Abstract
Compared to a few decades ago, adults, as well as children, sleep less. Sleeping as little as possible is often seen as an admirable behavior in contemporary society. However, sleep plays a major role in neuroendocrine function and glucose metabolism. Evidence that the curtailment of sleep duration may have adverse health effects has emerged in the past 10 years. Accumulating evidence from both epidemiologic studies and well-controlled laboratory studies indicates that chronic partial sleep loss may increase the risk of obesity and weight gain. The present chapter reviews epidemiologic studies in adults and children and laboratory studies in young adults indicating that sleep restriction results in metabolic and endocrine alterations, including decreased glucose tolerance, decreased insulin sensitivity, increased evening concentrations of cortisol, increased levels of ghrelin, decreased levels of leptin and increased hunger and appetite. Altogether, the evidence points to a possible role of decreased sleep duration in the current epidemic of obesity. Bedtime extension in short sleepers should be explored as a novel behavioral intervention that may prevent weight gain or facilitate weight loss. Avoiding sleep deprivation may help to prevent the development of obesity, particularly in children.
Hormones that Influence Glucose Regulation and Appetite Control Are Influenced by Sleep
The temporal organization of the release of the counterregulatory hormones growth hormone (GH) and cortisol as well as the release of hormones that play a major role in appetite regulation, such as leptin and ghrelin, is partly dependent on sleep timing, duration and quality. Glucose tolerance and insulin secretion are also markedly modulated by the sleep-wake cycle [1]. Sleep propensity and sleep architecture are in turn controlled by the interaction of two time-keeping mechanisms in the central nervous system, circadian rhythmicity (i.e. intrinsic effects of biological time, irrespective of the sleep or wake state) and sleep-wake homeostasis (i.e. a measure of the duration of prior wakefulness, irrespective of time of day).
Circadian rhythmicity is an endogenous oscillation with a near 24-hour period generated in the suprachiasmatic nuclei of the hypothalamus. The ability of the SCN nuclei to generate a circadian signal is not dependent on cell-to-cell interaction and synchronization. Instead, single SCN cells in culture can generate circadian neural signals [2]. The generation and maintenance of circadian oscillations in SCN neurons involve a series of clock genes (including at least per1, per 2, per3, cry1, cry2, tim, clock, B-mal1, CKIε/δ), often referred to as ‘canonical’, which interact in a complex feedback loop of transcription/translation [3, 4]. Circadian timing is transmitted to other areas of the brain and to the periphery via direct neuronal connections with other parts of the hypothalamus, via the control of sympathetic nervous activity and via hormonal signals, including melatonin. The molecular and neuronal mechanisms that measure the duration of prior wakefulness and are thus responsible for the homeo-static control of sleep have not been fully elucidated. Human sleep is comprised of rapid-eye-movement (REM) sleep and non-REM sleep. Deep non-REM sleep is characterized by ‘slow waves’ in the electroencephalogram (EEG), which reflect a mode of synchronous firing of thalamo-cortical neurons. The intensity of non-REM sleep may be quantified by slow wave activity (SWA; EEG spectral power in the 0.5–4 Hz frequency range). Slow waves of larger amplitude and greater regularity are reflected in higher SWA and in deeper sleep. Because SWA decreases in the course of the sleep period, is higher after sleep deprivation (i.e. extended wakefulness) and lower when the waking period has been interrupted by a long nap (i.e. shorter wakefulness), SWA is considered as the major marker of homeostatic sleep pressure. Converging evidence implicates adenosine, an inhibitory neurotransmitter, in sleep homeostasis in mammals [5]. Prolonged wakefulness results in increased levels of extracellular adenosine, which partly derive from ATP degradation, and adenosine levels decrease during sleep [6]. The adenosine receptor antagonist, caffeine, inhibits SWA [7]. It has been proposed that the restoration of brain energy during SWS involves the replenishment of glycogen stores [8]. The results of experiments testing this hypothesis have been mixed. A recent and well-supported hypothesis regarding sleep homeostasis is that the level of SWA in early sleep is a function of the strength of cortical synapses developed during wakefulness and that the decline in SWA across the sleep period reflects the downscaling of these synapses [9].
The major mechanisms by which the modulatory effects of circadian rhythmicity and sleep-wake homeostasis are exerted on peripheral physiological systems include the modulation of hypothalamic activating and inhibiting factors controlling the release of pituitary hormones and the modulation of sympathetic and parasympathetic nervous activity.
The relative contributions of the circadian signal versus homeostatic sleep pressure vary from endocrine axis to endocrine axis. It has been well-documented that GH is a hormone essentially controlled by sleep-wake homeostasis. Indeed, in men, the most reproducible pulse of GH occurs shortly after sleep onset, during slow wave sleep (SWS, stages 3 and 4) when SWA is high. In both young and older men, there is a ‘dose-response’ relationship between SWS and nocturnal GH release. When the sleep period is displaced, the major GH pulse is also shifted and nocturnal GH release during sleep deprivation is minimal or frankly absent. This impact of sleep pressure on GH is particularly clear in men but can also be detected in women.
The 24-hour profile of cortisol is characterized by an early morning maximum, declining levels throughout the daytime, a period of minimal levels in the evening and first part of the night, also called the quiescent period, and an abrupt circadian rise during the later part of the night. Manipulations of the sleep-wake cycle only minimally affect the wave shape of the cortisol profile. Sleep onset is associated with a short-term inhibition of cortisol secretion that may not be detectable when sleep is initiated in the morning, i.e. at the peak of corticotropic activity. Awakenings (final as well as during the sleep period) consistently induce a pulse in cortisol secretion. The cortisol rhythm is therefore primarily controlled by circadian rhythmicity. Modest effects of sleep deprivation are clearly present as will be shown below.
The 24-hour profiles of two hormones that play a major role in appetite regulation, leptin, a satiety hormone secreted by the adipocytes, and ghrelin, a hunger hormone released primarily from stomach cells, are also influenced by sleep. The human leptin profile is mainly dependent on meal intake and therefore shows a morning minimum and increasing levels throughout the daytime culminating in a nocturnal maximum. Under continuous enteral nutrition, a condition of constant caloric intake, a sleep-related elevation of leptin is observed, irrespective of the timing of sleep. Ghrelin levels decrease rapidly after meal ingestion and then increase in anticipation of the following meal. Both leptin and ghrelin concentrations are higher during nocturnal sleep than during wakefulness. Despite the absence of food intake, ghrelin levels decrease during the second part of the night suggesting an inhibitory effect of sleep per se. At the same time, leptin is elevated, maybe to inhibit hunger during the overnight fast.
The brain is almost entirely dependent on glucose for energy and is the major site of glucose disposal. Thus, it is not surprising that major changes in brain activity, such as those associated with sleep-wake and wake-sleep transitions, impact glucose tolerance. Cerebral glucose utilization represents 50% of total body glucose disposal during fasting conditions and 20–30% postprandially. During sleep, despite prolonged fasting, glucose levels remain stable or fall only minimally, contrasting with a clear decrease during fasting in the waking state. Thus, mechanisms operative during sleep must intervene to prevent glucose levels from falling during the overnight fast. Experimental protocols involving intravenous glucose infusion at a constant rate or continuous enteral nutrition during sleep have shown that glucose tolerance deteriorates as the evening progresses, reaches a minimum around mid sleep and then improves to return to morning levels [10, 11]. During the first part of the night, decreased glucose tolerance is due to decreased glucose utilization both by peripheral tissues (resulting from muscle relaxation and rapid hyperglycemic effects of sleep-onset GH secretion) and by the brain, as demonstrated by PET imaging studies that showed a 30–40% reduction in glucose uptake during SWS relative to waking or REM sleep. During the second part of the night, these effects subside as light non-REM sleep and REM sleep are dominant, awakenings are more likely to occur, GH is no longer secreted and insulin sensitivity increases, a delayed effect of low cortisol levels during the evening and early part of the night.
These important modulatory effects of sleep on hormonal levels and glucose regulation suggest that sleep loss may have adverse effects on endocrine function and metabolism. It is only during the past decade that a substantial body of evidence has emerged to support this hypothesis. Indeed, earlier work had only involved conditions of total sleep deprivation which are necessarily short term and therefore of dubious long-term clinical implication. The more recent focus on the highly prevalent condition of chronic partial sleep deprivation resulted in a major re-evaluation of the importance of sleep for health, and particularly for the risks of obesity and diabetes. In the two sections below, we first summarize the evidence from epidemiologic studies and then the evidence from laboratory studies.
Obesity and Sleep Loss: Epidemiologic Evidence
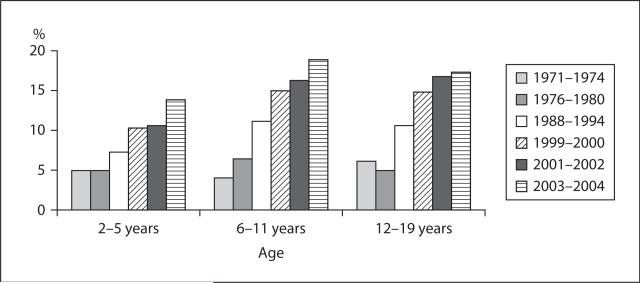
The increasing prevalence of obesity in both children and adults is affecting all industrialized countries. Figure 1 shows the change in the prevalence of overweight among American children per age category (2–5, 6–11 and 12–19 years) from 1971 to 2004 [12]. The prevalence of overweight went from about 5% in 1971 to about 15% in 2004 in each age category.
Fig. 1.
Prevalence of overweight (>95th percentile) among American children and adolescents ages 2 to 19 years old from 1971 to 2004.
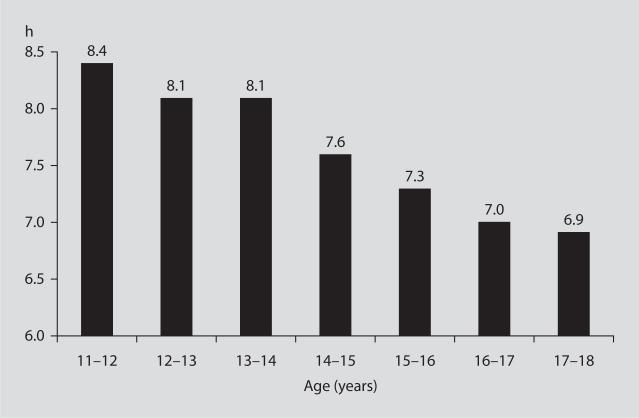
Increases in food intake and decreases in physical activity are the two most obvious reasons for the alarming increase in prevalence of obesity but experts agree that other factors must also be involved. Among those, reductions in sleep duration has been proposed to be one of the most likely contributing factors [13]. Over the past few decades, nightly sleep duration (by self-report) has decreased in a mirror image with the increase in the prevalence of obesity. In 2008, the poll conducted by the National Sleep Foundation [14] revealed that American adults sleep on average 6 h 40 min during weekdays and 7 h 25 min during the weekend. In contrast, in 1960, the average sleep duration was 8.5 h [15]. Thus, over less than 50 years, a reduction of sleep duration by 1.5–2 h seems to have occurred. Short sleep durations seems to be also typical in American adolescents. Well-documented laboratory studies have shown that, when given a 10-hour opportunity to sleep for several days, children between 10 and 17 years of age sleep for about 9 h, indicating that sleep need is not less than 9 h [16]. In stark contrast with this physiologic sleep need are the sleep durations self-reported by American children between 11 and 18 years old in 2006 [17]. Even in the youngest children, the amount of sleep is less than 9 hours and drops to 7 h or less in 16- to 18-year-olds (fig. 2).
Fig. 2.
Self-reported sleep duration in American adolescents in 2004.
Is there an association between the prevalence of obesity and the prevalence of short sleep duration? Cross-sectional studies have examined associations between sleep duration and BMI in both children and adults and prospective studies have tested the hypothesis that short sleep duration at baseline predicted weight gain or the incidence of obesity over the follow-up period. All studies controlled for a variety of potential confounders. In adults, as of May 2009, a total of 29 cross-sectional studies and 6 prospective studies originating from a wide variety of industrialized countries have been published. Thirty of these 35 studies had positive findings. Obesity risk generally increased for sleep durations under 6 h. There have been 20 cross-sectional studies in children and all had positive findings. Prospective studies are particularly important because they provide an indication regarding the direction of causality. Also, an overweight child is at higher risk of becoming an overweight or obese adult. Table 1 summarizes the 7 prospective epidemiologic studies so far that have examined sleep duration and obesity risk in boys and girls. All 7 studies showed a significant association between short sleep duration at baseline and weight gain or incidence of overweight or obesity over the follow-up period.
Table 1.
Prospective studies of sleep (reported by the parents) and obesity risk in boys and girls.
| Reference | Number of subjects and years of follow-up | Results | Country of origin |
|---|---|---|---|
| Lumeng et al. [18], 2007 | n = 785 aged 9–10 years (3rd grade) and 11–12 years (6th grade) |
short sleep duration in 3rd grade is associated with overweight in 6th grade | USA |
| Agras et al. [19], 2004 | n = 150 sleep reported at 3-5 years weight measured at 9.5 years |
less sleep time in childhood is a risk factor for childhood overweight | USA |
| Reilly et al. [20], 2005 | n = 7,758 sleep reported at 38 months obesity measured at 7 years |
short sleep duration (<10.5 h) at age 3 years is associated with a risk of obesity | UK |
| Taveras et al. [21], 2008 | n = 915 sleep reported at 6 months, 1 year and 2 years BMI z score measured at 3 years |
short sleep duration (<12 h/day) during infancy is associated with a higher BMI z score at 3 years | USA |
| Touchette et al. [22], 2008 | n = 1,138 sleep duration reported yearly from 2.5 to 6 years BMI measured at 2.5 and 6 years |
persistent short sleepers (<10 h) increases risk of overweight and obesity in later childhood | Canada |
| Sugimori et al. [23], 2004 | n = 8,170 sleep and BMI measured at ages 3 and 6 years |
short sleep duration (≤9 h) is associated with a risk of obesity in boys, not in girls | Japan |
| Snell et al. [24], 2007 | n = 2,281 aged 3–12 years at baseline and 5 years later |
less sleep is associated with higher BMI, 5 years later | USA |
In conclusion, the epidemiologic data consistently support a link between short sleep and obesity risk. Negative studies were mostly focusing on older adult populations. Of note, two cross-sectional studies used objectively recorded sleep, rather than self-report, and also found a significant association between short sleep and higher BMI. A major limitation of nearly all these studies is that there was no assessment of sleep quality or sleep disorders and therefore it is generally not known if short sleep was the result of bedtime restriction in a healthy sleeper or of the inability to achieve more sleep in an individual suffering from a sleep disorder.
Epidemiologic studies in adults have also shown associations between short sleep and diabetes risk [25]. Studies are needed to determine if the increased prevalence of type 2 diabetes in children and young adults is also partly predicted by short sleep.
Obesity, Diabetes and Sleep Loss: Evidence from Laboratory Studies
There has been no laboratory study so far that has examined the impact of experimental recurrent sleep restriction on hormones and metabolism in children. The existing laboratory studies were all conducted in young to middle-aged adults.
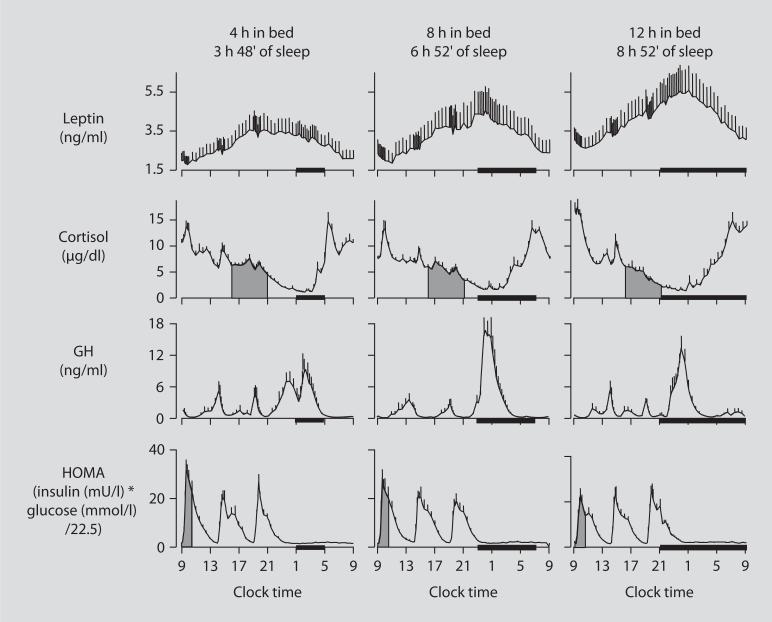
The first well-controlled laboratory study that tested the hypothesis that partial sleep deprivation could affect the metabolic and endocrine function was published 10 years ago [26]. Young lean subjects were studied (1) after building a state of sleep debt by restricting bedtime to 4 h for 6 nights, (2) after full recovery, obtained by extending the bedtime period to 12 h for 7 nights, and (3) under normal condition of 8 h in bed. This latter 8-hour bedtime condition was performed 1 year after the two other sleep conditions. Figure 3 shows the 24-hour profiles of leptin, cortisol, GH and HOMA (homeostatic model assessment, an integrated measure of glucose and insulin, that is the product of glucose concentration (mmol/l) by insulin concentration (mIU/l) divided by 22.5) under the 3 bedtime conditions [26]. Caloric intake was the same in the 3 conditions, i.e. 3 identical carbohydrate-rich meals. Posture and physical activity were also controlled as continuous bed rest was enforced during blood sampling. Clearly, overall leptin levels, evening cortisol levels, and the HOMA response to breakfast varied in a dose-response relationship with sleep duration. Shorter sleep duration was associated with greater disturbances in these hormonal and metabolic variables. Leptin levels were lowest when the subjects were in a state of sleep debt, signaling the brain an unnecessary need for extra caloric intake. Evening cortisol levels were highest when the subjects were in a state of sleep debt. A state of sleep debt therefore appears to delay the normal return to low levels of corticotropic activity. HOMA levels post-breakfast were the highest in a state of sleep debt indicating a decrease in glucose tolerance and/or a decrease in insulin sensitivity. The 24-hour GH profiles in the 8- and 12-hour bedtime conditions were qualitatively similar, with a trend for lower post-sleep peak values in the extended bedtime that is consistent with a reduced homeostatic drive for sleep with the decreased duration of the wake period. In the state of sleep debt, a GH pulse prior to sleep onset was observed, in addition to the normal post-sleep onset GH pulse. The elevation of GH concentrations during waking could have an adverse impact on glucose metabolism.
Fig. 3.
Relationship between sleep duration and leptin, cortisol, GH and HOMA.
A subsequent study [27] examined appetite regulation after 2 nights of 4 h in bed and after 2 nights of 10 h in bed, in a randomized cross-over design. This study confirmed the decrease in leptin levels seen in the previous study, with a 18% decrease of leptin levels after the short nights relative to the long nights. Furthermore, ghrelin was assayed and showed a 28% increase after the 2 nights of 4 h in bed. Questionnaires on hunger and appetite were completed and indicated a 24% increase in hunger and a 23% increase in global appetite after the 4-hour nights versus the 10-hour nights. Appetite for high carbohydrate nutrients was the most affected with a 32% increase. Importantly, the subjective report of increased hunger was correlated with the increase in ghrelin to leptin ratio (i.e. hunger factor/satiety factor). These observations suggest that in real life, when food is available everywhere and all the time, sleep deprived people may consume excessive amounts of calories, particularly from carbohydrates. A recent study tested this hypothesis using a randomized cross-over design with either extension or restriction of the usual bedtime period by 1.5 h for 2 weeks in the laboratory [28]. The subjects were middle-aged overweighed individuals who were exposed to unlimited amounts of palatable food presented in 3 meals per day and snacks were continuously available. The volunteers consumed excessive amounts of calories from meals under both sleep conditions but consumed more calories from snacks when sleep was restricted rather than extended.
Several studies have also shown that recurrent partial sleep restriction or experimentally reduced sleep quality results in decreased insulin resistance, another risk factor for weight gain and obesity. Remarkably, the decrease in insulin sensitivity was not associated with a compensatory increase in insulin release, and therefore diabetes risk was elevated.
The upper part of table 2 presents a re-analysis of the data from intravenous glucose tolerance testing (ivGTT) performed in the initial ‘sleep debt study’ [26] after 5 days of bedtime restriction to 4 h per night and when the subjects were fully rested at the end of the recovery period. Glucose tolerance was decreased by more than 40% when the subjects were in the state of sleep debt. This may be partly due to a decrease in brain glucose utilization as Sg (glucose effectiveness) which quantifies non-insulin-dependent glucose disposal, was significantly reduced. Insulin-dependent glucose disposal was also decreased since the glucose disposition index was markedly lower. Consistent findings have been observed in several follow-up studies [29, 30].
Table 2.
Alterations in glucose metabolism after sleep loss: 2 laboratory studies
| p | % change from well-rested condition | |
|---|---|---|
| 5 nights of 4-hour bedtimes (n = 11) | ||
| Glucose tolerance (% · min-1) | ≤0.003 | –43 ± 12 |
| Acute insulin response to glucose (μU · ml-1 · min) | ≤0.03 | –27 ±10 |
| Glucose effectiveness | ≤0.05 | –25 ± 19 |
| Insulin sensitivity, 104 min-1 (μU/ml)-1 | ≤0.04 | –24 ± 9 |
| Disposition index | 0.004 | –50 ± 6 |
| 3 nights of slow-wave sleep suppression (n = 9) | ||
| Glucose tolerance, % · min-1 | ≤0.03 | –23 ± 9 |
| Acute insulin response to glucose, μU · ml-1 · min | ≤0.73 | +11 ± 11 |
| Glucose effectiveness | ≤0.19 | –15 ± 10 |
| Insulin sensitivity, 104 min-1 (μU/ml)-1 | ≤0.009 | –25 ± 8 |
| Disposition index | ≤0.02 | –20 ± 7 |
Recently, a study showed that reduced sleep quality, without change in sleep duration, can also have adverse effects on glucose metabolism [31]. Slow-wave sleep was suppressed by delivering acoustic stimuli that replaced deep sleep SWS by shallow NREM sleep (stage 2) for 3 consecutive nights, mimicking the impact of four to five decades of aging. The lower part of table 2 shows the results of an ivGTT performed at baseline and after 3 nights of SWS suppression. The findings are qualitatively similar to those seen after 5 nights of bedtime curtailment but of lesser magnitude as would be expected since the intervention was of shorter duration.
Conclusions
Rapidly accumulating evidence suggests that sleep disturbances, including insufficient sleep due to bedtime curtailment and poor sleep quality, may represent novel risk factors for obesity and type 2 diabetes. While laboratory studies have been conducted in adults only, a large number of epidemiologic studies in pediatric populations have demonstrated associations between short sleep and adiposity that are often stronger than those seen in adult populations. Sleep curtailment appears to be an increasingly prevalent behavior in children and, in the United States, adolescents may well be the most sleep-deprived age group with a difference between self-reported sleep and estimated sleep need of more than 2 h daily. There is a paucity of knowledge regarding how insufficient sleep and sleep disorders may affect pubertal development and growth, despite the fact that it has been known for several decades that the release of sex steroids and GH is markedly dependent on sleep during the pubertal transition. An increasing number of children are obese and may suffer from obstructive sleep apnea. The impact of this sleep disorder, which is known to promote insulin resistance and reduced testosterone in adults, on neuroendocrine release and metabolic function in children is in urgent need of rigorous study.
Acknowledgements
Part of the work described in this article was supported by US National Institute of Health grants P01 AG-11412, R01 HL-075079, P60 DK-20595, R01 DK-0716960, R01 HL-075025 and M01 RR000055 and by US Department of Defense award W81XWH-07–2-0071.
References
- 1.Van Cauter E, Copinschi G. Endocrine and other biological rhythms. In: DeGroot L, Jameson J, editors. Endocrinology. Saunders; Philadelphia: 2006. pp. 235–256. [Google Scholar]
- 2.Welsh DK, et al. Individual neurons dissociated from rat suprachiasmatic nucleus express independently phased circadian firing rhythms. Neuron. 1995;14:697–706. doi: 10.1016/0896-6273(95)90214-7. [DOI] [PubMed] [Google Scholar]
- 3.Lowrey PL, Takahashi JS. Mammalian circadian biology: elucidating genome-wide levels of temporal organization. Annu Rev Genomics Hum Genet. 2004;5:407–441. doi: 10.1146/annurev.genom.5.061903.175925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vitaterna M, Pinto L, Turek F. Molecular genetic basis for mammalian circadian rhythms. In: Kryger MH, Dement WC, editors. Principles and Practices of Sleep Medicine. ed 4 Saunders; New York: 2005. pp. 363–374. [Google Scholar]
- 5.Landolt HP. Sleep homeostasis: a role for adenosine in humans? Biochem Pharmacol. 2008;75:2070–2079. doi: 10.1016/j.bcp.2008.02.024. [DOI] [PubMed] [Google Scholar]
- 6.Porkka-Heiskanen T, et al. Adenosine: A mediator of the sleep-inducing effects of prolonged wakefulness. Science. 1997;276:1255–1258. doi: 10.1126/science.276.5316.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Retey JV, et al. A functional genetic variation of adenosine deaminase affects the duration and intensity of deep sleep in humans. Proc Natl Acad Sci USA. 2005;102:15676–15681. doi: 10.1073/pnas.0505414102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Benington JH, Heller HC. Restoration of brain energy metabolism as the function of sleep. Prog Neurobiol. 1995;45:347–360. doi: 10.1016/0301-0082(94)00057-o. [DOI] [PubMed] [Google Scholar]
- 9.Tononi G, Cirelli C. Sleep function and synaptic homeostasis. Sleep Med Rev. 2006;10:49–62. doi: 10.1016/j.smrv.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 10.Van Cauter E, et al. Modulation of glucose regulation and insulin secretion by circadian rhythmicity and sleep. J Clin Invest. 1991;88:934–942. doi: 10.1172/JCI115396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Simon C, et al. Slow oscillations of plasma glucose and insulin secretion rate are amplified during sleep in humans under continuous enteral nutrition. Sleep. 1994;17:333–338. doi: 10.1093/sleep/17.4.333. [DOI] [PubMed] [Google Scholar]
- 12.Centers for Disease Control and Prevention [April 12, 2008];Prevalence of Overweight Among Children and Adolescents: United States, 2003–2004 [online] 2007 Available from: http://www.cdc.gov/nchs/products/pubs/pubd/hestats/overweight/overwght_child_03.htm.
- 13.Keith SW, et al. Putative contributors to the secular increase in obesity: exploring the roads less traveled. Int J Obes (Lond) 2006;30:1585–1594. doi: 10.1038/sj.ijo.0803326. [DOI] [PubMed] [Google Scholar]
- 14.National Sleep Foundation: Sleep in America Poll. Washington: 2008. [Google Scholar]
- 15.Kripke D, et al. Short and long sleep and sleeping pills. Is increased mortality associated? Arch Gen Psychiatry. 1979;36:103–116. doi: 10.1001/archpsyc.1979.01780010109014. [DOI] [PubMed] [Google Scholar]
- 16.Carskadon MA, Acebo C. Regulation of sleepiness in adolescents: update, insights, and speculation. Sleep. 2002;25:606–614. doi: 10.1093/sleep/25.6.606. [DOI] [PubMed] [Google Scholar]
- 17.National Sleep Foundation: Sleep in America Poll. Washington: 2006. [Google Scholar]
- 18.Lumeng JC, et al. Shorter sleep duration is associated with increased risk for being overweight at ages 9 to 12 years. Pediatrics. 2007;120:1020–1029. doi: 10.1542/peds.2006-3295. [DOI] [PubMed] [Google Scholar]
- 19.Agras WS, et al. Risk factors for childhood overweight: a prospective study from birth to 9.5 years. J Pediatr. 2004;145:20–25. doi: 10.1016/j.jpeds.2004.03.023. [DOI] [PubMed] [Google Scholar]
- 20.Reilly J, et al. Early life risk factors for obesity in childhood: cohort study. Br Med J. 2005;330:1357. doi: 10.1136/bmj.38470.670903.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taveras EM, et al. Short sleep duration in infancy and risk of childhood overweight. Arch Pediatr Adolesc Med. 2008;162:305–311. doi: 10.1001/archpedi.162.4.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Touchette E, et al. Associations between sleep duration patterns and overweight/obesity at age 6. Sleep. 2008;31:1507–1514. doi: 10.1093/sleep/31.11.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sugimori H, et al. Analysis of factors that influence body mass index from ages 3 to 6 years: a study based on the Toyama cohort study. Pediatr Int. 2004;46:302–310. doi: 10.1111/j.1442-200x.2004.01895.x. [DOI] [PubMed] [Google Scholar]
- 24.Snell EK, Adam EK, Duncan GJ. Sleep and the body mass index and overweight status of children and adolescents. Child Dev. 2007;78:309–323. doi: 10.1111/j.1467-8624.2007.00999.x. [DOI] [PubMed] [Google Scholar]
- 25.Knutson KL, Van Cauter E. Associations between sleep loss and increased risk of obesity and diabetes. Ann NY Acad Sci. 2008;1129:287–304. doi: 10.1196/annals.1417.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Spiegel K, Leproult R, Van Cauter E. Impact of sleep debt on metabolic and endocrine function. Lancet. 1999;354:1435–1439. doi: 10.1016/S0140-6736(99)01376-8. [DOI] [PubMed] [Google Scholar]
- 27.Spiegel K, et al. Sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels and increased hunger and appetite. Ann Intern Med. 2004;141:846–850. doi: 10.7326/0003-4819-141-11-200412070-00008. [DOI] [PubMed] [Google Scholar]
- 28.Nedeltcheva AV, Kilkus JM, Imperial J, Kasza K, Schoeller DA, Penev PD. Sleep curtailment is accompanied by increased intake of calories from snacks. Am J Clin Nutr. 2009;89:126–133. doi: 10.3945/ajcn.2008.26574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spiegel K, et al. Sleep loss: a novel risk factor for insulin resistance and Type 2 diabetes. J Appl Physiol. 2005;99:2008–2019. doi: 10.1152/japplphysiol.00660.2005. [DOI] [PubMed] [Google Scholar]
- 30.Buxton O, et al. Sleep restriction for one week reduces insulin sensitivity measured using the euglycemic hyperinsulinemic clamp technique. Sleep. 2008;31:A107. [Google Scholar]
- 31.Tasali E, et al. Slow-wave sleep and the risk of type 2 diabetes in humans. Proc Natl Acad Sci USA. 2008;105:1044–1049. doi: 10.1073/pnas.0706446105. [DOI] [PMC free article] [PubMed] [Google Scholar]