Abstract
Aims
To determine whether mothers of children with congenital toxoplasmosis have chorioretinal lesions consistent with toxoplasmosis.
Methods
Prospective cohort study. Ophthalmologists in our study have examined 173 children with congenital toxoplasmosis in a hospital outpatient setting. These children were referred to us by their primary care physicians. One hundred and thirty mothers of these children had retina examinations of both eyes at least once. Main outcome measure was lesion(s) consistent with ocular toxoplasmosis.
Results
Of 130 mothers examined between 1991–2005, 10 (7.7%, 95% Confidence Interval 3.8%, 13.7%) had chorioretinal lesions which likely represent resolved toxoplasmic chorioretinitis. Most of these were small peripheral chorioretinal lesions. None reactivated between 1991–2005.
Conclusions
Chorioretinal lesions consistent with quiescent ocular toxoplasmosis occur in mothers of children with congenital toxoplasmosis in the United States.
Keywords: Toxoplasma gondii; TOXOPLASMOSIS, CONGENITAL; TOXOPLASMOSIS, OCULAR; CHORIORETINITIS/complications; CHORIORETINITIS/diagnosis; CHICAGO; NCCCTS; MOTHERS
INTRODUCTION
The incidence of ocular lesions in persons who have acute acquired Toxoplasma gondii infection has been estimated to be less than one percent in the United States1 and varies at other times and places in the world.2–8 There are no studies of the incidence of and types of lesions in mothers of children with congenital toxoplasmosis. To determine how frequently eye lesions occurred, mothers of infants and children with congenital toxoplasmosis who were participants in the National Collaborative Chicago-based Congenital Toxoplasmosis Study (NCCCTS) were examined. Examination of mothers’ eyes was begun in a systematic manner in 1991. Children have been followed in the study since 1981.9–20 At present, each mother is examined at each visit of her child: at birth, 1, 3.5, 5, 7.5, 10, 15, 20, and >20 years of age. However, prior to 1991, not all mothers were examined at each visit.
METHODS
This study was conducted with the ethical standards for human experimentation established in the Declaration of Helsinki, with the prior approval of the Institutional Review Board of the University of Chicago and in accordance with the Health Insurance Portability and Accountability Act regulations. Informed consent was obtained from all adult participants and from parents or legal guardians of minors. One hundred seventy three children have been followed by the NCCCTS between December 1981 and January 2005. There are three sets of twins. Children are seen at birth, 1, 3.5, 5, 7.5, 10, 15, 20, and more than 20 years of age. Children have come to the University of Chicago at these time intervals from throughout the United States, and in some instances, Central and South America, Mexico, Canada, and Europe. They have been referred by their primary care physicians, either at birth or at time of diagnosis. Diagnosis of Toxoplasma gondii infection has been with serologic techniques as described before.9–23
During their child’s study visits, the mothers’ eyes were dilated with phenylephrine hydrochloride (2.5%) and tropicamide (0.5% or 1.0%) with or without cyclopentolate hydrochloride (0.5%). After dilation, the mothers’ eyes were evaluated by binocular indirect ophthalmoscopy. All the indirect ophthalmoscopic examinations in the earlier years of the study were performed by the same pediatric ophthalmologist (M.B.M.).9 The later examinations were done by three other pediatric ophthalmologists and three retina specialists.
A drawing of the retinal findings was completed for each mother at each examination and fundus photography was done. A narrative evaluation was also prepared. A standardized database was compiled that included each evaluation of each mother.
Abnormalities detected for the mothers are classified as either consistent with ocular toxoplasmosis or not. Lesions were classified as Toxoplasma chorioretinal scars if the lesion met clinical characteristics of chorioretinal scars consistent with ocular toxoplasmosis2–8 and were not consistent with other types of lesions such as lattice degeneration or congenital hypertrophy of the retina pigment epithelium (CHRPE). Lesions consistent with resolved toxoplasmic chorioretinitis are flat, well circumscribed, hypopigmented, hyperpigmented, or a mixture of hypopigmentation and hyperpigmentation. These chorioretinal lesions are also described by size. Small is defined as less than one-quarter disc diameter, medium is one quarter to less than one disc diameter, and large is greater than or equal to one disc diameter. Only the chorioretinal lesions suspicious for or consistent with ocular toxoplasmosis are included in the Tables, Figure and statistical analysis. Other lesions are described in the results section. The prevalence of chorioretinal lesions that are consistent with resolution of active toxoplasmic chorioretinitis in mothers who acquired acute Toxoplasma infection during pregnancy was determined.
All children who participated in this study at birth were stratified by severity of systemic/neurologic disease into one of three categories: None, Mild, and Moderate/Severe disease. Infants who had only systemic manifestations (such as jaundice, anemia, hepatomegaly or splenomegaly) at birth were placed in the Mild class. In contrast, infants with certain neurologic manifestations (such as greater than or equal to 3 calcifications on brain computed tomography [CT] scan, seizures near birth, motor abnormalities, abnormal density of white matter on brain CT scan, hydrocephalus or microcephalus, increased cerebrospinal fluid [CSF] protein, hypoglycorrachia, macular disease that threatened vision, microphthalmia, or optic atrophy at birth) were placed in the Moderate/Severe Neurologic disease category. The term historical children refers to those children seen for the first time after they were more than one year old who had not been treated during their first year of life.
At each visit, children were assigned a severity score for each eye as follows: 0 (normal vision, no lesions), 1 (normal vision, non-macular lesions), 2 (normal vision, macular lesions), 3 (impaired vision, non-macular lesions), 4 (impaired vision, macular lesions), 4.5 (impaired vision, inability to view the posterior pole because of cataracts or another etiology), 5 (no observable light perception). Children who have impaired vision due to a lesion secondary to toxoplasmosis have a score of 3 or higher.
Fisher’s exact test was used to compare the proportion of children with impaired vision between mothers with and without chorioretinal lesions suggestive of ocular toxoplasmosis. The Wilcoxon rank-sum test was used to compare children’s systemic/ neurologic disease severity at birth (None, Mild, or Moderate/Severe as defined previously14) between the two groups of mothers. Additionally, a 95% confidence interval for the proportion of mothers with chorioretinal lesions consistent with ocular toxolasmosis was calculated using exact binomial methods. All statistical analyses were performed using Stata (Stata Corp., College Station, TX).
RESULTS
There are 173 children (including three sets of twins) and 170 mothers followed in the NCCCTS between 1981 and January 2005. One hundred and thirty (76%) of these 170 mothers have had ophthalmologic evaluations at least once between 1991 and January 2005. Forty mothers were not present or declined examinations after 1991 when examinations of mothers’ eyes were initiated as part of the study. Ten children were adopted, including one set of twins whose biologic mother did not accompany them to the study visits.
There have been a total of 212 examinations performed for these 130 mothers. Examinations took place at all ages of the children. The number of children who visited at each age, the number of mothers who were examined at these visits, and the number of mothers whose retinal examinations were normal or abnormal are in Table 1.
Table 1.
Chorioretinal abnormalities observed in mothers at varying times after birth of a congenitally infected child.
| Age | Number of mothers: |
Percentage of abnormal exams¶ | ||||
|---|---|---|---|---|---|---|
| with children who have reached this age* | with children who have visited at this age† | who have been examined when child was this age†† | who had normal exams when child was this age§ | who had abnormal exams when child was this age|| | ||
| Birth | 170 | 121 | 71 | 66 | 5 | 7% |
| 1 year | 162 | 111 | 31 | 29 | 2 | 6% |
| 3.5 years | 149 | 85 | 18 | 17 | 1 | 6% |
| 5 years | 140 | 71 | 13 | 12 | 1 | 8% |
| 7.5 years | 126 | 49 | 15 | 15 | 0 | 0% |
| 10 years | 111 | 67 | 39 | 38 | 1 | 3% |
| 15 years | 52 | 34 | 19 | 18 | 1 | 5% |
| 20 years | 21 | 13 | 6 | 5 | 1 | 17% |
| >20 years | 8 | 7 | 0 | 0 | 0 | N/A |
| Total | 939* | 558† | 212†† | 200§ | 12|| | 6%¶ |
Total number of children’s visits possible. 173 children, 3 sets of twins.
Actual total number of children’s visits.
Total number of mothers’ examinations performed.
Number of examinations of mothers where no abnormalities consistent with ocular toxoplasmosis were found.
Number of examinations of mothers where abnormalities consistent with ocular toxoplasmosis were found (i.e., 10 mothers, 12 examinations).
%=Abnormal exams/exams performed at this age X100.
One hundred twenty one of the children in our study visited near birth, and 71 of their mothers were examined at this visit. Five mothers (7%) of these 71 had an abnormality detected shortly after the birth of their congenitally infected child.
The findings at each visit for mothers with lesions consistent with or suspicious for ocular toxoplasmosis are in Table 2. Descriptions of the specific lesions are in Table 3 and representative lesions are shown in Figure 1. The lesions consistent with having ocular toxoplasmosis as their etiology are defined in the Methods section. The chorioretinal lesions of the mothers are hyperpigmented, hypopigmented, or a mixture. The mothers’ chorioretinal lesions are flat, well circumscribed and are consistent with resolved acute, acquired toxoplasmic chorioretinitis. Most of the mothers had chorioretinal lesions that were unilateral, single, and small. Five mothers had peripheral hyperpigmented lesions: mothers of patients 2, 27, 78, 101, and 125. Two of the mothers had peripheral hypopigmented lesions: mothers of patients 161 and 171. Three of the mothers had peripheral lesions which were mixed hyperpigmentation and hypopigmentation: mothers of patients 118, 157, and 172. The mother of patient 118 had a small lesion with a mixture of hyperpigment peripherally and hypopigment centrally in the superior nasal periphery in the presence of high myopia (−7.50+0.75 at axis 83 degrees in the right eye and −6.75+0.75 at axis 80 degrees in the left eye). This chorioretinal lesion may be either a fundus change due to myopia or toxoplasmic chorioretinal scar. There are no photographs of this mother’s fundus prior to and after the acquired Toxoplasma infection to definitively classify this chorioretinal scar.
Table 2.
History of abnormal chorioretinal findings (described in Table 3) in mothers of children with congenital toxoplasmosis.
| Patient number | Eye | Patient Age |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| < 1 year | 1 year | 3.5 years | 5 years | 7.5 years | 10 years | 15 years | 20 years | ||
| 2 | Right | No exam | No exam | No exam | No exam | No exam | Normal | No exam | + |
| 27 | Right | No visit | No visit | No exam | No exam | Normal | No exam | + | N/A |
| 78 | Left | + | No exam | No visit | No visit | No visit | No exam | N/A | N/A |
| 125 | Right | + | No visit | No visit | + | N/A | N/A | N/A | N/A |
| 157 | Right | No visit | Normal | + | N/A | N/A | N/A | N/A | N/A |
| 161 | Right | + | + | N/A | N/A | N/A | N/A | N/A | N/A |
| 171 | Right | + | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| 172 | Left | + | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| 118 | Right | No visit | No visit | Normal | No exam | No exam | + | N/A | N/A |
| 101 | Right | No visit | + | No exam | No exam | No visit | * | N/A | N/A |
N/A = not applicable (child has not reached that age).
No exam = mother not present or not examined.
Normal = no abnormalities detected on mother’s examination which are consistent with or suspicious for ocular toxoplasmosis.
= chorioretinal lesions detected on mother’s examination which are consistent with possible resolved toxoplasmic chorioretinitis.
= examiner did not visualize inferior retina in this examination.
No visit = child did not visit at this age.
Table 3.
Descriptions of abnormal chorioretinal findings in mothers of children with congenital toxoplasmosis.
| Photograph in Figure 1 | Patient number | Description of abnormality |
|---|---|---|
| A | 2 | Small hyperpigmented choroidal lesion in the superior nasal periphery of the right eye |
| no photos | 27 | Small triangular abnormal pigment area in the superior temporal quadrant of the right eye |
| no photos | 78 | Small pigmented lesion superior to the arcade but inside the equator of the left eye |
| no photos | 125 | Small hyperpigmented lesion within the nasal arcade of the left eye |
| B | 157 | Small lesion, mix of hypopigmentation (mostly) and hyperpigmentation in the superior, nasal periphery of the right eye |
| C | 161 | Medium size, deep, hypopigmented lesion in the inferior periphery of the right eye |
| D | 171 | Small hypopigmented chorioretinal lesion in the far inferior nasal periphery of the right eye |
| E | 172 | Medium size, primarily hypopigmented chorioretinal lesion with a few areas of hyperpigmentation in the far superior nasal periphery of the left eye |
| no photos | 118 | Small chorioretinal lesion with a mixture of hyperpigment peripherally and hypopigment centrally in the superior nasal periphery of the right eye |
| no photos | 101 | Small lesion, mix of hypopigmentation and hyperpigmentation in the inferior, temporal periphery of right eye |
Figure 1.
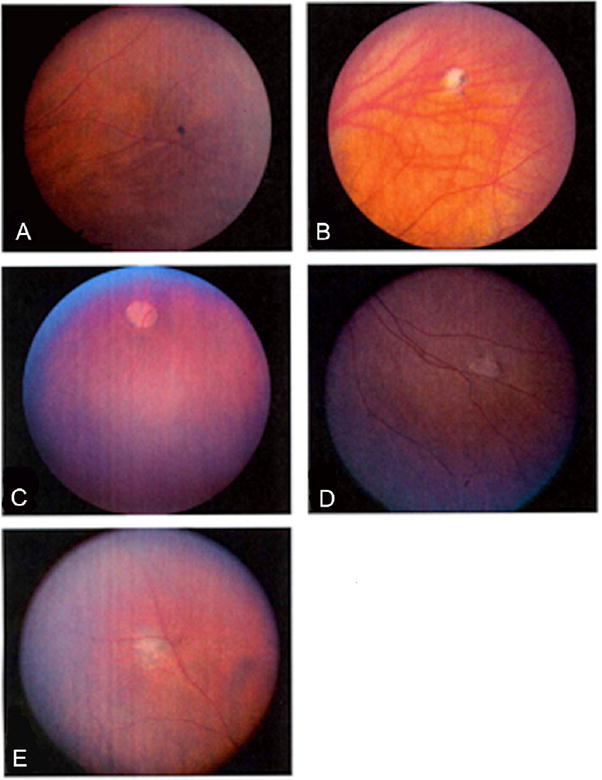
Photographs of mothers’ chorioretinal findings consistent with resolved toxoplasmic chorioretinitis. (A) Mother of Patient #2, small hyperpigmented chorioretinal lesion; (B) Mother of Patient #157, chorioretinal lesion with mix of primarily hypopigmentation and hyperpigmentation; (C) Mother of Patient #161, with deep hypopigmented lesion in right eye; (D) Mother of Patient #171, with hypopigmented chorioretinal lesion; (E) Mother of Patient # 172, chorioretinal lesion with mix of primarily hypopigmentation and a few areas of hyperpigmentation
There were no signs of active disease in any of the mothers at any of the 212 examinations. Moreover, no recurrences have been observed in any mother.
The mother of patient number 81 had a chorioretinal lesion suspicious for being a chorioretinal lesion due to Toxoplasma gondii infection seen at the first examination shortly after birth and it was not seen at the 10 year visit by two ophthalmologists. Shortly after birth, it was a small hyperpigmented lesion in the inferior nasal quadrant of the left eye. This lesion was not included in the Tables, Figure, and statistical analysis of this manuscript.
Other abnormalities detected in the mothers which are known to be not consistent with ocular toxoplasmosis include a single presumed cystic retinal tuft in the periphery (mother of patient 168) and two small areas of presumed cystic retinal tufts/vitreous condensation in the periphery of each eye (mother of patient 30). There also are other findings detected in the mothers that are not ascribed to Toxoplasma gondii ocular infection. In addition to the chorioretinal scars consistent with resolved toxoplasmic chorioretinitis (included in the Tables, Figure, and statistical analysis), there was a small size choroidal nevus deep to the superior temporal vascular arcade in the right eye of the mother of patient number 101 detected at the 10 year visit of the child. There was a medium size choriodal nevus detected in the right eye of the mother of patient number 161. Also, there was a medium size choroidal nevus deep to the superior temporal vascular arcade in the right eye of the mother of patient number 111. Additional findings detected in the mothers include cataracts in each eye of the 34 year old mother of patient number 94 and severe hypertensive retinopathy in the mother of patient number 156 whose blood pressure was 200/140. None of these abnormalities described as incidental findings are included in the Tables, Figure, and statistical analysis.
Surprisingly, 10 (7.7%, 95% CI 3.8%, 13.7%) of 130 mothers examined at any time in our study had lesions consistent with or suspicious for ocular toxoplasmosis. Five (7.0%, 95% CI 2.3%, 15.7%) of the 71 mothers examined shortly after childbirth had lesions consistent with ocular toxoplasmosis. Infants whose mothers had chorioretinal lesions consistent with ocular toxoplasmosis included those children with none or mild eye disease (mothers of patient numbers 2, 161, 171, 172) as well as ophthalmic manifestations across the spectrum of severity. Three of the mothers with eye lesions consistent with toxoplasmic chorioretinal scars had children in the None/Mild severity category (mothers of patient numbers 2, 171, 172) and three mothers had children who were characterized as having Moderate/Severe neurologic disease (mothers of patient numbers 78, 125, 161). Two of these mothers with chorioretinal lesions had children who were first seen when their child was one year old (mothers of patients 101, 157) and two were first seen when their child was 3.5 years old (mother of patients 27, 118). When we compared children’s disease severity, as defined in Methods, in the group of mothers with chorioretinal abnormalities to the group without chorioretinal abnormalities, there was a statistically significant difference with worse disease severity in the group whose mothers did not have ocular abnormalities (p-value = 0.04 from Wilcoxon rank-sum test).
There was not a statistically significant association between a child’s eye disease severity at initial visit (impaired vision or not) and whether their mother had an abnormality or not (p-value = 0.74 from Fisher’s exact test). Four (8.5%) of 47 children without impaired vision had a mother with a chorioretinal lesion suspicious for ocular toxoplasmosis. Six (7.1%) of 85 children with visual impairment had a mother with a chorioretinal scar consistent with resolved toxoplasmic chorioretinitis.
DISCUSSION
The prevalence of chorioretinal lesions suspicious for ocular toxoplasmosis in mothers of children with congenital toxoplasmosis in our cohort was determined. The incidence of ocular lesions in persons who have acute acquired Toxoplasma gondii infection earlier was estimated to be less than one percent in the United States1 and varies at other times and places in the world.2–8 Surprisingly, 7.7% (10 of 130 mothers) who were examined at any time in this study had chorioretinal lesions consistent with or suspicious for ocular toxoplasmosis and 7.0% (5 of 71 mothers) who were examined shortly after childbirth had chorioretinal lesions consistent with ocular toxoplasmosis. This is a higher incidence than had been estimated earlier for presence of eye lesions in patients in the United States with postnatally acquired Toxoplasma gondii infection.1,25 Our data suggests that there may be a higher incidence of toxoplasmic chorioretinitis in immunologically normal adults with acute acquired Toxoplasma gondii infection in this country than estimated earlier for all individuals with acquired Toxoplasma infections. Our data also might instead reflect that the TH2 lymphocyte shift 7 that occurs during pregnancy could alter the occurrence of ocular toxoplasmosis in pregnancy. It will be of interest to determine which of these two possibilities actually occur. Interestingly, Bosch-Driessen et al. have reported that 4 (57%) of 7 mothers who had reactivated eye lesions in one pregnancy developed recurrence in a subsequent pregnancy.27 When these women acquired the infection for the first time was not stated.
Our study demonstrates that mothers of infants and children with congenital toxoplasmosis had an approximately 7–8% incidence of chorioretinal lesions consistent with ocular toxoplasmosis. The data decrease inpresented herein are important for the mothers of infants with congenital toxoplasmosis. None of the mothers in our study had recurrences of active ocular toxoplasmosis which threatened vision, thus the incidence of sight-threatening recurrences appears small. These examinations have almost all taken place when the mothers are not pregnant. There are, however, studies (discussed above)27 in which a significant proportion of the mothers with Toxoplasma gondii associated eye disease did have reactivation/recrudescence of their ocular toxoplasmosis in subsequent pregnancies. Further follow-up will determine the rate of reactivation and the natural history of eye lesions in our cohort of mothers over time.
Other studies have shown that 9% (7 of 82) of female patients with ocular toxoplasmosis had recurrence of ocular toxoplasmosis during pregnancy.27 It is important for a pregnant woman with previous Toxoplasma gondii infection to have an eye examination during subsequent pregnancies. Such pregnant women should be aware that if they experience new onset of ocular pain, blurred or decreased vision, increased photosensitivity, increased floaters (which are signs and symptoms of adults with mild posterior uveitis), they should have an eye examination as soon as possible. This is also the case for a pregnant woman who acquires toxoplasmosis for the first time during pregnancy. A thorough posterior segment examination at the slit lamp is indicated to evaluate for cells in the vitreous as well as a dilated ophthalmoscopic examination to carefully evaluate the retina looking for active lesions or small, flat, well circumscribed chorioretinal lesions in the periphery which are hyperpigmented, hypopigmented, or a mixture of hyperpigment and hypopigment.
If a pregnant woman without a history of previous ocular toxoplasmosis has a suspicious chorioretinal lesion on an eye examination or has signs or symptoms of posterior uveitis (described above), with or without systemic signs and symptoms of acute acquired toxoplasmosis (described above), we recommend a diagnostic work up of the pregnant woman (serologic tests) for toxoplasmosis. If this woman has acute acquired toxoplasmosis, then additional evaluation of the fetus for congenital toxoplasmosis is indicated.
Treatment of the pregnant woman’s ocular disease is dependent on activity of her eye lesions and the signs and symptoms of the disease. Treatment of a pregnant woman who has serology indicative of acute, acquired toxoplasmosis is critical to the well being of the fetus.10–12,14,17,28–30 Prompt treatment of the fetus and newborn with congenital toxoplasmosis results in significant decrease in both systemic, neurologic, and ocular disease12,14,28–30 and may profoundly affect the well being of the infant for the remainder of his/ her lifetime. The findings we describe herein also suggest that it may be prudent to examine mothers of congenitally infected children at the time infection is diagnosed, at the time of birth of a child, and also in subsequent pregnancies if lesions are detected with the earlier examination.
Acknowledgments
This work was supported by the Research to Prevent Blindness Foundation, NIH grant NIAID AI 27530, and gifts from the Kieweit, Blackmon, Brennan, Koshland, Langel, Morel, Rosenstein, Rooney-Alden, Taub, Kapnick, Engel, Mann and Dominique Cromwell, Singer and Schilling families. J.K. would like to thank Professor Roman Gos for his support. We thank A. Esquivel and M. Sautter for their assistance in preparation of this manuscript.
References
- 1.Montoya JG, Remington JS. Toxoplasmic Chorioretinitis in the Setting of Acute Acquired Toxoplasmosis. Clin Infect Dis. 1996;23:277–82. doi: 10.1093/clinids/23.2.277. [DOI] [PubMed] [Google Scholar]
- 2.Perkins ES. Ocular Toxoplasmosis. Brit J Ophthal. 1973;57:1–17. doi: 10.1136/bjo.57.1.1. [Review] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Melamed J. Contributions to the history of ocular toxoplasmosis in Southern Brazil. Mem Inst Oswaldo Cruz. 2009;104:358–63. doi: 10.1590/s0074-02762009000200032. [DOI] [PubMed] [Google Scholar]
- 4.Hogan MJ. Ocular toxoplasmosis. Trans Pac Coast Ophthalmol Soc Annu Meet. 1969;50:1–21. [Review] [PubMed] [Google Scholar]
- 5.Phan L, Kasza K, Jalbrzikowski J, et al. Longitudinal study of new eye lesions in treated congenital toxoplasmosis. Ophthalmology. 2008;115:553–8. doi: 10.1016/j.ophtha.2007.06.022. [DOI] [PubMed] [Google Scholar]
- 6.Phan L, Kasza K, Jalbrzikowski J, et al. Longitudinal study of new eye lesions in children with toxoplasmosis who were not treated during the first year of life. Am J Ophthalmol. 2008;146:375–84. doi: 10.1016/j.ajo.2008.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roberts CW, Gazzinelli R, Khan I, Nowakowska D, McLeod R. Adaptive Immunity and genetics of the host immune response. In: Kim K, Weiss L, editors. Toxoplasma gondii: The Model Apicomplexan: Perspectives and Methods. London: Academic Press; 2007. pp. 609–720. [Google Scholar]
- 8.Roberts F, McLeod R. Pathogenesis of Toxoplasmic Retinochoroiditis. Parasitology Today. 1999;15:51–7. doi: 10.1016/s0169-4758(98)01377-5. [DOI] [PubMed] [Google Scholar]
- 9.Mets MB, Holfels E, Boyer KM, et al. Eye Manifestations of Congenital Toxoplasmosis. Am J Ophthalmol. 1996;122:309–24. doi: 10.1016/s0002-9394(14)72057-4. [DOI] [PubMed] [Google Scholar]
- 10.McAuley J, Boyer KM, Patel D, et al. Early and Longitudinal Evaluations of Treated Clinical Outcome in CongenitalInfants and Children and Untreated Historical Patients with Congenital Toxoplasmosis: the Chicago Collaborative Treatment Trial. Clin Infect Dis. 1994;18:38–72. doi: 10.1093/clinids/18.1.38. Erratum in: Clin Infect Dis 1994;19:820. [DOI] [PubMed] [Google Scholar]
- 11.Remington JS, McLeod R, Thulliez P, Desmonts G. Toxoplasmosis. In: Remington J, Klein J, editors. Infectious Diseases of the Fetus and Newborn Infant. 6. Philadelphia: WB Saunders; 2006. pp. 947–1091. [Google Scholar]
- 12.McLeod R, Kieffer F, Sautter M, et al. Why prevent, diagnose and treat congenital toxoplasmosis? Mem Inst Oswaldo Cruz. 2009;104:320–344. doi: 10.1590/s0074-02762009000200029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arun V, Noble AG, Latkany P, et al. Cataracts in congenital toxoplasmosis. J AAPOS. 2007;11:551–4. doi: 10.1016/j.jaapos.2007.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McLeod R, Boyer K, Karrison T, et al. Outcome of treatment for congenital toxoplasmosis, 1981–2004: The National Collaborative Chicago-based Congenital Toxoplasmosis Study (NCCCTS) Clin Infect Dis. 2006;42:1383–94. doi: 10.1086/501360. [DOI] [PubMed] [Google Scholar]
- 15.Boyer K, Holfels E, Roizen N, et al. Risk factors for Toxplasma gondii infection in mothers of infants with congenital toxoplasmosis: implications for prenatal management and screening. AJOG. 2005;192:564–71. doi: 10.1016/j.ajog.2004.07.031. [DOI] [PubMed] [Google Scholar]
- 16.Patel DV, Holfels EM, Vogel NP, et al. Resolution of intracranial calcifications in infants with treated congenital toxoplasmosis. Radiology. 1996;199:433–40. doi: 10.1148/radiology.199.2.8668790. [DOI] [PubMed] [Google Scholar]
- 17.McLeod R, Mack D, Foss R, et al. Levels of pyrimethamine in sera and cerebrospinal and ventricular fluids from infants treated for congenital toxoplasmosis. Antimicrob Ag Chemother. 1992;36:1040–8. doi: 10.1128/aac.36.5.1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roizen N, Swisher C, Stein M, et al. Neurologic and Developmental Outcome in Treated Congenital Toxoplasmosis. Pediatrics. 1995;95:11–20. [PubMed] [Google Scholar]
- 19.Swisher CN, Boyer K, McLeod R. Congenital toxoplasmosis. The Toxoplasmosis Study Group. Sem Ped Neurol. 1994;1:4–25. [PubMed] [Google Scholar]
- 20.Roizen N, Kasza K, Karrison T, et al. Impact of visual impairment on measures of cognitive function for children with congenital toxoplasmosis: implications for compensatory intervention strategies. Pediatrics. 2006;118:e379–90. doi: 10.1542/peds.2005-1530. [DOI] [PubMed] [Google Scholar]
- 21.Jamieson SE, de Roubaix L-A, Cortina-Borja M, et al. Genetic and Epigenetic Factors at COL2A1 and ABCA4 influence Clinical Outcome in Congenital Toxoplasmosis. PLoS ONE. 2008;3:e2285. doi: 10.1371/journal.pone.0002285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mack D, Johnson J, Roberts F, et al. HLA-Class II genes modify outcome of Toxoplasma gondii infection. Int J Parasitol. 1999;29:1351–8. doi: 10.1016/s0020-7519(99)00152-6. [Rapid Communication] [DOI] [PubMed] [Google Scholar]
- 23.McLeod R, Mack DG, Boyer K, et al. Phenotypes and functions of lymphocytes in congenital toxoplasmosis. J Lab Clin Immunol. 1990;116:623–35. [PubMed] [Google Scholar]
- 24.Brezin AP, Thulliez P, Couvreur J, et al. Ophthalmic outcomes after prenatal and postnatal treatment of congenital toxoplasmosis. Am J Ophthalmol. 2003;135:779–84. doi: 10.1016/s0002-9394(02)01704-x. [DOI] [PubMed] [Google Scholar]
- 25.Holland GN, Lewis KG. An update on current practices in the management of ocular toxoplasmosis. Am J Ophthalmol. 2002;134:102–14. doi: 10.1016/s0002-9394(02)01526-x. [DOI] [PubMed] [Google Scholar]
- 26.Roberts F, Mets MB, Ferguson DJP, et al. Histopathology of congenital ocular toxoplasmosis in the human infant and fetus. Arch Ophthalmol. 2001;119:51–8. [PubMed] [Google Scholar]
- 27.Bosch-Driessen LEH, Berendschot TTJM, Ongkosuwito JV, et al. Ocular Toxoplasmosis Clinical Features and Prognosis of 154 patients. Ophthalmology. 2002;109:869–78. doi: 10.1016/s0161-6420(02)00990-9. [DOI] [PubMed] [Google Scholar]
- 28.Foulon W, Villena I, Stray-Pedersen B, et al. Treatment of toxoplasmosis during pregnancy: a multicenter study of impact on fetal transmission and children’s sequelae at age 1 year. Am JObstet Gynecol. 1999;180:410–5. doi: 10.1016/s0002-9378(99)70224-3. [DOI] [PubMed] [Google Scholar]
- 29.Hohlfeld P, Daffos F, Costa JM, et al. Prenatal diagnosis of congenital toxoplasmosis with a polymerase-chain-reaction test on amniotic fluid. N Engl J Med. 1994;331:695–9. doi: 10.1056/NEJM199409153311102. [DOI] [PubMed] [Google Scholar]
- 30.Kieffer F, Wallon M, Garcia P, et al. Risk factors for retinochoroiditis during the first 2 years of life in infants with treated congenital toxoplasmosis. Pediatr Infect Dis J. 2008;27:27–32. doi: 10.1097/INF.0b013e318134286d. [DOI] [PubMed] [Google Scholar]


