Oukachbi Z, Totet A, Raccurt CP. 2010. Cryptosporidium hominis and Isospora belli diarrhea in travelers returning from West Africa. J. Travel Med. 17:141–142 [DOI] [PubMed] [Google Scholar]
- 3. Agnamey P, et al. 2011. Evaluation of four commercial rapid immunochromatographic assays for detection of Cryptosporidium antigens in stool samples: a blind multicenter trial. J. Clin. Microbiol. 49:1605–1607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ahmed W, Gardner T, Toze S. 2011. Microbiological quality of roof-harvested rainwater and health risks: a review. J. Environ. Qual. 40:13–21 [DOI] [PubMed] [Google Scholar]
- 5. Ahmed W, Vieritz A, Goonetilleke A, Gardner T. 2010. Health risk from the use of roof-harvested rainwater in Southeast Queensland, Australia, as potable or nonpotable water, determined using quantitative microbial risk assessment. Appl. Environ. Microbiol. 76:7382–7391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Aikawa NE, et al. 2011. Intestinal microsporidiosis: a hidden risk in rheumatic disease patients undergoing anti-tumor necrosis factor therapy combined with disease-modifying anti-rheumatic drugs? Clinics 66:1171–1175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ali IKM, Clark CG, Petri WA., Jr 2008. Molecular epidemiology of amebiasis. Infect. Genet. Evol. 8:698–707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Aliaga-Leyton A, et al. 2011. An observational study on the prevalence and impact of Isospora suis in suckling piglets in southwestern Ontario, and risk factors for shedding oocysts. Can. Vet. J. 52:184–188 [PMC free article] [PubMed] [Google Scholar]
- 9. Alonso JL, Amorós I, Cañigral I. 2011. Development and evaluation of a real-time PCR assay for quantification of Giardia and Cryptosporidium in sewage samples Appl. Microbiol. Biotechnol. 89:1203–1211 [DOI] [PubMed] [Google Scholar]
- 10. Alum A, Rubino JR, Ijaz MK. 2010. The global war against intestinal parasites—should we use a holistic approach? Int. J. Infect. Dis. 14:e732–e738 [DOI] [PubMed] [Google Scholar]
- 11. Amar C, et al. 2007. Detection by PCR of eight groups of enteric pathogens in 4,627 faecal samples: re-examination of the English case-control Infectious Intestinal Disease Study (1993–1996). Eur. J. Clin. Microbiol. Infect. Dis. 26:311–323 [DOI] [PubMed] [Google Scholar]
- 12. Amin OM. 1998. Seasonal prevalence and host relationships of Cyclospora cayetanensis in North America during 1996. Parasitol. Int. 47:53–58 [Google Scholar]
- 13. Anane S, Attouchi H. 2010. Microsporidiosis: epidemiology, clinical data and therapy. Gastroenterol. Clin. Biol. 34:450–464 [DOI] [PubMed] [Google Scholar]
- 14. Anaya-Velazquez F, Padilla-Vaca F. 2011. Virulence of Entamoeba histolytica: a challenge for human health research. Future Microbiol. 6:255–258 [DOI] [PubMed] [Google Scholar]
- 15. Anderson KA, et al. 2004. Impact of Giardia vaccination on asymptomatic Giardia infections in dogs at a research facility. Can. Vet. J. 45:924–930 [PMC free article] [PubMed] [Google Scholar]
- 16. Areeshi M, et al. 2008. Cryptosporidium species causing acute diarrhoea in children in Antananarivo, Madagascar. Ann. Trop. Med. Parasitol. 102:309–315 [DOI] [PubMed] [Google Scholar]
- 17. Armson A, et al. 2009. Giardia genotypes in pigs in Western Australia: prevalence and association with diarrhea. Exp. Parasitol. 121:381–383 [DOI] [PubMed] [Google Scholar]
- 18. Ashbolt NJ, Grabow WOK, Snozzi M. 2001. Indicators of microbial water quality, p 289–316 In Fewtrell L, Bartram J. (ed), Water quality: guidelines, standards and health. IWA Publishing, London, United Kingdom [Google Scholar]
- 19. Ashley DVM, Walters C, Dockery-Brown C, McNab A, Ashley DEC. 2004. Interventions to prevent and control food-borne diseases associated with a reduction in traveler's diarrhea in tourists to Jamaica. J. Travel Med. 11:364–369 [DOI] [PubMed] [Google Scholar]
- 20. Assavasilavasukul P, Lau BLT, Harrington GW, Hoffman RM, Borchardt MA. 2008. Effect of pathogen concentrations on removal of Cryptosporidium and Giardia by conventional drinking water treatment. Water Res. 42:2678–2690 [DOI] [PubMed] [Google Scholar]
- 21. Bakheit MA, et al. 2008. Sensitive and specific detection of Cryptosporidium species in PCR-negative samples by loop-mediated isothermal DNA amplification and confirmation of generated LAMP products by sequencing. Vet. Parasitol. 158:11–22 [DOI] [PubMed] [Google Scholar]
- 22. Banik GR, et al. 2011. A case-controlled study of Dientamoeba fragilis infections in children. Parasitology 138:819–823 [DOI] [PubMed] [Google Scholar]
- 23. Barceló D, Petrovic M, Fuerhacker M, Haile T. 2011. Treatment and reuse of sludge, p 63–92 In Waste water treatment and reuse in the Mediterranean region, vol 14. Springer, Berlin, Germany [Google Scholar]
- 24. Barnes GL, Uren E, Stevens KB, Bishop RF. 1998. Etiology of acute gastroenteritis in hospitalized children in Melbourne, Australia, from April 1980 to March 1993. J. Clin. Microbiol. 36:133–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Barratt J, Harkness J, Marriott D, Ellis J, Stark D. 2011. The ambiguous life of Dientamoeba fragilis: the need to investigate current hypotheses on transmission. Parasitology 138:557–572 [DOI] [PubMed] [Google Scholar]
- 26. Barratt J, Harkness J, Marriott D, Ellis J, Stark D. 2011. A review of Dientamoeba fragilis carriage in humans: several reasons why this organism should be considered in the diagnosis of gastrointestinal illness. Gut Microbes 2:3–12 [DOI] [PubMed] [Google Scholar]
- 27. Barratt JLN, et al. 2010. Newly defined conditions for the in vitro cultivation and cryopreservation of Dientamoeba fragilis: new techniques set to fast track molecular studies on this organism. Parasitology 137:1867–1878 [DOI] [PubMed] [Google Scholar]
- 28. Bean C, et al. 2007. Class B alkaline stabilization to achieve pathogen inactivation. Int. J. Environ. Res. Public Health 4:53–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bern C, et al. 2005. The epidemiology of intestinal microsporidiosis in patients with HIV/AIDS in Lima, Peru. J. Infect. Dis. 191:1658–1664 [DOI] [PubMed] [Google Scholar]
- 30. Betancourt WQ, Rose JB. 2004. Drinking water treatment processes for removal of Cryptosporidium and Giardia. Vet. Parasitol. 126:219–234 [DOI] [PubMed] [Google Scholar]
- 31. Björkman C, Svensson C, Christensson B, de Verdier K. 2003. Cryptosporidium parvum and Giardia intestinalis in calf diarrhoea in Sweden. Acta Vet. Scand. 44:145–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Boorom K, et al. 2008. Oh my aching gut: irritable bowel syndrome, Blastocystis, and asymptomatic infection. Parasit. Vectors 1:40 doi:10.1186/1756-3305-1-40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Boreham PFL, Upcroft JA, Upcroft P. 1990. Changing approaches to the study of Giardia epidemiology: 1681–2000. Int. J. Parasitol. 20:479–487 [DOI] [PubMed] [Google Scholar]
- 34. Bridge J, et al. 2010. Engaging with the water sector for public health benefits: waterborne pathogens and diseases in developed countries. Bull. World Health Organ. 88:873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Britton E, Hales S, Venugopal K, Baker MG. 2010. Positive association between ambient temperature and salmonellosis notifications in New Zealand, 1965–2006. Aust. N. Zeal. J. Public Health 34:126–129 [DOI] [PubMed] [Google Scholar]
- 36. Bumb R, Mehta R. 2006. Amoebiasis cutis in HIV positive patient. Indian J. Dermatol. Venereol. Leprol. 72:224–226 [DOI] [PubMed] [Google Scholar]
- 37. Busatti HG, Santos JF, Gomes MA. 2009. The old and new therapeutic approaches to the treatment of giardiasis: where are we? Biologics 3:273–287 [PMC free article] [PubMed] [Google Scholar]
- 38. Buzby JC, Roberts T. 2009. The economics of enteric infections: human foodborne disease costs. Gastroenterology 136:1851–1862 [DOI] [PubMed] [Google Scholar]
- 39. Cabada MM, White ACJ. 2010. Treatment of cryptosporidiosis: do we know what we think we know? Curr. Opin. Infect. Dis. 23:494–499 [DOI] [PubMed] [Google Scholar]
- 40. Cacciò SM, Ryan U. 2008. Molecular epidemiology of giardiasis. Mol. Biochem. Parasitol. 160:75–80 [DOI] [PubMed] [Google Scholar]
- 41. Calderaro A, et al. 2006. Entamoeba histolytica and Entamoeba dispar: comparison of two PCR assays for diagnosis in a non-endemic setting. Trans. R. Soc. Trop. Med. Hyg. 100:450–457 [DOI] [PubMed] [Google Scholar]
- 42. Cama V, Ortega YR. 2006. Coccidian parasites: Cyclospora cayetanensis, Isospora belli, Sarcocystis hominis/suihominis, p 33–55 In Ortega YR. (ed), Foodborne parasites. Springer, New York, NY [Google Scholar]
- 43. Carey CM, Lee H, Trevors JT. 2004. Biology, persistence and detection of Cryptosporidium parvum and Cryptosporidium hominis oocyst. Water Res. 38:818–862 [DOI] [PubMed] [Google Scholar]
- 44. Carlin EP, Bowman DD, Scarlett JM. 2006. Prevalence of Giardia in symptomatic dogs and cats in the United States. Vet. Ther. 7:199–206 [PubMed] [Google Scholar]
- 45. Carmena D. 2010. Waterborne transmission of Cryptosporidium and Giardia: detection, surveillance and implications for public health, p 3–14 In Mendez-Vilas A. (ed), Current research, technology and education topics in applied microbiology and microbial biotechnology, 2nd ed Formatex Research Center, Badajoz, Spain [Google Scholar]
- 46. Carmena D, Aguinagalde X, Zigorraga C, Fernández-Crespo JC, Ocio JA. 2007. Presence of Giardia cysts and Cryptosporidium oocysts in drinking water supplies in northern Spain. J. Appl. Microbiol. 102:619–629 [DOI] [PubMed] [Google Scholar]
- 47. Carvalho-Costa FA, et al. 2007. Detection of Cryptosporidium spp and other intestinal parasites in children with acute diarrhea and severe dehydration in Rio de Janeiro. Rev. Soc. Bras. Med. Trop. 40:346–348 [DOI] [PubMed] [Google Scholar]
- 48. Castro-Hermida JA, García-Presedo I, González-Warleta M, Mezo M. 2010. Cryptosporidium and Giardia detection in water bodies of Galicia, Spain. Water Res. 44:5887–5896. [DOI] [PubMed] [Google Scholar]
- 49. CDC 2010. Laboratory diagnosis of amebiasis: Entamoeba histolytica and Entamoeba dispar. CDC, Atlanta, GA [Google Scholar]
- 50. Certad G, et al. 2010. Fulminant cryptosporidiosis associated with digestive adenocarcinoma in SCID mice infected with Cryptosporidium parvum TUM1 strain. Int. J. Parasitol. 40:1469–1475 [DOI] [PubMed] [Google Scholar]
- 51. Certad G, et al. 2010. Development of Cryptosporidium parvum-induced gastrointestinal neoplasia in severe combined immunodeficiency (SCID) mice: severity of lesions is correlated with infection intensity. Am. J. Trop. Med. Hyg. 82:257–265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Certad G, et al. 2007. Cryptosporidium parvum, a potential cause of colic adenocarcinoma. Infect. Agents Cancer 2:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Chacín-Bonilla L. 2010. Epidemiology of Cyclospora cayetanensis: a review focusing in endemic areas. Acta Trop. 115:181–193 [DOI] [PubMed] [Google Scholar]
- 54. Chacín-Bonilla L. 2008. Transmission of Cyclospora cayetanensis infection: a review focusing on soil-borne cyclosporiasis. Trans. R. Soc. Trop. Med. Hyg. 102:215–216 [DOI] [PubMed] [Google Scholar]
- 55. Chalmers R, Smith R, Hadfield S, Elwin K, Giles M. 2011. Zoonotic linkage and variation in Cryptosporidium parvum from patients in the United Kingdom. Parasitol. Res. 108:1321–1325 [DOI] [PubMed] [Google Scholar]
- 56. Chalmers RM, Campbell BM, Crouch N, Charlett A, Davies AP. 2011. Comparison of diagnostic sensitivity and specificity of seven Cryptosporidium assays used in the UK. J. Med. Microbiol. 60:1598–1604 [DOI] [PubMed] [Google Scholar]
- 57. Chalmers RM, Davies AP. 2010. Clinical cryptosporidiosis. Exp. Parasitol. 124:138–146 [DOI] [PubMed] [Google Scholar]
- 58. Champion L, et al. 2010. Fumagillin for treatment of intestinal microsporidiosis in renal transplant recipients. Am. J. Transplant. 10:1925–1930 [DOI] [PubMed] [Google Scholar]
- 59. Charron DF, et al. 2004. Vulnerability of waterborne diseases to climate change in Canada: a review. J. Toxicol. Environ. Health 67:1667–1677 [DOI] [PubMed] [Google Scholar]
- 60. Cheun H-I, et al. 2010. Infection status of hospitalized diarrheal patients with gastrointestinal protozoa, bacteria, and viruses in the Republic of Korea. Korean J. Parasitol. 48:113–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Chomel B, Sun B. 2011. Zoonoses in the bedroom. Emerg. Infect. Dis. 17:167–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Chou W-C, et al. 2010. Modeling the impact of climate variability on diarrhea-associated diseases in Taiwan (1996–2007). Sci. Total Environ. 409:43–51 [DOI] [PubMed] [Google Scholar]
- 63. Clarke S. 2011. Reducing the public health risk of cryptosporidiosis by optimizing treatment processes at a military water system. U.S. Army Med. Dep. J. Jul-Sep:50–61 [PubMed] [Google Scholar]
- 64. Coffey R, Bergin D, Cummins E. 2010. Use of meta-analysis to assess the effect of conventional water treatment methods on the prevalence of Cryptosporidium spp. in drinking water. Human Ecol. Risk Assess. 16:1360–1378 [Google Scholar]
- 65. Coklin T, Farber J, Parrington L, Dixon B. 2007. Prevalence and molecular characterization of Giardia duodenalis and Cryptosporidium spp. in dairy cattle in Ontario, Canada. Vet. Parasitol. 150:297–305 [DOI] [PubMed] [Google Scholar]
- 66. Collier SA, et al. 2011. Cryptosporidiosis outbreak at a summer camp—North Carolina, 2009. MMWR Morb. Mortal. Wkly. Rep. 60:918–922 [PubMed] [Google Scholar]
- 67. Collignon PJ. 2009. Water recycling—forwards or backwards for public health? Med. J. Aust. 191:238–239 [DOI] [PubMed] [Google Scholar]
- 68. Collin C. 2009. Biosand filtration of high turbidity water: modified filter design and safe filtrate storage. Massachusetts Institute of Technology, Cambridge, MA [Google Scholar]
- 69. Corradi N, Keeling PJ. 2009. Microsporidia: a journey through radical taxonomical revisions. Fungal Biol. Rev. 23:1–8 [Google Scholar]
- 70. Costello A, et al. 2009. Managing the health effects of climate change. Lancet 373:1693–1733 [DOI] [PubMed] [Google Scholar]
- 71. Coyle CM, Varughese J, Weiss LM, Tanowitz HB. 2012. Blastocystis: to treat or not to treat. Clin. Infect. Dis. 54:105–110 [DOI] [PubMed] [Google Scholar]
- 72. Craun GF, et al. 2010. Causes of outbreaks associated with drinking water in the United States from 1971 to 2006. Clin. Microbiol. Rev. 23:507–528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Cretikos M, Telfer B, McAnulty J. 2008. Evaluation of the system of surveillance for enteric disease outbreaks, New South Wales, Australia, 2000 to 2005. N. S. W. Public Health Bull. 19:8–14 [DOI] [PubMed] [Google Scholar]
- 74. Creusy C, Certad G, Guyot K, Dei-Cas E. 2008. Parasites and oncogenesis with a special reference to gastro-intestinal neoplasia induced by Cryptosporidium parvum, p 381–388 In Magni MV. (ed), Detection of bacteria, viruses, parasites and fungi: bioterrorism prevention. Springer, Dordrecht, The Netherlands [Google Scholar]
- 75. Cummins E, Kennedy R, Cormican M. 2010. Quantitative risk assessment of Cryptosporidium in tap water in Ireland. Sci. Total Environ. 408:740–753 [DOI] [PubMed] [Google Scholar]
- 76. Current WL, et al. 1983. Human cryptosporidiosis in immunocompetent and immunodeficient persons. N. Engl. J. Med. 308:1252–1257 [DOI] [PubMed] [Google Scholar]
- 77. Curriero FC, Patz JA, Rose JB, Lele S. 2001. The association between extreme precipitation and waterborne disease outbreaks in the United States, 1948–1994. Am. J. Public Health 91:1194–1199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Curry A, Canning EU. 1993. Human microsporidiosis. J. Infect. 27:229–236 [DOI] [PubMed] [Google Scholar]
- 79. Dawson D. 2005. Foodborne protozoan parasites. Int. J. Food Microbiol. 103:207–227 [DOI] [PubMed] [Google Scholar]
- 80. Demirci M, Delibas N, Altuntas I, Oktem F, Yönden Z. 2003. Serum iron, zinc and copper levels and lipid peroxidation in children with chronic giardiasis. J. Health Popul. Nutr. 21:72–75 [PubMed] [Google Scholar]
- 81. Dengjel B, et al. 2001. Zoonotic potential of Enterocytozoon bieneusi. J. Clin. Microbiol. 39:4495–4499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Denoeud F, et al. 2011. Genome sequence of the stramenopile Blastocystis, a human anaerobic parasite. Genome Biol. 12:R29 doi:10.1186/gb-2011-12-3-r29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Derouin F, Lagrange-Xelot M. 2008. Treatment of parasitic diarrhea in HIV-infected patients. Expert Rev. Anti Infect. 6:337–349 [DOI] [PubMed] [Google Scholar]
- 84. de Wit M, et al. 2001. Gastroenteritis in sentinel general practices, The Netherlands. Emerg. Infect. Dis. 7:82–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. de Wit M, et al. 2001. Etiology of gastroenteritis in sentinel general practices, The Netherlands. Clin. Infect. Dis. 33:280–288 [DOI] [PubMed] [Google Scholar]
- 86. Didier ES. 2005. Microsporidiosis: an emerging and opportunistic infection in humans and animals. Acta Trop. 94:61–76 [DOI] [PubMed] [Google Scholar]
- 87. Didier ES, Weiss LM. 2011. Microsporidiosis: not just in AIDS patients. Curr. Opin. Infect. Dis. 24:490–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Didier ES, Weiss LM. 2008. Overview of microsporidia and microsporidiosis. Protistology 5:243–255 [Google Scholar]
- 89. Dixon B, et al. 2011. The potential for zoonotic transmission of Giardia duodenalis and Cryptosporidium spp. from beef and dairy cattle in Ontario, Canada. Vet. Parasitol. 175:20–26 [DOI] [PubMed] [Google Scholar]
- 90. Dogruman-Al F, et al. 2009. Blastocystis subtypes in irritable bowel syndrome and inflammatory bowel disease in Ankara, Turkey. Mem. Inst. Oswaldo Cruz 104:724–727 [DOI] [PubMed] [Google Scholar]
- 91. Dogruman-Al F, et al. 2010. Comparison of methods for detection of Blastocystis infection in routinely submitted stool samples, and also in IBS/IBD patients in Ankara, Turkey. PLoS One 5:e15484 doi:10.1371/journal.pone.0015484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Döller PC, et al. 2002. Cyclosporiasis outbreak in Germany associated with the consumption of salad. Emerg. Infect. Dis. 8:992–994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Dong J, Olano JP, McBride JW, Walker DH. 2008. Emerging pathogens: challenges and successes of molecular diagnostics. J. Mol. Diagn. 10:185–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Dorny P, Praet N, Deckers N, Gabriel S. 2009. Emerging food-borne parasites. Vet. Parasitol. 163:196–206 [DOI] [PubMed] [Google Scholar]
- 95. Duffy G, Lynch OA, Cagney C. 2008. Tracking emerging zoonotic pathogens from farm to fork. Meat Sci. 78:34–42 [DOI] [PubMed] [Google Scholar]
- 96. Dworkin MS, et al. 2007. Prevalence of intestinal microsporidiosis in human immunodeficiency virus-infected patients with diarrhea in major United States cities. Rev. Inst. Med. Trop. Sao Paulo 49:339–342 [DOI] [PubMed] [Google Scholar]
- 97. Ebi K. 2008. Adaptation costs for climate change-related cases of diarrhoeal disease, malnutrition, and malaria in 2030. Global Health 4:9 doi:10.1186/1744-8603-4-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Ebi K. 2008. Healthy people 2100: modeling population health impacts of climate change. Climatic Change 88:5–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Ebi KL, Paulson JA. 2010. Climate change and child health in the United States. Curr. Probl. Pediatr. Adolesc. Health Care 40:2–18 [DOI] [PubMed] [Google Scholar]
- 100. Edvinsson B, Lappalainen M, Anttila V-J, Paetau A, Evengård B. 2009. Toxoplasmosis in immunocompromized patients. Scand. J. Infect. Dis. 41:368–371 [DOI] [PubMed] [Google Scholar]
- 101. Egyed Z, Sréter T, Széll Z, Varga I. 2003. Characterization of Cryptosporidium spp.—recent developments and future needs. Vet. Parasitol. 111:103–114 [DOI] [PubMed] [Google Scholar]
- 102. El-Moamly AA, El-Sweify MA. 2012. ImmunoCard STAT!cartridge antigen detection assay compared to microplate enzyme immunoassay and modified Kinyoun's acid-fast staining technique for detection of Cryptosporidium in fecal specimens. Parasitol. Res. 110:1037–1041 [DOI] [PubMed] [Google Scholar]
- 103. Environment Protection and Heritage Council, National Health and Medical Research Council, Natural Resource Management Ministerial Council 2008. Australian guidelines for water recycling: managing health and environmental risks (phase 2). Stormwater harvesting and reuse. Biotext, Canberra, Australia [Google Scholar]
- 104. Environment Protection Authority 2000. Environmental guidelines: use and disposal of biosolids products. EPA guideline 97/62. Environment Protection Authority, Sydney, NSW, Australia [Google Scholar]
- 105. Environmental Protection Agency 1993. 40 CFR parts 257, 403, 503 Standards for use or disposal of sewage sludge, vol 58. FR 9248. Office of Water, Washington, DC [Google Scholar]
- 106. Environmental Protection Agency 2005. Method 1623: Cryptosporidium and Giardia in water by filtration/IMS/FA. Office of Water, Washington, DC [Google Scholar]
- 107. Erickson MC, Ortega YR. 2006. Inactivation of protozoan parasites in food, water, and environmental systems. J. Food Prot. 69:2786–2808 [DOI] [PubMed] [Google Scholar]
- 108. ESCCAP (ed). 2011. Control of intestinal protozoa in dogs and cats. Guideline no. 6. ESCCAP, Worcestershire, United Kingdom [Google Scholar]
- 109. Escobedo AA, et al. 2009. Treatment of intestinal protozoan infections in children. Arch. Dis. Child. 94:478–482 [DOI] [PubMed] [Google Scholar]
- 110. Escobedo AA, et al. 2010. Giardiasis: the ever-present threat of a neglected disease. Infect. Disord. Drug Targets 10:329–348 [DOI] [PubMed] [Google Scholar]
- 111. Esteban J, Aguirre C, Angles R, Ash L, Mas-Coma S. 1998. Balantidiasis in Aymara children from the northern Bolivian Altiplano. Am. J. Trop. Med. Hyg. 59:922–927 [DOI] [PubMed] [Google Scholar]
- 112. European Environment Agency 1986. Council directive 86/278/EEC of 12 June 1986 on the protection of the environment, and in particular of the soil, when sewage sludge is used in agriculture. European Environment Agency, Copenhagen, Denmark [Google Scholar]
- 113. FAO and WHO 2008. Microbiological hazards associated with fresh produce. Food and Agriculture Organization of the United Nations and World Health Organization, Geneva, Switzerland [Google Scholar]
- 114. Farthing MJG, Kelly P. 2005. Protozoal gastrointestinal infections. Medicine 33:81–83 [Google Scholar]
- 115. Fayer R, Dubey JP, Lindsay DS. 2004. Zoonotic protozoa: from land to sea. Trends Parasitol. 20:531–536 [DOI] [PubMed] [Google Scholar]
- 116. Fayer R, Morgan U, Upton SJ. 2000. Epidemiology of Cryptosporidium: transmission, detection and identification. Int. J. Parasitol. 30:1305–1322 [DOI] [PubMed] [Google Scholar]
- 117. Feasey NA, Healey P, Gordon MA. 2011. Review article: the aetiology, investigation and management of diarrhoea in the HIV-positive patient. Aliment. Pharmacol. Ther. 34:587–603 [DOI] [PubMed] [Google Scholar]
- 118. Feng Y, Xiao L. 2011. Zoonotic potential and molecular epidemiology of Giardia species and giardiasis. Clin. Microbiol. Rev. 24:110–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Ferreira CS, Amato Neto V, Alarcan RSR, Gakiya E. 2001. Identification of Cryptosporidium spp. oocysts in fecal smears stained with Heidenhain's iron hematoxylin. Rev. Inst. Med. Trop. Sao Paulo 43:341–342 [DOI] [PubMed] [Google Scholar]
- 120. Ferry T, et al. 2004. Severe peritonitis due to Balantidium coli acquired in France. Eur. J. Clin. Microbiol. Infect. Dis. 23:393–395 [DOI] [PubMed] [Google Scholar]
- 121. Field KG, Samadpour M. 2007. Fecal source tracking, the indicator paradigm, and managing water quality. Water Res. 41:3517–3538 [DOI] [PubMed] [Google Scholar]
- 122. Field V, et al. 2010. Travel and migration associated infectious diseases morbidity in Europe, 2008. BMC Infect. Dis. 10:330 doi:10.1186/1471-2334-10-330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Figueras MJ, Borrego JJ. 2010. New perspectives in monitoring drinking water microbial quality. Int. J. Environ. Res. Public Health 7:4179–4202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Reference deleted.
- 125. Fletcher SM, Maharaj SR, James K. 2009. Description of the food safety system in hotels and how it compares with HACCP standards. J. Travel Med. 16:35–41 [DOI] [PubMed] [Google Scholar]
- 126. Fletcher SM, Stark D, Ellis J. 2011. Prevalence of gastrointestinal pathogens in sub-Saharan Africa. Systematic review and meta-analysis. J. Public Health Afr. 2:127–137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Fletcher SM, Stark D, Ellis J, Harkness J. 2010. Etiology of gastrointestinal illness in patients in Sydney. 74th Annu. Educ. Conf. Exhib 2010, 6 to 9 June 2010, Denver, CO [Google Scholar]
- 128. Fleury MD, Stratton J, Tinga C, Charron DF, Aramini J. 2008. A descriptive analysis of hospitalization due to acute gastrointestinal illness in Canada, 1995–2004. Can. J. Public Health 99:489–493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Ford A, Duke T, Campbell H. 2009. Evidence behind the WHO guidelines: hospital care for children. What is the aetiology and treatment of chronic diarrhoea in children with HIV? J. Trop. Pediatr. 55:349–355 [DOI] [PubMed] [Google Scholar]
- 130. Foronda P, et al. 2008. Identification of genotypes of Giardia intestinalis of human isolates in Egypt. Parasitol. Res. 103:1177–1181 [DOI] [PubMed] [Google Scholar]
- 131. Fotedar R, et al. 2007. Laboratory diagnostic techniques for Entamoeba species. Clin. Microbiol. Rev. 20:511–532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Fotedar R, Stark DJ, Marriott DJ, Ellis JT, Harkness JL. 2008. Entamoeba moshkovskii infections in Sydney, Australia. Eur. J. Clin. Microbiol. Infect. Dis. 27:133–137 [DOI] [PubMed] [Google Scholar]
- 133. Fox LM, Saravolatz LD. 2005. Nitazoxanide: a new thiazolide antiparasitic agent. Clin. Infect. Dis. 40:1173–1180 [DOI] [PubMed] [Google Scholar]
- 134. Franzen O, et al. 2009. Draft genome sequencing of Giardia intestinalis assemblage B isolate GS: is human giardiasis caused by two different species? PLoS Pathog. 5:e1000560 doi:10.1371/journal.ppat.1000560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Gallay A, et al. 2006. A large multi-pathogen waterborne community outbreak linked to faecal contamination of a groundwater system, France, 2000. Clin. Microbiol. Infect. 12:561–570 [DOI] [PubMed] [Google Scholar]
- 136. Galvan AL, et al. 2011. First cases of microsporidiosis in transplant recipients in Spain and review of the literature. J. Clin. Microbiol. 49:1301–1306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Garcia LS. 1994. Diagnostic medical parasitology: an update. Clin. Microbiol. Newsl. 16:105–110 [Google Scholar]
- 138. Garcia LS, Bruckner DA, Brewer TC, Shimizu RY. 1983. Techniques for the recovery and identification of Cryptosporidium oocysts from stool specimens. J. Clin. Microbiol. 18:185–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Garcia LS, Shimizu RY. 1997. Evaluation of nine immunoassay kits (enzyme immunoassay and direct fluorescence) for detection of Giardia lamblia and Cryptosporidium parvum in human fecal specimens. J. Clin. Microbiol. 35:1526–1529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Garcia LS, Shimizu RY, Bernard CN. 2000. Detection of Giardia lamblia, Entamoeba histolytica/Entamoeba dispar, and Cryptosporidium parvum antigens in human fecal specimens using the Triage parasite panel enzyme immunoassay. J. Clin. Microbiol. 38:3337–3340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Garcia LS, Shimizu RY, Novak S, Carroll M, Chan F. 2003. Commercial assay for detection of Giardia lamblia and Cryptosporidium parvum antigens in human fecal specimens by rapid solid-phase qualitative immunochromatography. J. Clin. Microbiol. 41:209–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Gerba C, Pepper I, Whitehead L. 2002. A risk assessment of emerging pathogens of concern in the land application of biosolids. Water Sci. Technol. 46:225–230 [PubMed] [Google Scholar]
- 143. Gerba C, Tamimi A, Pettigrew C, Weisbrod A, Rajagopalan V. 2011. Sources of microbial pathogens in municipal solid waste landfills in the United States of America. Waste Manag Res. 29:781–790 [DOI] [PubMed] [Google Scholar]
- 144. Germani Y, et al. 1994. Two-year study of endemic enteric pathogens associated with acute diarrhea in New Caledonia. J. Clin. Microbiol. 32:1532–1536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Geurden T, et al. 2009. Multilocus genotyping of Cryptosporidium and Giardia in non-outbreak related cases of diarrhoea in human patients in Belgium. Parasitology 136:1161–1168 [DOI] [PubMed] [Google Scholar]
- 146. Giangaspero A, Berrilli F, Brandonisio O. 2007. Giardia and Cryptosporidium and public health: the epidemiological scenario from the Italian perspective. Parasitol. Res. 101:1169–1182 [DOI] [PubMed] [Google Scholar]
- 147. Giangaspero A, et al. 2009. Giardia and Cryptosporidium in inflowing water and harvested shellfish in a lagoon in Southern Italy. Parasitol. Int. 58:12–17 [DOI] [PubMed] [Google Scholar]
- 148. Giangaspero A, et al. 2005. Cryptosporidium parvum oocysts in seawater clams (Chamelea gallina) in Italy. Prev. Vet. Med. 69:203–212 [DOI] [PubMed] [Google Scholar]
- 149. Gilbert D, Moellering R, Eliopoulos G, Sande M. (ed). 2009. The Sanford guide to antimicrobial therapy. Antimicrobial Therapy, Inc., Sperryville, VA [Google Scholar]
- 150. Gill EE, Fast NM. 2006. Assessing the microsporidia-fungi relationship: combined phylogenetic analysis of eight genes. Gene 375:103–109 [DOI] [PubMed] [Google Scholar]
- 151. Gill ON. 1997. Cyclosporiasis in Europe: underdiagnosed or simply not a problem? Euro Surveill. 1:1061 [Google Scholar]
- 152. Girginkardeşler N, et al. 2003. Dientamoeba fragilis, a neglected cause of diarrhea, successfully treated with secnidazole. Clin. Microbiol. Infect. 9:110–113 [DOI] [PubMed] [Google Scholar]
- 153. Girginkardesler N, et al. 2008. Transmission of Dientamoeba fragilis: evaluation of the role of Enterobius vermicularis. Parasitol. Int. 57:72–75 [DOI] [PubMed] [Google Scholar]
- 154. Girones R, et al. 2010. Molecular detection of pathogens in water—the pros and cons of molecular techniques. Water Res. 44:4325–4339 [DOI] [PubMed] [Google Scholar]
- 155. Godfree A, Farrell J. 2005. Processes for managing pathogens. J. Environ. Qual. 34:105–113 [DOI] [PubMed] [Google Scholar]
- 156. Goodgame R. 2003. Emerging causes of traveler's diarrhea: Cryptosporidium, Cyclospora, Isospora, and Microsporidia. Curr. Infect. Dis. Rep. 5:66–73 [DOI] [PubMed] [Google Scholar]
- 157. Goodgame RW. 1996. Understanding intestinal spore-forming protozoa: Cryptosporidia, Microsporidia, Isospora, and Cyclospora. Ann. Intern. Med. 124:429–441 [DOI] [PubMed] [Google Scholar]
- 158. Graczyk TK, Cranfield MR, Fayer R. 1996. Evaluation of commercial enzyme immunoassay (EIA) and immunofluorescent antibody (IFA) test kits for detection of Cryptosporidium oocysts of species other than Cryptosporidium parvum. Am. J. Trop. Med. Hyg. 54:274–279 [DOI] [PubMed] [Google Scholar]
- 159. Graczyk TK, et al. 2008. Occurrence of Cryptosporidium and Giardia in sewage sludge and solid waste landfill leachate and quantitative comparative analysis of sanitization treatments on pathogen inactivation. Environ. Res. 106:27–33 [DOI] [PubMed] [Google Scholar]
- 160. Graczyk TK, Knight R, Tamang L. 2005. Mechanical transmission of human protozoan parasites by insects. Clin. Microbiol. Rev. 18:128–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Gregory R. 2002. Bench-marking pool water treatment for coping with Cryptosporidium. J. Environ. Health Res. 1:11–19 [Google Scholar]
- 162. Greig JD, Ravel A. 2009. Analysis of foodborne outbreak data reported internationally for source attribution. Int. J. Food Microbiol. 130:77–87 [DOI] [PubMed] [Google Scholar]
- 163. Gruz F, et al. 2010. Isospora belli infection after isolated intestinal transplant. Transpl. Infect. Dis. 12:69–72 [DOI] [PubMed] [Google Scholar]
- 164. Gualdieri L, et al. 2011. Intestinal parasites in immigrants in the city of Naples (southern Italy). Acta Trop. 117:196–201 [DOI] [PubMed] [Google Scholar]
- 165. Guk S-M, et al. 2005. Parasitic infections in HIV-infected patients who visited Seoul National University Hospital during the period 1995–2003. Korean J. Parasitol. 43:1–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Gunnarsdottir MJ, Gissurarson LR. 2008. HACCP and water safety plans in Icelandic water supply: preliminary evaluation of experience. J. Water Health 6:377–382 [DOI] [PubMed] [Google Scholar]
- 167. Gutiérrez-Cisneros MJ, et al. 2010. Application of real-time PCR for the differentiation of Entamoeba histolytica and E. dispar in cyst-positive faecal samples from 130 immigrants living in Spain. Ann. Trop. Med. Parasitol. 104:145–149 [DOI] [PubMed] [Google Scholar]
- 168. Hadfield SJ, Robinson G, Elwin K, Chalmers RM. 2011. Detection and differentiation of Cryptosporidium spp. in human clinical samples by use of real-time PCR. J. Clin. Microbiol. 49:918–924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Haghighi A, Kobayashi S, Takeuchi T, Masuda G, Nozaki T. 2002. Remarkable genetic polymorphism among Entamoeba histolytica isolates from a limited geographic area. J. Clin. Microbiol. 40:4081–4090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Hajdu A, et al. 2008. Investigation of Swedish cases reveals an outbreak of cryptosporidiosis at a Norwegian hotel with possible links to in-house water systems. BMC Infect. Dis. 8:152 doi:10.1186/1471-2334-8-152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Hall G, Vally H, Kirk M, Kris H. 2008. Foodborne illnesses: overview, p 638–653 In International encyclopedia of public health. Academic Press, Oxford, United Kingdom [Google Scholar]
- 172. Hall GV, D'Souza RM, Kirk MD. 2002. Foodborne disease in the new millennium: out of the frying pan and into the fire? Med. J. Aust. 177:614–618 [DOI] [PubMed] [Google Scholar]
- 173. Hall R, Jones J, Herwaldt B. 2008. Surveillance for laboratory-confirmed sporadic cases of cyclosporiasis—United States, 1997–2008. MMWR Surveill. Summ. 60:1–11 [PubMed] [Google Scholar]
- 174. Hamano S, Petri WA, Jr, Kris H. 2008. Protozoan diseases: amebiasis, p 335–341 In International encyclopedia of public health. Academic Press, Oxford, United Kingdom [Google Scholar]
- 175. Hamer DH, Gorbach SL, Kris H. 2008. Intestinal infections: overview, p 683–695 In International encyclopedia of public health. Academic Press, Oxford, United Kingdom [Google Scholar]
- 176. Hanevik K, Dizdar V, Langeland N, Hausken T. 2009. Development of functional gastrointestinal disorders after Giardia lamblia infection. BMC Gastroenterol. 9:27 doi:10.1186/1471-230X-9-27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Haque R, Ali IKM, Akther S, Petri WA. 1998. Comparison of PCR, isoenzyme analysis, and antigen detection for diagnosis of Entamoeba histolytica infection. J. Clin. Microbiol. 36:449–452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Haque R, et al. 2003. Epidemiologic and clinical characteristics of acute diarrhoea with emphasis on Entamoeba histolytica infections in preschool children in an urban slum of Dhaka, Bangladesh. Am. J. Trop. Med. Hyg. 69:398–405 [PubMed] [Google Scholar]
- 179. Haque R, Neville LM, Hahn P, Petri WA. 1995. Rapid diagnosis of Entamoeba infection by using Entamoeba and Entamoeba histolytica stool antigen detection kits. J. Clin. Microbiol. 33:2558–2561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Haque R, et al. 2007. Multiplex real-time PCR assay for detection of Entamoeba histolytica, Giardia intestinalis, and Cryptosporidium spp. Am. J. Trop. Med. Hyg. 76:713–717 [PubMed] [Google Scholar]
- 181. Harper S, Edge V, Schuster-Wallace C, Berke O, McEwen S. 2011. Weather, water quality and infectious gastrointestinal illness in two Inuit communities in Nunatsiavut, Canada: potential implications for climate change. EcoHealth 2011:1–16 [DOI] [PubMed] [Google Scholar]
- 182. Hashizume M, et al. 2007. Association between climate variability and hospital visits for non-cholera diarrhoea in Bangladesh: effects and vulnerable groups. Int. J. Epidemiol. 36:1030–1037 [DOI] [PubMed] [Google Scholar]
- 183. Havelaar AH, et al. 2010. Future challenges to microbial food safety. Int. J. Food Microbiol. 139(Suppl 1):S79–S94 [DOI] [PubMed] [Google Scholar]
- 184. Hellard ME, Sinclair MI, Harris AH, Kirk M, Fairley CK. 2003. Cost of community gastroenteritis. J. Gastroenterol. Hepatol. 18:322–328 [DOI] [PubMed] [Google Scholar]
- 185. Helmreich B, Horn H. 2009. Opportunities in rainwater harvesting. Desalination 248:118–124 [Google Scholar]
- 186. Henriques-Gil N, Haro M, Izquierdo F, Fenoy S, Del Aguila C. 2010. Phylogenetic approach to the variability of the microsporidian Enterocytozoon bieneusi and its implications for inter- and intrahost transmission. Appl. Environ. Microbiol. 76:3333–3342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Hlavsa MC, Watson JC, Beach MJ. 2005. Cryptosporidiosis surveillance—United States, 1999–2002. MMWR Morb. Mortal. Wkly. Rep. 54:1–8 [PubMed] [Google Scholar]
- 188. Hlavsa MC, Watson JC, Beach MJ. 2005. Giardiasis surveillance—United States, 1998–2002. MMWR Morb. Mortal. Wkly. Rep. 54:9–16 [PubMed] [Google Scholar]
- 189. Ho AY, et al. 2002. Outbreak of cyclosporiasis associated with imported raspberries, Philadelphia, Pennsylvania, 2000. Emerg. Infect. Dis. 8:783–788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Horman A, et al. 2004. Campylobacter spp., Giardia spp., Cryptosporidium spp., noroviruses, and indicator organisms in surface water in southwestern Finland, 2000–2001. Appl. Environ. Microbiol. 70:87–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Hotez P. 2000. The other intestinal protozoa: enteric infections caused by Blastocystis hominis, Entamoeba coli, and Dientamoeba fragilis. Semin. Pediatr. Infect. Dis. 11:178–181 [Google Scholar]
- 192. Huh JW, Moon SG, Lim YH. 2009. A survey of intestinal protozoan infections among gastroenteritis patients during a 3-year period (2004–2006) in Gyeonggi-do (Province), South Korea. Korean J. Parasitol. 47:303–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Huhulescu S, et al. 2009. Etiology of acute gastroenteritis in three sentinel general practices, Austria 2007. Infection 37:103–108 [DOI] [PubMed] [Google Scholar]
- 194. Hung C-C, et al. 2008. Increased risk for Entamoeba histolytica infection and invasive amebiasis in HIV seropositive men who have sex with men in Taiwan. PLoS Negl. Trop. Dis. 2:e175 doi:10.1371/journal.pntd.0000175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Hunter PR, Pond K, Jagals P, Cameron J. 2009. An assessment of the costs and benefits of interventions aimed at improving rural community water supplies in developed countries. Sci. Total Environ. 407:3681–3685 [DOI] [PubMed] [Google Scholar]
- 196. Hunter PR, Thompson RCA. 2005. The zoonotic transmission of Giardia and Cryptosporidium. Int. J. Parasitol. 35:1181–1190 [DOI] [PubMed] [Google Scholar]
- 197. Imhoff B, et al. 2004. Burden of self-reported acute diarrheal illness in FoodNet surveillance areas, 1998 and 1999. Clin. Infect. Dis. 38:S219–S226 [DOI] [PubMed] [Google Scholar]
- 198. Insulander M, Svenungsson B, Lebbad M, Karlsson L, de Jong B. 2010. A foodborne outbreak of Cyclospora infection in Stockholm, Sweden. Foodborne Pathog. Dis. 7:1585–1587 [DOI] [PubMed] [Google Scholar]
- 199. Iwamoto M, Ayers T, Mahon BE, Swerdlow DL. 2010. Epidemiology of seafood-associated infections in the United States. Clin. Microbiol. Rev. 23:399–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Izquierdo F, et al. 2011. Detection of microsporidia in drinking water, wastewater and recreational rivers. Water Res. 45:4837–4843 [DOI] [PubMed] [Google Scholar]
- 201. Jackson TFHG. 1998. Entamoeba histolytica and Entamoeba dispar are distinct species; clinical, epidemiological and serological evidence. Int. J. Parasitol. 28:181–186 [DOI] [PubMed] [Google Scholar]
- 202. Jagals C, Jagals P. 2004. Application of HACCP principles as a management tool for monitoring and controlling microbiological hazards in water treatment facilities. Water Sci. Technol. 50:69–76 [PubMed] [Google Scholar]
- 203. James R, Barratt J, Marriott D, Harkness J, Stark D. 2010. Seroprevalence of Entamoeba histolytica infection among men who have sex with men in Sydney, Australia. Am. J. Trop. Med. Hyg. 83:914–916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Jansen A, et al. 2008. Aetiology of community-acquired, acute gastroenteritis in hospitalised adults: a prospective cohort study. BMC Infect. Dis. 8:143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Jayalakshmi J, Appalaraju B, Mahadevan K. 2008. Evaluation of an enzyme-linked immunoassay for the detection of Cryptosporidium antigen in fecal specimens of HIV/AIDS patients. Indian J. Pathol. Microbiol. 51:137–138 [DOI] [PubMed] [Google Scholar]
- 206. Jaykus L-A, et al. 2009. Climate change: implications for food safety. FAO, Rome, Italy [Google Scholar]
- 207. Jerlstrom-Hultqvist J, Ankarklev J, Svard SG. 2010. Is human giardiasis caused by two different Giardia species? Gut Microbes 1:379–382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Jernigan J, Guerrant RL, Pearson RD. 1994. Parasitic infections of the small intestine. Gut 35:289–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Jex AR, Smith HV, Monis PT, Campbell BE, Gasser RB. 2008. Cryptosporidium—biotechnological advances in the detection, diagnosis and analysis of genetic variation. Biotechnol. Adv. 26:304–317 [DOI] [PubMed] [Google Scholar]
- 210. Jex AR, et al. 2012. Detection of diarrhoeal pathogens in human faeces using an automated, robotic platform. Mol. Cell. Probes 26:11–15 [DOI] [PubMed] [Google Scholar]
- 211. Jimenez-Gonzalez D, et al. 2012. Blastocystis infection is associated with irritable bowel syndrome in a Mexican patient population. Parasitol. Res. 110:1269–1275 [DOI] [PubMed] [Google Scholar]
- 212. Johnson J, Samarasinghe B, Buddle R, Armson A, Ryan U. 2008. Molecular identification and prevalence of Isospora sp. in pigs in Western Australia using a PCR-RFLP assay. Exp. Parasitol. 120:191–193 [DOI] [PubMed] [Google Scholar]
- 213. Johnston AR, et al. 2010. Molecular epidemiology of cross-species Giardia duodenalis transmission in western Uganda. PLoS Negl. Trop. Dis. 4:e683 doi:10.1371/journal.pntd.0000683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Johnston SP, Ballard MM, Beach MJ, Causer L, Wilkins PP. 2003. Evaluation of three commercial assays for detection of Giardia and Cryptosporidium organisms in fecal specimens. J. Clin. Microbiol. 41:623–626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Jokipii L, Pohjola S, Jokipii AMM. 1983. Cryptosporidium: a frequent finding in patients with gastrointestinal symptoms. Lancet 322:358–361 [DOI] [PubMed] [Google Scholar]
- 216. Jones M, Whipps C, Ganac R, Hudson N, Boroom K. 2009. Association of Blastocystis subtype 3 and 1 with patients from an Oregon community presenting with chronic gastrointestinal illness. Parasitol. Res. 104:341–345 [DOI] [PubMed] [Google Scholar]
- 217. Jones P, Martin M. 2003. The occurrence and survival of pathogens of animals and humans in green compost. Institute for Animal Health, Waste and Resources Action Program, Banbury, Oxon, United Kingdom [Google Scholar]
- 218. Kappagoda S, Singh U, Blackburn B. 2011. Antiparasitic therapy. Mayo Clin. Proc. 86:561–583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. Karanis P, Kourenti C, Smith H. 2007. Waterborne transmission of protozoan parasites: a worldwide review of outbreaks and lessons learnt. J. Water Health 5:1–38 [DOI] [PubMed] [Google Scholar]
- 220. Keller SE. 2009. Microbial contamination of fresh produce, p 25–45 In Intentional and unintentional contaminants in food and feed, vol 1020 American Chemical Society, Washington, DC [Google Scholar]
- 221. Kenner BM, Rosen T. 2006. Cutaneous amebiasis in a child and review of the literature. Pediatr. Dermatol. 23:231–234 [DOI] [PubMed] [Google Scholar]
- 222. Kenny JM, Kelly P. 2009. Protozoal gastrointestinal infections. Medicine 37:599–602 [Google Scholar]
- 223. Khaldi S, et al. 2011. Intensive exploitation of a karst aquifer leads to Cryptosporidium water supply contamination. Water Res. 45:2906–2914 [DOI] [PubMed] [Google Scholar]
- 224. Khasnis AA, Nettleman MD. 2005. Global warming and infectious disease. Arch. Med. Res. 36:689–696 [DOI] [PubMed] [Google Scholar]
- 225. Klein E, et al. 2006. Diarrhea etiology in a children's hospital emergency department: a prospective cohort study. Clin. Infect. Dis. 43:807–813 [DOI] [PubMed] [Google Scholar]
- 226. Kniel KE, Shearer AEH, Cascarino JL, Wilkins GC, Jenkins MC. 2007. High hydrostatic pressure and UV light treatment of produce contaminated with Eimeria acervulina as a Cyclospora cayetanensis surrogate. J. Food Prot. 70:2837–2842 [DOI] [PubMed] [Google Scholar]
- 227. Koru O, et al. 2007. Case report: Isospora belli infection in a renal transplant recipent. Turkiye Parazitol. Derg. 31:98–100 [PubMed] [Google Scholar]
- 228. Kucerova Z, et al. 2011. Microsporidiosis and cryptosporidiosis in HIV/AIDS patients in St. Petersburg, Russia: serological identification of microsporidia and Cryptosporidium parvum in sera samples from HIV/AIDS patients. AIDS Res. Hum. Retroviruses 27:13–15 [DOI] [PubMed] [Google Scholar]
- 229. Lagacé-Wiens PR, VanCaeseele PG, Koschik C. 2006. Dientamoeba fragilis: an emerging role in intestinal disease. CMAJ 175:468–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. Lagrange-Xélot M, et al. 2008. Isosporiasis in patients with HIV infection in the highly active antiretroviral therapy era in France. HIV Med. 9:126–130 [DOI] [PubMed] [Google Scholar]
- 231. Lake IR, et al. 2009. Using infectious intestinal disease surveillance data to explore illness aetiology; a cryptosporidiosis case study. Health Place 15:333–339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232. Lamps LW. 2010. Entamoeba histolytica, p 169–175 In Surgical pathology of the gastrointestinal system: bacterial, fungal, viral, and parasitic infections. Springer, New York, NY [Google Scholar]
- 233. Lasek-Nesselquist E, Welch DM, Sogin ML. 2010. The identification of a new Giardia duodenalis assemblage in marine vertebrates and a preliminary analysis of G. duodenalis population biology in marine systems. Int. J. Parasitol. 40:1063–1074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234. Laupland K, Church D. 2005. Population-based laboratory surveillance for Giardia sp. and Cryptosporidium sp. infections in a large Canadian health region. BMC Infect. Dis. 5:72 doi:10.1186/1471-2334-5-72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235. Leav BA, Mackay M, Ward HD. 2003. Cryptosporidium species: new insights and old challenges. Clin. Infect. Dis. 36:903–908 [DOI] [PubMed] [Google Scholar]
- 236. Lebbad M. 2010. Molecular diagnosis and characterization of two intestinal protozoa: Entamoeba histolytica and Giardia intestinalis. Ph.D. thesis. Karolinska Institutet, Stockholm, Sweden [Google Scholar]
- 237. Lebbad M, et al. 2011. Multilocus genotyping of human Giardia isolates suggests limited zoonotic transmission and association between assemblage B and flatulence in children. PLoS Negl. Trop. Dis. 5:e1262 doi:10.1371/journal.pntd.0001262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238. Leclerc H, Schwartzbrod L, Dei-Cas E. 2002. Microbial agents associated with waterborne diseases. Crit. Rev. Microbiol. 28:371–409 [DOI] [PubMed] [Google Scholar]
- 239. Leder KS, O'Toole JE, Sinclair MI. 2009. Water recycling—forwards or backwards for public health? Med. J. Aust. 190:293–294 [DOI] [PubMed] [Google Scholar]
- 240. Lee JY, Yang J-S, Han M, Choi J. 2010. Comparison of the microbiological and chemical characterization of harvested rainwater and reservoir water as alternative water resources. Sci. Total Environ. 408:896–905 [DOI] [PubMed] [Google Scholar]
- 241. Lemarchand K, Lebaron P. 2003. Occurrence of Salmonella spp. and Cryptosporidium spp. in a French coastal watershed: relationship with fecal indicators. FEMS Microbiol. Lett. 218:203–209 [DOI] [PubMed] [Google Scholar]
- 242. Leone A, Ripabelli G, Sammarco M, Grasso G. 2009. Detection of Cryptosporidium spp. from human faeces by PCR-RFLP, cloning and sequencing. Parasitol. Res. 104:583–587 [DOI] [PubMed] [Google Scholar]
- 243. Lewis DL, Gattle DK. 2002. Pathogen risks from applying sewage sludge to land. Environ. Sci. Technol 36:286A–293A. [DOI] [PubMed] [Google Scholar]
- 244. Reference deleted.
- 245. Li Y, et al. 2009. Removal of waterborne particles by electrofiltration: pilot-scale testing. Environ. Eng. Sci. 26:1795–1803 [Google Scholar]
- 246. Li Z, Boyle F, Reynolds A. 2010. Rainwater harvesting and greywater treatment systems for domestic application in Ireland. Desalination 260:1–8 [Google Scholar]
- 247. Logar J, Andlovic A, Poljsak-Prijatelj M. 1994. Incidence of Blastocystis hominis in patients with diarrhoea. J. Infect. 28:151–154 [DOI] [PubMed] [Google Scholar]
- 248. Lores B, et al. 2002. Intestinal microsporidiosis due to Enterocytozoon bieneusi in elderly human immunodeficiency virus negative patients from Vigo, Spain. Clin. Infect. Dis. 34:918–921 [DOI] [PubMed] [Google Scholar]
- 249. Lowther SA, et al. 2000. Entamoeba histolytica/Entamoeba dispar infections in human immunodeficiency virus-infected patients in the United States. Clin. Infect. Dis. 30:955–959 [DOI] [PubMed] [Google Scholar]
- 250. Lynch M, Painter J, Woodruff R, Braden C. 2006. Surveillance for foodborne-disease outbreaks—United States, 1998–2002. MMWR Morb. Mortal. Wkly. Rep. 55:1–48 [PubMed] [Google Scholar]
- 251. Machado ECL, Stamford TLM, Alves LC, Melo RG, Shinohara NKS. 2006. Effectiveness of Cryptosporidium spp. oocysts detection and enumeration methods in water and milk samples. Arq. Bras. Med. Vet. Zootecnol. 58:432–439 [Google Scholar]
- 252. MacKenzie WR, et al. 1995. Massive outbreak of waterborne Cryptosporidium infection in Milwaukee, Wisconsin: recurrence of illness and risk of secondary transmission. Clin. Infect. Dis. 21:57–62 [DOI] [PubMed] [Google Scholar]
- 253. MacPherson DW, McQueen R. 1993. Cryptosporidiosis: multiattribute evaluation of six diagnostic methods. J. Clin. Microbiol. 31:198–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254. Madoni P. 2010. Protozoa in wastewater treatment processes: a minireview. Ital. J. Zool. 78:3–11 [Google Scholar]
- 255. Magliani W, et al. 1985. Epidemiological survey on bacterial, viral and parasitic agents in patients affected by acute enteritis. Eur. J. Epidemiol. 1:127–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256. Majuru B, Mokoena M, Jagals P, Hunter PR. 2011. Health impact of small-community water supply reliability. Int. J. Hyg. Environ. Health 214:162–166 [DOI] [PubMed] [Google Scholar]
- 257. Maldonado-Barrera C, et al. 2012. Clinical case of cerebral amebiasis caused by Entamoeba histolytica. Parasitol. Res. 110:1291–1296 [DOI] [PubMed] [Google Scholar]
- 258. Mangen M-JJ, et al. 2010. Integrated approaches for the public health prioritization of foodborne and zoonotic pathogens. Risk Anal. 30:782–797 [DOI] [PubMed] [Google Scholar]
- 259. Mansfield LS, Gajadhar AA. 2004. Cyclospora cayetanensis, a food- and waterborne coccidian parasite. Vet. Parasitol. 126:73–90 [DOI] [PubMed] [Google Scholar]
- 260. Marimoutou C, et al. 2011. Self-reporting compared to prospective surveillance to evaluate the incidence of diarrhea among French army personnel deployed to N′djamena, Chad. J. Travel Med. 18:217–220 [DOI] [PubMed] [Google Scholar]
- 261. Marques A, Nunes ML, Moore SK, Strom MS. 2010. Climate change and seafood safety: human health implications. Food Res. Int. 43:1766–1779 [Google Scholar]
- 262. Martínez-Díaz R, et al. 2011. Occurrence and genetic characterization of Giardia duodenalis from captive nonhuman primates by multi-locus sequence analysis. Parasitol. Res. 109:539–544 [DOI] [PubMed] [Google Scholar]
- 263. Mathis A, Weber R, Deplazes P. 2005. Zoonotic potential of the microsporidia. Clin. Microbiol. Rev. 18:423–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264. McIver CJ, et al. 2001. Diagnosis of enteric pathogens in children with gastroenteritis. Pathology 33:353–358 [PubMed] [Google Scholar]
- 265. Mead P, et al. 1999. Food-related illness and death in the United States. Emerg. Infect. Dis. 5:607–625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266. Meamar AR, et al. 2009. Severe diarrhea due to Isospora belli in a patient with thymoma. J. Microbiol. Immunol. Infect. 42:526–529 [PubMed] [Google Scholar]
- 267. Meeusen ENT, Walker J, Peters A, Pastoret P-P, Jungersen G. 2007. Current status of veterinary vaccines. Clin. Microbiol. Rev. 20:489–510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268. Menounos PG, et al. 2008. Direct detection of Blastocystis sp. in human faecal samples and subtype assignment using single strand conformational polymorphism and sequencing. Mol. Cell. Probes 22:24–29 [DOI] [PubMed] [Google Scholar]
- 269. Meusburger S, et al. 2007. Outbreak of acute gastroenteritis of unknown etiology caused by contaminated drinking water in a rural village in Austria, August 2006. Wien. Klin. Wochenschr. 119:717–721 [DOI] [PubMed] [Google Scholar]
- 270. Miller WA, et al. 2005. Clams (Corbicula fluminea) as bioindicators of fecal contamination with Cryptosporidium and Giardia spp. in freshwater ecosystems in California. Int. J. Parasitol. 35:673–684 [DOI] [PubMed] [Google Scholar]
- 271. Milord F, Lampron-Goulet E, St Amour M, Levac E, Ramsay D. 2011. Cyclospora cayetanensis: a description of clinical aspects of an outbreak in Quebec, Canada. Epidemiol. Infect. 27:1–7 [DOI] [PubMed] [Google Scholar]
- 272. Mirza H, Teo JDW, Upcroft J, Tan KSW. 2011. A rapid, high-throughput viability assay for Blastocystis spp. reveals metronidazole resistance and extensive subtype-dependent variations in drug susceptibilities. Antimicrob. Agents Chemother. 55:637–648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273. Mody R, et al. 2007. Intestinal parasites, p 273–307 In Immigrant medicine (1st ed). W. B. Saunders, Edinburgh, United Kingdom [Google Scholar]
- 274. Moe KT, et al. 1997. Experimental Blastocystis hominis infection in laboratory mice. Parasitol. Res. 83:319–325 [DOI] [PubMed] [Google Scholar]
- 275. Moghaddam DD, Ghadirian E, Azami M. 2005. Blastocystis hominis and the evaluation of efficacy of metronidazole and trimethoprim/sulfamethoxazole. Parasitol. Res. 96:273–275 [DOI] [PubMed] [Google Scholar]
- 276. Molina J-M, et al. 2002. Fumagillin treatment of intestinal microsporidiosis. N. Engl. J. Med. 346:1963–1969 [DOI] [PubMed] [Google Scholar]
- 277. Mondal D, Haque R, Sack RB, Kirkpatrick BD, Petri WA., Jr 2009. Attribution of malnutrition to cause-specific diarrheal illness: evidence from a prospective study of preschool children in Mirpur, Dhaka, Bangladesh. Am. J. Trop. Med. Hyg. 80:824–826 [PMC free article] [PubMed] [Google Scholar]
- 278. Mor Siobhan M, Tzipori S. 2008. Cryptosporidiosis in children in sub-Saharan Africa: a lingering challenge. Clin. Infect. Dis. 47:915–921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279. Moreno A. 2006. Climate change and human health in Latin America: drivers, effects, and policies. Reg. Environ. Change J. 6:157–164 [Google Scholar]
- 280. Mortimer L, Chadee K. 2010. The immunopathogenesis of Entamoeba histolytica. Exp. Parasitol. 126:366–380 [DOI] [PubMed] [Google Scholar]
- 281. Mundaca CC, et al. 2008. Use of PCR to improve diagnostic yield in an outbreak of cyclosporiasis in Lima, Peru. Trans. R. Soc. Trop. Med. Hyg. 102:712–717 [DOI] [PubMed] [Google Scholar]
- 282. Mundt HC, et al. 2005. Occurrence of Isospora suis in Germany, Switzerland and Austria. J. Vet. Med. B Infect. Dis. Vet. Public Health 52:93–97 [DOI] [PubMed] [Google Scholar]
- 283. Murphy SC, et al. 2011. Molecular diagnosis of cystoisosporiasis using extended-range PCR screening. J. Mol. Diagn. 13:359–362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284. Nair G, et al. 2010. Emerging trends in the etiology of enteric pathogens as evidenced from an active surveillance of hospitalized diarrhoeal patients in Kolkata, India. Gut Pathog. 2:4 doi:10.1186/1757-4749-2-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285. Nash TE, et al. 1985. Restriction-endonuclease analysis of DNA from 15 Giardia isolates obtained from humans and animals. J. Infect. Dis. 152:64–73 [DOI] [PubMed] [Google Scholar]
- 286. Navarro I, Jiménez B. 2011. Evaluation of the WHO helminth eggs criteria using a QMRA approach for the safe reuse of wastewater and sludge in developing countries. Water Sci. Technol. 63:1499–1505 [DOI] [PubMed] [Google Scholar]
- 287. Navarro I, Jiménez B, Lucario S, Cifuentes E. 2009. Application of helminth ova infection dose curve to estimate the risks associated with biosolid application on soil. J. Water Health 7:31–44 [DOI] [PubMed] [Google Scholar]
- 288. Newell DG, et al. 2010. Food-borne diseases: the challenges of 20 years ago still persist while new ones continue to emerge. Int. J. Food Microbiol. 139:S3–S15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289. Newman RD, et al. 2001. A longitudinal study of Giardia lamblia infection in north-east Brazilian children. Trop. Med. Int. Health 6:624–634 [DOI] [PubMed] [Google Scholar]
- 290. Ng J, Yang R, Whiffin V, Cox P, Ryan U. 2011. Identification of zoonotic Cryptosporidium and Giardia genotypes infecting animals in Sydney's water catchments. Exp. Parasitol. 128:138–144 [DOI] [PubMed] [Google Scholar]
- 291. Nichols GL. 1999. Food-borne protozoa. Br. Med. Bull. 55:209–235 [DOI] [PubMed] [Google Scholar]
- 292. Nichols RAB, Connelly L, Sullivan CB, Smith HV. 2010. Identification of Cryptosporidium species and genotypes in Scottish raw and drinking waters during a one-year monitoring period. Appl. Environ. Microbiol. 76:5977–5986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293. Noel C, et al. 2005. Molecular phylogenies of Blastocystis isolates from different hosts: implications for genetic diversity, identification of species, and zoonosis. J. Clin. Microbiol. 43:348–355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294. Noordeen F, Rajapakse RPVJ, Faizal ACM, Horadagoda NU, Arulkanthan A. 2000. Prevalence of Cryptosporidium infection in goats in selected locations in three agroclimatic zones of Sri Lanka. Vet. Parasitol. 93:95–101 [DOI] [PubMed] [Google Scholar]
- 295. NSW Department of Health 2009. Further increase in Cryptosporidiosis cases. NSW Department of Health, North Sydney, NSW Australia [Google Scholar]
- 296. Nuchjangreed C, Boonrod K, Ongerth J, Karanis P. 2008. Prevalence and molecular characterization of human and bovine Cryptosporidium isolates in Thailand. Parasitol. Res. 103:1347–1353 [DOI] [PubMed] [Google Scholar]
- 297. Nygård K, et al. 2006. A large community outbreak of waterborne giardiasis—delayed detection in a non-endemic urban area. BMC Res. Notes 6:141 doi:10.1186/1471-2458-6-141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298. O'Brien TF, Stelling J. 2011. Integrated multilevel surveillance of the world's infecting microbes and their resistance to antimicrobial agents. Clin. Microbiol. Rev. 24:281–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299. Oda T, et al. 2000. Size selective continuous flow filtration method for detection of Cryptosporidium and Giardia. Water Res. 34:4477–4481 [Google Scholar]
- 300. O'Donoghue PJ. 1995. Cryptosporidium and cryptosporidiosis in man and animals. Int. J. Parasitol. 25:139–195 [DOI] [PubMed] [Google Scholar]
- 301. Olesen B, et al. 2005. Etiology of diarrhea in young children in Denmark: a case-control study. J. Clin. Microbiol. 43:3636–3641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302. Olson ME, Leonard NJ, Strout J. 2010. Prevalence and diagnosis of Giardia infection in dogs and cats using a fecal antigen test and fecal smear. Can. Vet. J. 51:640–642 [PMC free article] [PubMed] [Google Scholar]
- 303. O'Reilly CE, et al. 2007. A waterborne outbreak of gastroenteritis with multiple etiologies among resort island visitors and residents: Ohio, 2004. Clin. Infect. Dis. 44:506–512 [DOI] [PubMed] [Google Scholar]
- 304. Orlandi PA, Lampel KA. 2000. Extraction-free, filter-based template preparation for rapid and sensitive PCR detection of pathogenic parasitic protozoa. J. Clin. Microbiol. 38:2271–2277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305. Ortega YR, Eberhard ML, Kris H. 2008. Protozoan diseases: cryptosporidiosis, giardiasis and other intestinal protozoan diseases, p 354–366 In International encyclopedia of public health. Academic Press, Oxford, United Kingdom [Google Scholar]
- 306. Ortega YR, Sanchez R. 2010. Update on Cyclospora cayetanensis, a food-borne and waterborne parasite. Clin. Microbiol. Rev. 23:218–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307. Ortega YR, Sterling CR. 1996. Cyclospora cayetanensis: epidemiology and diagnosis. Clin. Microbiol. Newsl. 18:169–172 [Google Scholar]
- 308. Overgaauw PAM, et al. 2009. Zoonotic parasites in fecal samples and fur from dogs and cats in the Netherlands. Vet. Parasitol. 163:115–122 [DOI] [PubMed] [Google Scholar]
- 309. Özyurt M, et al. 2008. Molecular epidemiology of Blastocystis infections in Turkey. Parasitol. Int. 57:300–306 [DOI] [PubMed] [Google Scholar]
- 310. Payment P, Berte A, Prevost M, Menard B, Barbeau B. 2000. Occurence of pathogenic microorganisims in the Saint Lawrence River (Canada) and comparison of health risks for populations using it as their source of drinking water. Can. J. Microbiol. 46:565–576 [PubMed] [Google Scholar]
- 311. Pérez-Ayala A, et al. 2011. Self-limited travelers' diarrhea by Isospora belli in a patient with dengue infection. J. Travel Med. 18:212–213 [DOI] [PubMed] [Google Scholar]
- 312. Petrášová J, et al. 2011. Diversity and host specificity of Blastocystis in syntopic primates on Rubondo Island, Tanzania. Int. J. Parasitol. 41:1113–1120 [DOI] [PubMed] [Google Scholar]
- 313. Petry F, et al. 1997. Cyclospora cayetanensis: first imported infections in Germany. Infection 25:167–170 [DOI] [PubMed] [Google Scholar]
- 314. Pieniazek NJ, Slemenda SB, da Silva AJ, Alfano EM, Arrowood MJ. 1996. PCR confirmation of infection with Cyclospora cayetanensis. Emerg. Infect. Dis. 2:357–359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315. Pierce K, Huston CD, Moselio S. 2009. Protozoan, intestinal, p 696–705 In Encyclopedia of microbiology. Academic Press, Oxford, United Kingdom [Google Scholar]
- 316. Pillai DR, et al. 1999. Entamoeba histolytica and Entamoeba dispar: epidemiology and comparison of diagnostic methods in a setting of nonendemicity. Clin. Infect. Dis. 29:1315–1318 [DOI] [PubMed] [Google Scholar]
- 317. Pitkanen T, et al. 2011. Microbial contamination of groundwater at small community water supplies in Finland. AMBIO 40:377–390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318. Plutzer J, Ongerth J, Karanis P. 2010. Giardia taxonomy, phylogeny and epidemiology: facts and open questions. Int. J. Hyg. Environ. Health 213:321–333 [DOI] [PubMed] [Google Scholar]
- 319. Plutzer J, Tomor B. 2009. The role of aquatic birds in the environmental dissemination of human pathogenic Giardia duodenalis cysts and Cryptosporidium oocysts in Hungary. Parasitol. Int. 58:227–231 [DOI] [PubMed] [Google Scholar]
- 320. Poirier P, et al. 2011. Development and evaluation of a real-time PCR assay for detection and quantification of Blastocystis parasites in human stool samples: prospective study of patients with hematological malignancies. J. Clin. Microbiol. 49:975–983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321. Poirier P, Wawrzyniak I, Vivares CP, Delbac F, El Alaoui H. 2012. New insights into Blastocystis spp.: a potential link with irritable bowel syndrome. PLoS Pathog. 8:e1002545 doi:10.1371/journal.ppat.1002545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322. Pönka A, et al. 2009. A foodborne outbreak due to Cryptosporidium parvum in Helsinki, November 2008. Euro Surveill. 14:14–16 http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=19269 [DOI] [PubMed] [Google Scholar]
- 323. Posch C, et al. 2011. Perianal ulcer—amebiasis cutis. J. Dtsch. Dermatol. Ges. 9:649–650 [DOI] [PubMed] [Google Scholar]
- 324. Pritt BS, Clark CG. 2008. Amebiasis. Mayo Clinic Proc. 83:1154–1160 [DOI] [PubMed] [Google Scholar]
- 325. Puech MC, et al. 2001. A statewide outbreak of cryptosporidiosis in New South Wales associated with swimming at public pools. Epidemiol. Infect. 126:389–396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326. Pupulin ÁR, Carvalho PG, Nishi L, Nakamura CV, Guilherme AL. 2009. Enteropathogens relating to diarrhea in HIV patients on antiretroviral therapy. Rev. Soc. Bras. Med. Trop. 42:551–555 [DOI] [PubMed] [Google Scholar]
- 327. Putignani L, Menichella D. 2010. Global distribution, public health and clinical impact of the protozoan pathogen Cryptosporidium. Interdiscip. Perspect. Infect. Dis. 2010:1–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328. Quihui L, et al. 2010. Could giardiasis be a risk factor for low zinc status in schoolchildren from northwestern Mexico? A cross-sectional study with longitudinal follow-up. BMC Public Health 10:85 doi:10.1186/1471-2458-10-85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329. Quílez J, Sánchez-Acedo C, Clavel A, del Cacho E, López-Bernad F. 1996. Prevalence of Cryptosporidium infections in pigs in Aragón (northeastern Spain). Vet. Parasitol. 67:83–88 [DOI] [PubMed] [Google Scholar]
- 330. Quintero-Betancourt W, Peele ER, Rose JB. 2002. Cryptosporidium parvum and Cyclospora cayetanensis: a review of laboratory methods for detection of these waterborne parasites. J. Microbiol. Methods 49:209–224 [DOI] [PubMed] [Google Scholar]
- 331. Rabold JG, et al. 1994. Cyclospora outbreak associated with chlorinated drinking water. Lancet 344:1360–1361 [DOI] [PubMed] [Google Scholar]
- 332. Ralston KS, Petri WA., Jr 2011. Tissue destruction and invasion by Entamoeba histolytica. Trends Parasitol. 27:254–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333. Ramasamy R, Surendran S. 2011. Possible impact of rising sea levels on vector-borne infectious diseases. BMC Infect. Dis. 11:18 doi:10.1186/1471-2334-11-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334. Ramirez-Miranda M, et al. 2010. Parasites in Mexican patients with irritable bowel syndrome: a case-control study. Parasites Vectors 3:96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335. Rautelin HI, et al. 1989. Prospective study of the etiology of diarrhea in adult outpatients and inpatients. Scand. J. Gastroenterol. 24:329–333 [DOI] [PubMed] [Google Scholar]
- 336. Reeders JW, Yee J, Gore RM, Miller FH, Megibow AJ. 2004. Gastrointestinal infection in the immunocompromised (AIDS) patient. Eur. Radiol. 14(Suppl):E84–E102 [DOI] [PubMed] [Google Scholar]
- 337. Resende D, et al. 2011. Polymorphisms in the 18S rDNA gene of Cystoisospora belli and clinical features of cystoisosporosis in HIV-infected patients. Parasitol. Res. 108:679–685 [DOI] [PubMed] [Google Scholar]
- 338. Reynolds KA, et al. 2008. Risk of waterborne illness via drinking water in the United States. Rev. Environ. Contam. Toxicol. 192:117–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339. Rimhanen-Finne R, Vuorinen A, Marmo S, Malmberg S, Hänninen ML. 2004. Comparative analysis of Cryptosporidium, Giardia and indicator bacteria during sewage sludge hygienization in various composting processes. Lett. Appl. Microbiol. 38:301–305 doi:10.1111/j.1472-765X.2004.01487.x [DOI] [PubMed] [Google Scholar]
- 340. Rimseliene G, et al. 2011. An outbreak of gastroenteritis among schoolchildren staying in a wildlife reserve: thorough investigation reveals Norway's largest cryptosporidiosis outbreak. Scand. J. Public Health 39:287–295 [DOI] [PubMed] [Google Scholar]
- 341. Roberts T, Barratt J, Harkness J, Ellis J, Stark D. 2011. Comparison of microscopy, culture, and conventional polymerase chain reaction for detection of Blastocystis sp. in clinical stool samples. Am. J. Trop. Med. Hyg. 84:308–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342. Robinson G, et al. 2011. A whole water catchment approach to investigating the origin and distribution of Cryptosporidium species. J. Appl. Microbiol. 111:717–730 [DOI] [PubMed] [Google Scholar]
- 343. Roy SL, et al. 2004. Risk factors for sporadic cryptosporidiosis among immunocompetent persons in the United States from 1999 to 2001. J. Clin. Microbiol. 42:2944–2951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344. Rudrapatna JS, Kumar V, Sridhar H. 1997. Intestinal parasitic infections in patients with malignancy. J. Diarrhoeal Dis. Res. 15:71–74 [PubMed] [Google Scholar]
- 345. Ryan U. 2010. Cryptosporidium in birds, fish and amphibians. Exp. Parasitol. 124:113–120 [DOI] [PubMed] [Google Scholar]
- 346. Sagebiel D, Weitzel T, Stark K, Leitmeyer K. 2009. Giardiasis in kindergartens: prevalence study in Berlin, Germany, 2006. Parasitol. Res. 105:681–687 [DOI] [PubMed] [Google Scholar]
- 347. Samarasinghe B, Johnson J, Ryan U. 2008. Phylogenetic analysis of Cystoisospora species at the rRNA ITS1 locus and development of a PCR-RFLP assay. Exp. Parasitol. 118:592–595 [DOI] [PubMed] [Google Scholar]
- 348. Samie A, et al. 2006. Cryptosporidium species: preliminary descriptions of the prevalence and genotype distribution among school children and hospital patients in the Venda region, Limpopo Province, South Africa. Exp. Parasitol. 114:314–322 [DOI] [PubMed] [Google Scholar]
- 349. Samie A, Obi CL, Tzipori S, Weiss LM, Guerrant RL. 2007. Microsporidiosis in South Africa: PCR detection in stool samples of HIV-positive and HIV-negative individuals and school children in Vhembe District, Limpopo Province. Trans. R. Soc. Trop. Med. Hyg. 101:547–554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350. Sancak B, Akyon Y, Erguven S. 2006. Cyclospora infection in five immunocompetent patients in a Turkish university hospital. J. Med. Microbiol. 55:459–462 [DOI] [PubMed] [Google Scholar]
- 351. Santos H, et al. 2010. Differential identification of Entamoeba spp. based on the analysis of 18S rRNA. Parasitol. Res. 106:883–888 [DOI] [PubMed] [Google Scholar]
- 352. Savarino SJ, Bourgeois AL. 1993. Epidemiology of diarrhoeal diseases in developed countries. Trans. R. Soc. Trop. Med. Hyg. 87:7–11 [DOI] [PubMed] [Google Scholar]
- 353. Savichtcheva O, Okabe S. 2006. Alternative indicators of fecal pollution: relations with pathogens and conventional indicators, current methodologies for direct pathogen monitoring and future application perspectives. Water Res. 40:2463–2476 [DOI] [PubMed] [Google Scholar]
- 354. Savioli L, Smith H, Thompson A. 2006. Giardia and Cryptosporidium join the Neglected Diseases Initiative. Trends Parasitol. 22:203–208 [DOI] [PubMed] [Google Scholar]
- 355. Sawangjaroen N, Luke R, Prociv P. 1993. Diagnosis by faecal culture of Dientamoeba fragilis infections in Australian patients with diarrhoea. Trans. R. Soc. Trop. Med. Hyg. 87:163–165 [DOI] [PubMed] [Google Scholar]
- 356. Scallan E, et al. 2011. Foodborne illness acquired in the United States—major pathogens. Emerg. Infect. Dis. 17:7–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357. Scallan E, et al. 2006. Factors associated with seeking medical care and submitting a stool sample in estimating the burden of foodborne illness. Foodborne Pathog. Dis. 3:432–438 [DOI] [PubMed] [Google Scholar]
- 358. Schaefer FW, Marshall MM, Clancy JL. 2004. Inactivation and removal of enteric protozoa in water, p 117–127 In Sterling CR, Adam RD. (ed), The pathogenic enteric protozoa: Giardia, Entamoeba, Cryptosporidium and Cyclospora. Kluwer Academic Publishers, Boston, MA [Google Scholar]
- 359. Schuster FL, Ramirez-Avila L. 2008. Current world status of Balantidium coli. Clin. Microbiol. Rev. 21:626–638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360. Schuster FL, Visvesvara GS. 2004. Amebae and ciliated protozoa as causal agents of waterborne zoonotic disease. Vet. Parasitol. 126:91–120 [DOI] [PubMed] [Google Scholar]
- 361. Schwartz MD, Nelson ME. 2003. Dientamoeba fragilis infection presenting to the emergency department as acute appendicitis. J. Emerg. Med. 25:17–21 [DOI] [PubMed] [Google Scholar]
- 362. Semenza JC, Menne B. 2009. Climate change and infectious diseases in Europe. Lancet Infect. Dis. 9:365–375 [DOI] [PubMed] [Google Scholar]
- 363. Shah N, DuPont HL, Ramsey DJ. 2009. Global etiology of travelers' diarrhea: systematic review from 1973 to the present. Am. J. Trop. Med. Hyg. 80:609–614 [PubMed] [Google Scholar]
- 364. Shannon MA, et al. 2008. Science and technology for water purification in the coming decades. Nature 452:301–310 [DOI] [PubMed] [Google Scholar]
- 365. Sherif M. 2010. Water availability and quality in the Gulf Cooperation Council countries: implications for public health. Asia Pac. J. Public Health 22:40S–47S [DOI] [PubMed] [Google Scholar]
- 366. Sheth M, Obrah M. 2004. Diarrhea prevention through food safety education. Indian J. Pediatr. 71:879–882 [DOI] [PubMed] [Google Scholar]
- 367. Shirazi S, Lin C-J, Chen D. 2009. Inorganic fouling of pressure-driven membrane processes: a critical review. Desalination 250:236–248 [Google Scholar]
- 368. Shoff WH, et al. 26 February 2010, posting date. Cyclospora: differential diagnoses & workup. Medscape. http://emedicine.medscape.com/article/236105-workup [Google Scholar]
- 369. Sidhu JPS, Toze SG. 2009. Human pathogens and their indicators in biosolids: a literature review. Environ. Int. 35:187–201 [DOI] [PubMed] [Google Scholar]
- 370. Sinclair MI, et al. 2005. Pathogens causing community gastroenteritis in Australia. J. Gastroenterol. Hepatol. 20:1685–1690 [DOI] [PubMed] [Google Scholar]
- 371. Singh M, Suresh K, Ho LC, Ng GC, Yap EH. 1995. Elucidation of the life cycle of the intestinal protozoan Blastocystis hominis. Parasitol. Res. 81:446–450 [DOI] [PubMed] [Google Scholar]
- 372. Skotarczak B. 2010. Progress in the molecular methods for the detection and genetic characterization of Cryptosporidium in water samples. Ann. Agric. Environ. Med. 17:1–8 [PubMed] [Google Scholar]
- 373. Skovgaard N. 2007. New trends in emerging pathogens. Int. J. Food Microbiol. 120:217–224 [DOI] [PubMed] [Google Scholar]
- 374. Slifko TR, Smith HV, Rose JB. 2000. Emerging parasite zoonoses associated with water and food. Int. J. Parasitol. 30:1379–1393 [DOI] [PubMed] [Google Scholar]
- 375. Smith HV, Nichols RAB. 2010. Cryptosporidium: detection in water and food. Exp. Parasitol. 124:61–79. [DOI] [PubMed] [Google Scholar]
- 376. Reference deleted.
- 377. Smith HV, Simjee S. 2007. Cyclospora, p 277–301 In Georgiev VS. (ed), Foodborne diseases. Humana Press, Totowa, NJ [Google Scholar]
- 378. Snelling WJ, Moore JE, McKenna JP, Lecky DM, Dooley JSG. 2006. Bacterial-protozoa interactions; an update on the role these phenomena play towards human illness. Microbes Infect. 8:578–587 [DOI] [PubMed] [Google Scholar]
- 379. Snow M, Chen M, Guo J, Atkinson J, Stanley SL. 2008. Differences in complement-mediated killing of Entamoeba histolytica between men and women: an explanation for the increased susceptibility of men to invasive amebiasis? Am. J. Trop. Med. Hyg. 78:922–923 [PubMed] [Google Scholar]
- 380. Sobsey MD, Stauber CE, Casanova LM, Brown JM, Elliott MA. 2008. Point of use household drinking water filtration: a practical, effective solution for providing sustained access to safe drinking water in the developing world. Environ. Sci. Technol. 42:4261–4267 [DOI] [PubMed] [Google Scholar]
- 381. Sohail MR, Fischer PR. 2005. Blastocystis hominis and travelers. Travel Med. Infect. Dis. 3:33–38 [DOI] [PubMed] [Google Scholar]
- 382. Sokolova OI, et al. 2011. Emerging microsporidian infections in Russian HIV-infected patients. J. Clin. Microbiol. 49:2102–2108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383. Solaymani-Mohammadi S, Petri JWA. 2006. Zoonotic implications of the swine-transmitted protozoal infections. Vet. Parasitol. 140:189–203 [DOI] [PubMed] [Google Scholar]
- 384. Souppart L, et al. 2010. Subtype analysis of Blastocystis isolates from symptomatic patients in Egypt. Parasitol. Res. 106:505–511 [DOI] [PubMed] [Google Scholar]
- 385. Stanley JSL. 2003. Amoebiasis. Lancet 361:1025–1034 [DOI] [PubMed] [Google Scholar]
- 386. Stark D, et al. 2011. Evaluation of multiplex tandem real-time PCR for detection of Cryptosporidium spp., Dientamoeba fragilis, Entamoeba histolytica, and Giardia intestinalis in clinical stool samples. J. Clin. Microbiol. 49:257–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387. Stark D, Barratt J, Ellis J, Harkness J, Marriott D. 2009. Repeated Dientamoeba fragilis infections: a case report of two families from Sydney, Australia. Infect. Dis. Rep. 1:7–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388. Stark D, et al. 2010. Comparison of microscopy, two xenic culture techniques, conventional and real-time PCR for the detection of Dientamoeba fragilis in clinical stool samples. Eur. J. Clin. Microbiol. Infect. Dis. 29:411–416 [DOI] [PubMed] [Google Scholar]
- 389. Stark D, et al. 2010. A review of the clinical presentation of dientamoebiasis. Am. J. Trop. Med. Hyg. 82:614–619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390. Stark D, et al. 2009. Clinical significance of enteric protozoa in the immunosuppressed human population. Clin. Microbiol. Rev. 22:634–650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391. Stark D, Beebe N, Marriott D, Ellis J, Harkness J. 2005. Detection of Dientamoeba fragilis in fresh stool specimens using PCR. Int. J. Parasitol. 35:57–62 [DOI] [PubMed] [Google Scholar]
- 392. Stark D, Beebe N, Marriott D, Ellis J, Harkness J. 2007. Dientamoeba fragilis as a cause of traveler's diarrhea: report of seven cases. J. Travel Med. 14:72–73 [DOI] [PubMed] [Google Scholar]
- 393. Stark D, Beebe N, Marriott D, Ellis J, Harkness J. 2006. Evaluation of three diagnostic methods, including real-time PCR, for detection of Dientamoeba fragilis in stool specimens. J. Clin. Microbiol. 44:232–235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394. Stark D, Beebe N, Marriott D, Ellis J, Harkness J. 2005. Prospective study of the prevalence, genotyping, and clinical relevance of Dientamoeba fragilis infections in an Australian population. J. Clin. Microbiol. 43:2718–2723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 395. Stark D, et al. 2007. Prevalence of enteric protozoa in human immunodeficiency virus (HIV)-positive and HIV negative men who have sex with men from Sydney, Australia. Am. J. Trop. Med. Hyg. 76:549–552 [PubMed] [Google Scholar]
- 396. Stark D, et al. 2008. Gorillas are a host for Dientamoeba fragilis: an update on the life cycle and host distribution. Vet. Parasitol. 151:21–26 [DOI] [PubMed] [Google Scholar]
- 397. Stark D, et al. 2010. Entamoeba histolytica, p 363–367 In Schuller M, Sloots TP, James GS, Halliday CL, Carter IWJ. (ed), PCR for clinical microbiology: an Australian and international perspective. Springer, Dordrecht, Netherlands: doi:10.1007/978-90-481-9039-3_62 [Google Scholar]
- 398. Stark D, et al. 2009. Limited genetic diversity among genotypes of Enterocytozoon bieneusi strains isolated from HIV-infected patients from Sydney, Australia. J. Med. Microbiol. 58:355–357 [DOI] [PubMed] [Google Scholar]
- 399. Stark D, van Hal S, Marriott D, Ellis J, Harkness J. 2007. Irritable bowel syndrome: a review on the role of intestinal protozoa and the importance of their detection and diagnosis. Int. J. Parasitol. 37:11–20 [DOI] [PubMed] [Google Scholar]
- 400. Stark D, van Hal S, Matthews G, Marriott D, Harkness J. 2008. Invasive amebiasis in men who have sex with men, Australia. Emerg. Infect. Dis. 14:1141–1143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 401. Stark DJ, Beebe N, Marriott D, Ellis JT, Harkness J. 2006. Dientamoebiasis: clinical importance and recent advances. Trends Parasitol. 22:92–96 [DOI] [PubMed] [Google Scholar]
- 402. Stensvold CR, Arendrup MC, Jespersgaard C, Mølbak K, Nielsen HV. 2007. Detecting Blastocystis using parasitologic and DNA-based methods: a comparative study. Diagn. Microbiol. Infect. Dis. 59:303–307 [DOI] [PubMed] [Google Scholar]
- 403. Stensvold CR, Arendrup MC, Mølbak K, Nielsen HV. 2007. The prevalence of Dientamoeba fragilis in patients with suspected enteroparasitic disease in a metropolitan area in Denmark. Clin. Microbiol. Infect. 13:839–842 [DOI] [PubMed] [Google Scholar]
- 404. Stensvold CR, Nielsen HV, Mølbak K, Smith HV. 2009. Pursuing the clinical significance of Blastocystis: diagnostic limitations. Trends Parasitol. 25:23–29. [DOI] [PubMed] [Google Scholar]
- 405. Reference deleted.
- 406. Stensvold CR, et al. 2011. The prevalence and clinical significance of intestinal parasites in HIV-infected patients in Denmark. Scand. J. Infect. Dis. 43:129–135 [DOI] [PubMed] [Google Scholar]
- 407. Stensvold CR, Smith HV, Nagel R, Olsen KEP, Traub RJ. 2010. Eradication of Blastocystis carriage with antimicrobials: reality or delusion? J. Clin. Gastroenterol. 44:85–90 [DOI] [PubMed] [Google Scholar]
- 408. Stensvold CR, et al. 2007. Terminology for Blastocystis subtypes: a consensus. Trends Parasitol. 23:93–96 [DOI] [PubMed] [Google Scholar]
- 409. Stenzel D, Boreham P. 1996. Blastocystis hominis revisited. Clin. Microbiol. Rev. 9:563–584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 410. Sterling CR, Adam RD. (ed). 2004. The pathogenic enteric protozoa: Giardia, Entamoeba, Cryptosporidium and Cyclospora. Kluwer Academic Publishers, Boston, MA [Google Scholar]
- 411. Stern N. January 2007. The economics of climate change: the Stern review. Cambridge University Press, Cambridge, United Kingdom [Google Scholar]
- 412. St. Louis ME, Hess JJ. 2008. Climate change: impacts on and implications for global health. Am. J. Prev. Med. 35:527–538 [DOI] [PubMed] [Google Scholar]
- 413. Sturdee AP, Bodley-Tickell AT, Archer A, Chalmers RM. 2003. Long-term study of Cryptosporidium prevalence on a lowland farm in the United Kingdom. Vet. Parasitol. 116:97–113 [DOI] [PubMed] [Google Scholar]
- 414. Surangsrirat S, et al. 2010. Assessment of the association between Blastocystis infection and irritable bowel syndrome. J. Med. Assoc. Thailand 93(Suppl 6):S119–S124 [PubMed] [Google Scholar]
- 415. Suresh K, Smith HV. 2004. Tropical organisms in Asia/Africa/South America, p 93–112 In Cotruvo JA, et al. (ed), Waterborne zoonoses: identification, causes and control. IWA Publishing, London, United Kingdom [Google Scholar]
- 416. Svenungsson B, et al. 2000. Enteropathogens in adult patients with diarrhea and healthy control subjects: a 1-year prospective study in a Swedish clinic for infectious diseases. Clin. Infect. Dis. 30:770–778 [DOI] [PubMed] [Google Scholar]
- 417. Swaminathan A, et al. 2009. A global study of pathogens and host risk factors associated with infectious gastrointestinal disease in returned international travellers. J. Infect. 59:19–27 [DOI] [PubMed] [Google Scholar]
- 418. Tan K, Mirza H, Teo J, Wu B, MacAry P. 2010. Current views on the clinical relevance of Blastocystis spp. Curr. Infect. Dis. Rep. 12:28–35 [DOI] [PubMed] [Google Scholar]
- 419. Tan KSW. 2004. Blastocystis in humans and animals: new insights using modern methodologies. Vet. Parasitol. 126:121–144 [DOI] [PubMed] [Google Scholar]
- 420. Tan KSW. 2008. New insights on classification, identification, and clinical relevance of Blastocystis spp. Clin. Microbiol. Rev. 21:639–665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 421. Tan KSW, Singh M, Yap EH. 2002. Recent advances in Blastocystis hominis research: hot spots in terra incognita. Int. J. Parasitol. 32:789–804 [DOI] [PubMed] [Google Scholar]
- 422. Tan T, Suresh K. 2006. Predominance of amoeboid forms of Blastocystis hominis in isolates from symptomatic patients. Parasitol. Res. 98:189–193 [DOI] [PubMed] [Google Scholar]
- 423. Tang YW, et al. 2009. Diagnostic microbiology, p 308–320 In Encyclopedia of microbiology, 3rd ed Academic Press, Oxford, United Kingdom: [Google Scholar]
- 424. Tangtrongsup S, Scorza V. 2010. Update on the diagnosis and management of Giardia spp infections in dogs and cats. Top. Companion Anim. Med. 25:155–162 [DOI] [PubMed] [Google Scholar]
- 425. Tebbutt GM, Wilson D, Holtby I. 2009. A study of 279 general outbreaks of gastrointestinal infection in the north-east region of England. Int. J. Environ. Res. Public Health 6:547–557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 426. ten Hove R, et al. 2009. Molecular diagnostics of intestinal parasites in returning travellers. Eur. J. Clin. Microbiol. Infect. Dis. 28:1045–1053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 427. ten Hove RJ. 2009. Molecular detection of intestinal parasites for clinical diagnosis and epidemiology. Ph.D. thesis. Leiden University, Leiden, Netherlands [Google Scholar]
- 428. Thomas KM, et al. 2006. A role of high impact weather events in waterborne disease outbreaks in Canada, 1975–2001. Int. J. Environ. Res. Public Health 16:167–180 [DOI] [PubMed] [Google Scholar]
- 429. Thompson R, Kutz S, Smith A. 2009. Parasite zoonoses and wildlife: emerging issues. Int. J. Environ. Res. Public Health 6:678–693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 430. Thompson RCA. 2000. Giardiasis as a re-emerging infectious disease and its zoonotic potential. Int. J. Parasitol. 30:1259–1267 [DOI] [PubMed] [Google Scholar]
- 431. Thompson RCA. 2004. The zoonotic significance and molecular epidemiology of Giardia and giardiasis. Vet. Parasitol. 126:15–35 [DOI] [PubMed] [Google Scholar]
- 432. Thompson RCA, Lymbery AJ, Smith A. 2010. Parasites, emerging disease and wildlife conservation. Int. J. Parasitol. 40:1163–1170 [DOI] [PubMed] [Google Scholar]
- 433. Thompson RCA, Smith A. 2011. Zoonotic enteric protozoa. Vet. Parasitol. 182:70–78 [DOI] [PubMed] [Google Scholar]
- 434. Tian H-F, Chen B, Wen J-F. 2010. Giardiasis, drug resistance, and new target discovery. Infect. Disord. Drug Targets 10:295–302 [DOI] [PubMed] [Google Scholar]
- 435. Tirado MC, Clarke R, Jaykus LA, McQuatters-Gollop A, Frank JM. 2010. Climate change and food safety: a review. Food Res. Int. 43:1745–1765 [Google Scholar]
- 436. Tiwari S-SK, Schmidt W-P, Darby J, Kariuki ZG, Jenkins MW. 2009. Intermittent slow sand filtration for preventing diarrhoea among children in Kenyan households using unimproved water sources: randomized controlled trial. Trop. Med. Int. Health 14:1374–1382 [DOI] [PubMed] [Google Scholar]
- 437. Tomley FM, Shirley MW. 2009. Livestock infectious diseases and zoonoses. Philos. Trans. R. Soc. Lond. B Biol. Sci. 364:2637–2642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 438. Torgerson PR, Macpherson CNL. 2011. The socioeconomic burden of parasitic zoonoses: global trends. Vet. Parasitol. 182:79–95 [DOI] [PubMed] [Google Scholar]
- 439. Tuli L, Singh D, Gulati A, Sundar S, Mohapatra T. 2010. A multiattribute utility evaluation of different methods for the detection of enteric protozoa causing diarrhea in AIDS patients. BMC Microbiol. 10:11 doi:10.1186/1471-2180-10-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 440. Tungtrongchitr A, et al. 2004. Blastocystis hominis infection in irritable bowel syndrome patients. Southeast Asian J. Trop. Med. Public Health 35:705–710 [PubMed] [Google Scholar]
- 441. Tzipori S, Ward H. 2002. Cryptosporidiosis: biology, pathogenesis and disease. Microbes Infect. 4:1047–1058 [DOI] [PubMed] [Google Scholar]
- 442. Uhnoo I, et al. 1986. Aetiology and epidemiology of acute gastro-enteritis in Swedish children. J. Infect. 13:73–89 [DOI] [PubMed] [Google Scholar]
- 443. van den Akker B, et al. 2011. Estimating the risk from sewage treatment plant effluent in the Sydney catchment area. Water Sci. Technol. 63:1707–1715 [DOI] [PubMed] [Google Scholar]
- 444. Vandenberg O, et al. 2006. Clinical and microbiological features of dientamoebiasis in patients suspected of suffering from a parasitic gastrointestinal illness: a comparison of Dientamoeba fragilis and Giardia lamblia infections. Int. J. Infect. Dis. 10:255–261 [DOI] [PubMed] [Google Scholar]
- 445. Vandenberg O, et al. 2007. Treatment of Dientamoeba fragilis infection with paromomycin. Pediatr. Infect. Dis. J. 26:88–90 [DOI] [PubMed] [Google Scholar]
- 446. van den Brandhof W, Bartelds A, Koopmans M, van Duynhoven Y. 2006. General practitioner practices in requesting laboratory tests for patients with gastroenteritis in the Netherlands, 2001–2002. BMC Fam. Pract. 7:56 doi:10.1186/1471-2296-7-56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 447. Van der Bruggen B, Vandecasteele C, Van Gestel T, Doyenb W, Leysen R. 2003. A review of pressure-driven membrane processes in wastewater treatment and drinking water production. Environ. Prog. 22:46–55 [Google Scholar]
- 448. van Hal SJ, et al. 2007. Declining incidence of intestinal microsporidiosis and reduction in AIDS-related mortality following introduction of HAART in Sydney, Australia. Trans. R. Soc. Trop. Med. Hyg. 101:1096–1100 [DOI] [PubMed] [Google Scholar]
- 449. van Hal SJ, et al. 2007. Amoebiasis: current status in Australia. Med. J. Aust. 186:412–416 [DOI] [PubMed] [Google Scholar]
- 450. Varma M, Hester JD, Schaefer FW, Ware MW, Lindquist HDA. 2003. Detection of Cyclospora cayetanensis using a quantitative real-time PCR assay. J. Microbiol. Methods 53:27–36 [DOI] [PubMed] [Google Scholar]
- 451. Vassalos CM, Vakalis N, Papadopoulou C. 2008. Blastocystis and its pathogenic potential: latest aspects. Rev. Med. Microbiol. 19:87–97 [Google Scholar]
- 452. Velasco AC, et al. 1984. Three-year prospective study of intestinal pathogens in Madrid, Spain. J. Clin. Microbiol. 20:290–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 453. Velasquez J, et al. 2011. Molecular characterization of Cystoisospora belli and unizoite tissue cyst in patients with acquired immunodeficiency syndrome. Parasitology 138:279–286 [DOI] [PubMed] [Google Scholar]
- 454. Verma GK, et al. 2010. Amoebiasis cutis: clinical suspicion is the key to early diagnosis. Australas. J. Dermatol. 51:52–55 [DOI] [PubMed] [Google Scholar]
- 455. Verweij JJ, et al. 2004. Simultaneous detection of Entamoeba histolytica, Giardia lamblia, and Cryptosporidium parvum in fecal samples by using multiplex real-time PCR. J. Clin. Microbiol. 42:1220–1223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 456. Verweij JJ, Laeijendecker D, Brienen EAT, van Lieshout L, Polderman AM. 2003. Detection of Cyclospora cayetanensis in travellers returning from the tropics and subtropics using microscopy and real-time PCR. Int. J. Med. Microbiol. 293:199–202 [DOI] [PubMed] [Google Scholar]
- 457. Vesy CJ, Peterson WL. 1999. Review article: the management of giardiasis. Aliment. Pharmacol. Ther. 13:843–850 [DOI] [PubMed] [Google Scholar]
- 458. Villarreal EL, Dixon A. 2005. Analysis of a rainwater collection system for domestic water supply in Ringdansen, Norrköping, Sweden. Build. Environ. 40:1174–1184 [Google Scholar]
- 459. Viola Magni M, Creusy C, Certad G, Guyot K, Dei-Cas E. 2010. Parasites and oncogenesis with a special reference to gastro-intestinal neoplasia induced by Cryptosporidium parvum, p 381–388 In Detection of bacteria, viruses, parasites and fungi. Springer, Dordrecht, Netherlands [Google Scholar]
- 460. Visser LG, et al. 2006. Diagnostic methods for differentiation of Entamoeba histolytica and Entamoeba dispar in carriers: performance and clinical implications in a non-endemic setting. Int. J. Med. Microbiol. 296:397–403 [DOI] [PubMed] [Google Scholar]
- 461. Visvesvara G, Moura H, Kovacs-Nace E, Wallace S, Eberhard M. 1997. Uniform staining of Cyclospora oocysts in fecal smears by a modified safranin technique with microwave heating. J. Clin. Microbiol. 35:730–733. (Erratum, 35:1648.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 462. Reference deleted.
- 463. Waldron LS, et al. 2011. Molecular epidemiology and spatial distribution of a waterborne cryptosporidiosis outbreak, Australia. Appl. Environ. Microbiol. 77:7766–7771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 464. Water UK. 2001. Guidelines for the application of sewage sludge to industrial crops, p 1–4 Water UK, Westminster, United Kingdom [Google Scholar]
- 465. Weitzel T, Dittrich S, Möhl I, Adusu E, Jelinek T. 2006. Evaluation of seven commercial antigen detection tests for Giardia and Cryptosporidium in stool samples. Clin. Microbiol. Infect. 12:656–659 [DOI] [PubMed] [Google Scholar]
- 466. Wenjun S, Wenjun L. 2008. Impact of the ultraviolet disinfection process on biofilm control in a model drinking water distribution system. 26:809–816 [Google Scholar]
- 467. Wheeler J, et al. 1999. Study of infectious intestinal disease in England: rates in the community, presenting to general practice, and reported to national surveillance. BMJ 318:1046–1050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 468. WHO 2008. The global burden of disease: 2004 update. WHO Press, Geneva, Switzerland [Google Scholar]
- 469. WHO 2006. Guidelines for safe recreational water environments, vol 2. Swimming pools and similar environments. WHO Press, Geneva, Switzerland [Google Scholar]
- 470. WHO, PAHO, UNESCO 1997. Report: a consultation with experts on amoebiasis: Mexico City. Epidemiol. Bull. 18:13–14 [PubMed] [Google Scholar]
- 471. Wilson N, Baker M, Edwards R, Simmons G. 2008. Case-case analysis of enteric diseases with routine surveillance data: potential use and example results. Epidemiol. Perspect. Innov. 5:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 472. Wingender J, Flemming H-C. 2011. Biofilms in drinking water and their role as reservoir for pathogens. Int. J. Hyg. Environ. Health 214:417–423 [DOI] [PubMed] [Google Scholar]
- 473. Xiao L. 2010. Molecular epidemiology of cryptosporidiosis: an update. Exp. Parasitol. 124:80–89 [DOI] [PubMed] [Google Scholar]
- 474. Xiao L, Cama V, Ortega YR. 2006. Cryptosporidium and cryptosporidiosis, p 57–108 In Foodborne parasites. Springer, New York, NY [Google Scholar]
- 475. Yang R, Reid A, Lymbery A, Ryan U. 2010. Identification of zoonotic Giardia genotypes in fish. Int. J. Parasitol. 40:779–785 [DOI] [PubMed] [Google Scholar]
- 476. Yoder JS, Harral C, Beach MJ. 2010. Cryptosporidiosis surveillance—United States, 2006–2008. MMWR Morb. Mortal. Wkly. Rep. 59:1–14 [PubMed] [Google Scholar]
- 477. Yoder JS, Harral C, Beach MJ. 2010. Giardiasis surveillance—United States, 2006–2008. MMWR Morb. Mortal. Wkly. Rep. 59:15–25 [PubMed] [Google Scholar]
- 478. Yoder JS, et al. 2008. Surveillance for waterborne disease and outbreaks associated with recreational water use and other aquatic facility-associated health events—United States, 2005–2006. MMWR Morb. Mortal. Wkly. Rep. 57:1–30 [PubMed] [Google Scholar]
- 479. Yoshikawa H, et al. 2004. Polymerase chain reaction-based genotype classification among human Blastocystis hominis populations isolated from different countries. Parasitol. Res. 92:22–29 [DOI] [PubMed] [Google Scholar]
- 480. Yoshiuchi R, et al. 2010. Survey and molecular characterization of Cryptosporidium and Giardia spp. in owned companion animal, dogs and cats, in Japan. Vet. Parasitol. 174:313–316 [DOI] [PubMed] [Google Scholar]
- 481. Zhang X, et al. 2012. In vitro culture of Blastocystis hominis in three liquid media and its usefulness in the diagnosis of blastocystosis. Int. J. Infect. Dis. 16:e23–e28 [DOI] [PubMed] [Google Scholar]
- 482. Zhang Y, Bi P, Hiller JE. 2010. Climate variations and Salmonella infection in Australian subtropical and tropical regions. Sci. Total Environ. 408:524–530 [DOI] [PubMed] [Google Scholar]
- 483. Zhang Y, Bi P, Hiller JE, Sun Y, Ryan P. 2007. Climate variations and bacillary dysentery in northern and southern cities of China. J. Infect. 55:194–200 [DOI] [PubMed] [Google Scholar]
- 484. Zmirou-Navier D, Gofti-Laroche L, Hartemann P. 2006. Waterborne microbial risk assessment: a population-based dose-response function for Giardia spp. (EMIRA study). BMC Public Health 6:122 doi:10.1186/1471-2458-6-122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 485. Zuel-Fakkar NM, Abdel Hameed DM, Hassanin OM. 2011. Study of Blastocystis hominis isolates in urticaria: a case-control study. Clin. Exp. Dermatol. 36:908–910 [DOI] [PubMed] [Google Scholar]