Abstract
The thymus contributes naïve, self MHC reactive, self tolerant T cells to the peripheral immune system throughout life, albeit with a log-linear decline with age. Quantification of thymic function is clinically relevant in the setting of lymphoablation, but a phenotypic marker distinguishing recent thymic emigrants from long lived naïve T cells remains elusive. T cell receptor excision circles (TREC) are present in thymocytes exiting the thymus and quantification of the most frequent of these, the δrec-ψJα rearrangement has been widely used as a measure of recent thymic function. However, interpretation of results presented as TREC per cell has been criticised on the basis that extra-thymic cellular proliferation impacts on peripherally determined TREC numbers. TREC/ml is now considered to be more representative of thymic function than TREC/cell, especially where significant cellular proliferation occurs (e.g. during reconstitution following stem cell transplantation). Here we describe the validation of a novel variation to the established assay, directly quantifying TREC/ml from 300 µl whole blood. We show the assay to be reproducible, robust and stable longitudinally and we show equivalence of performance when compared with more standard assays. This assay particularly lends itself to the measurement of thymic function in children and where monitoring clinical variables is limited by tissue availability.
Keywords: TREC, Thymic function, Recent thymic emigrants, Stem cell transplantation, Thymic reconstitution, Paediatric
1. Introduction
An important feature of the adaptive immune response is the broad repertoire of T cell receptor (TCR) specificities (Le Douarin et al., 1996). TCR diversity is generated through rearrangements of TCR alpha (TCRα) chain and TCR beta (TCRβ) chain genes, and subsequent random pairing of α and β chain polypeptides (Arstila et al., 1999; Schlissel, 2003; Petrie et al., 1995). The TCR delta (TCRδ) chain locus lies within the TCRα chain locus and its excision forms the first step in TCRα chain gene rearrangement (de Villartay et al., 1988). One particularly frequent rearrangement occurs between the δrec and ψJalpha element recombination sequences. The intervening excised DNA is circularised by the formation of a ‘signal joint’ forming a DNA episome, termed a signal joint T cell receptor excision circle (sjTREC). Approximately 70% of T cells emerging from the thymus contain one or two sjTRECs (depending on whether one or both TCRα loci genes are rearranged) (Verschuren et al., 1997). As these T cells mature and proliferate their sjTRECs, which are stable (Livak and Schatz, 1996) and do not divide, are distributed amongst their progeny (Takeshita et al., 1989).
In humans there is no specific phenotypic marker for recent thymic emigrants. Although thymic imaging can provide information about thymic capacity (Mackall et al., 1995) it is inconvenient, may involve radiation exposure, and does not provide specific functional information. In 1998 Douek et al. (1998) published data suggesting that the absolute number of TREC molecules measured per microgram (μg) of DNA derived from isolated CD4+ and CD8+ T cells represented a valid biomarker of thymic function. The original semi-quantitative hybridisation assay has since been superseded by assays that use real time quantitative PCR for absolute TREC quantification but the underlying principle is that the proportion of peripheral blood T cells containing a TREC provides an approximation of recent thymic function.
TREC measurements have been presented in a number of different ways. In some methods a TREC standard enables the reporting of the absolute number of TREC molecules per unit number of cells or TREC molecules per μg of DNA within PBMC or T-lymphocytes (Nobile et al., 2004; Hug et al., 2003; Zhang et al., 1999). In an alternative approach, TREC DNA has been reported relative to genomic DNA within a particular cellular subset (PBMC, T-lymphocytes, naïve T-lymhocytes) (Ponchel et al., 2003) and yet others have sorted individual T cell subsets and measured TREC within that subset (Hazenberg et al., 2000). The different methods and units used to measure TRECs affect the interpretation and comparison of TREC data. Additionally, T cell proliferation, T cell death and intra-cellular TREC degradation influence the different assays in distinct ways. In particular, any assay that uses a cellular subset as denominator, whether absolute cell number or DNA content, will be influenced by cell proliferation within that subset as illustrated by Hazenberg et al. (2000). For instance if an assay reports TREC per cell, an increase in circulating T- lymphocytes as a consequence of peripheral division will artificially dilute the assay readout regardless of recent thymic function. As thymic involution occurs a proportional decline in peripheral blood TREC numbers occurs and a coincident increase in naïve T cell proliferation maintains the size of this T cell compartment (Murray et al., 2003; Douek et al., 2001). Memory T cells (CD45RO+) contain few if any detectable TREC (Kimmig et al., 2002). Numerous mathematical models have been described which attempt, using the available clinical data, to enhance the interpretation of TREC data in the face of these confounding parameters (Hazenberg et al., 2000; Lewin et al., 2002; Ye and Kirschner, 2002). Recently, several authors have concluded that measuring TREC per millilitre of blood overcomes the problem induced by cellular proliferation in this assay, since the absolute number of TREC molecules, assuming there is not significant specific death of TREC containing T cells, remains independent of total cell number (Krenger et al., 2004; Ribeiro and Perelson, 2007).
In the current study we describe and evaluate a method which directly quantifies TREC from whole blood, using a modification of the currently well described real time PCR assay. The value generated is TREC/ml and is therefore measured without reference to cellular populations, removing any confounding attributable to cell proliferation. Moreover, it is performed using very small quantities of whole blood (300 µl or less) and removes the necessity for separating PBMC and other T cell subsets. The assay is validated and has been tested in a large sample of healthy control children and adults.
2. Materials and methods
2.1. Samples
Ethical approval for the study was given by the Newcastle & North Tyneside Research Ethics Committee. 9 ml blood samples were obtained from healthy laboratory controls, individuals attending local blood transfusion donation sessions and from healthy children attending the local hospital for simple surgical procedures such as hernia repair, circumcision and correction of strabismus. All samples were collected in to Vacuette EDTA K3 tubes (Greiner Bio-one, Austria) with informed consent from the individual or a parent or guardian.
2.2. DNA extraction
DNA was extracted from 300 µl of whole blood using the Wizard Genomic DNA extraction kit® (Promega) according to the manufacturer's instructions and stored at 4 °C until use. Sample purity and quantity was determined by spectrophotometry (Nanodrop® ND-100).
2.3. Isolation of PBMC and CD4+ T cells
PBMC were isolated according to a standard, sucrose density gradient (Lymphoprep®) protocol. CD4+ T cells were isolated from PBMC with CD4+ positive selection beads (MACS®—Miltenyi Biotec) using a semi-automated positive selection protocol according to the manufacturers instructions yielding CD3+CD4+ T cells with 95–98% purity.
2.4. Real time quantitative PCR
The TREC content of each sample was determined by quantitative real time PCR (RQ-PCR) using a standard curve derived from plasmid constructs encoding the sjTREC sequence (Douek et al., 2000). Standards were diluted over the range 107–101 and a curve was run in each experiment together with standard samples, positive and negative controls. RQ-PCR was performed using an ABI Prism 7900HT Sequence Detector System and data analysed using SDS2.2 software from the manufacturer (Applied Biosystems, Warrington, UK). Taqman™ Hydrolysis technology was used in a 25 μl reaction mixture containing 700 nM of each primer CACATCCCTTTCAACCATGCT and GCCAGCTGCAGGGTTTAGG, 150 nM Taqman hydrolysis probe (6-FAM-ACACCTCTGGTTTTTGTAAAGGTGCCCACT-TAMRA) and 12.5 µl JumpStart™ Taq ReadyMix (SIGMA). Each reaction contained 200 μg DNA. Thermal cycling conditions were 50 °C for 2 min then 95 °C for 10 min then 95 °C for 15 s and 60 °C for 1 min for 40 cycles. Experimental samples were run in duplicate and the replicate average value taken as the sample result. Primers and probes were all from SIGMA-Aldrich.
2.5. Statistics
All statistical analysis was performed using SPSS© 11th edition software. Values for TREC/ml were log transformed as described in the text prior to statistical analysis. Population distributions were tested with a one sample Kolmogorov–Smirnov test to confirm normality. Correlations for normally distributed data were tested for significance using Pearson's correlation coefficient unless otherwise stated. Analysis of covariance (ANCOVA) was used to test for differences between groups where other covariates were present. Results were considered significant when p < 0.05.
3. Results
3.1. Direct quantification of TREC per millilitre of whole blood
We devised a mathematical description for the determination of TREC per millilitre of whole blood, where DNA was extracted from a 300 μl aliquot of the sample. TREC per ml was derived using Eq.(1).
| (1) |
Eq. (1)—Calculation of TREC per ml of whole blood (WB—whole blood; RQ-PCR—real time quantitative PCR, No. of TREC is absolute value derived from RQ-PCR standard curve).
Calculated vales for TREC/ml were log transformed since the size of the error in individual results (which had a range of 1.5Log10) was proportional to the size of the mean (Bland, 2000). This also allowed statistical analysis to be performed on normally distributed data.
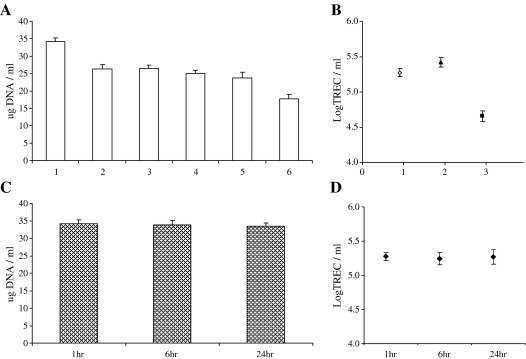
From Eq. (1), four sources of experimental variability were identified that could contribute to measurement error in the assay. Such methodological considerations are true for most systems of this nature but infrequently described in the literature. We therefore sought to confirm that we could a) reproducibly extract the same quantity of DNA from a single donor sample on multiple occasions; b) that we could reproducibly quantify TREC molecules from single donor samples on multiple occasions; c) that there was no effect of time from sample donation to sample preparation on values obtained and d) that TREC values were independent of total white cell count—since granulocytes, B cells and NK cells are all nucleated cells present in whole blood. Fig. 1(A) shows that DNA was reproducibly extracted from whole blood samples and Fig. 1(B) that TREC/ml was reproducibly calculated in three of six of these samples. Over a 24 h period there was no effect of time from the sample being drawn to being processed on the quantity of DNA extracted—Fig. 1(C) or the determined TREC value for that sample—Fig. 1(D). The coefficient of variation for the DNA extraction was calculated to be 5.07% from six samples extracted 10 times each.
Fig. 1.
Determination of assay reproducibility. DNA was extracted from ten 300 µl replicate aliquots of whole blood from six healthy donors (A). WBTREC/ml was then calculated for five of these replicates in three donors (B). To examine the potential effect of a delay in sample processing, DNA was extracted from five replicates of a single sample at 1 h, 6 h and 24 h (C). WBLogTREC/ml was then quantified in all five aliquots for each time point (D). All plotted values are mean ± SD.
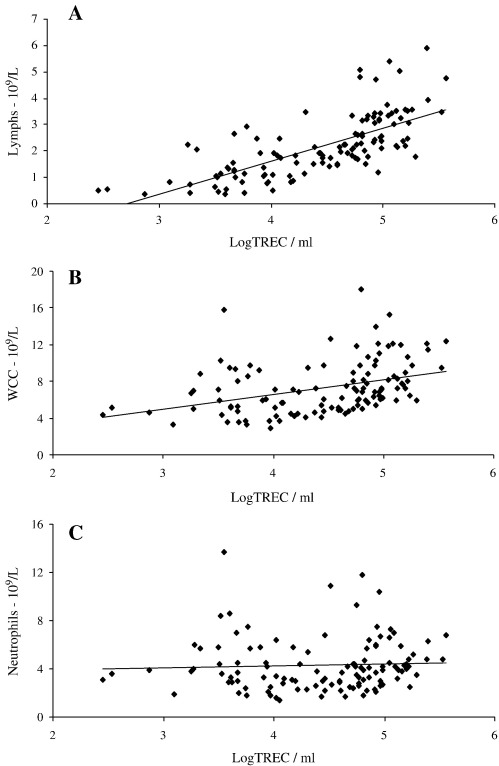
All white cells (granulocytes, monocytes, B cells, NK cells and T cells) in whole blood are nucleated and thus in this assay contribute to the total DNA extracted from any sample. Since the number of white cells (WC) varies between individuals the total WC count may alter the sensitivity of TREC measurements. We therefore assessed the relationships between total WC count, lymphocyte count, neutrophil count and WBLogTREC/ml. WC count and subset counts were determined in a clinical laboratory for a small sample of healthy controls. Samples from patients with rheumatological autoimmune diseases who had blood counts available were also included. We showed that WBLogTREC/ml was significantly correlated with lymphocyte count (sum of T cells (60–80%), B cells (12–20%), NK cells (10–15%)) (r = 0.71; p < 0.01). WC count was correlated to a lesser extent, reflecting the lymphocyte content of total WC (approximately one third lymphocytes), (r = 0.38; p < 0.05). Neutrophil count was not correlated with WBLogTREC/ml—Fig. 2.
Fig. 2.
WBLogTREC/ml is positively correlated with lymphocyte count (A) (Pearson's correlation coefficient r = 0.67; p < 0.01) and weakly with white cell count (r = 0.38; p < 0.05) (B) but not neutrophil count (p = n.s.) (C) when directly quantified from whole blood.
3.2. Determining assay precision
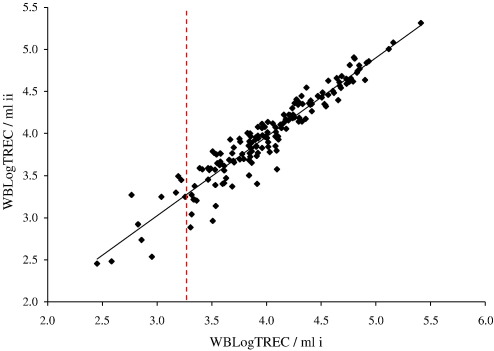
The precision of any assay to detect the presence or absence of a quantifiable unit is reduced as the limit of detection for the assay is approached. Here, as the number of TREC molecules detected per reaction approaches one, the precision of the assay declines, reflecting the limit of detection of real time PCR (Ginzinger, 2002). To assess this experimentally we quantified TREC from 175 individual samples in duplicate. The overall correlation coefficient for the comparison was 0.96; (p < 0.01). However, as the number of TREC quantified per sample fell below 10, variation in the final result was increased. Fig. 3 shows that values to the left of the dashed line (which represents the values of WBLogTREC/ml given by samples in which ‘10 or less TREC’ were quantified in the real time PCR) are more widely dispersed from the regression line than values to the right which represent samples in which ‘11 or more TREC’ were measured.
Fig. 3.
Duplicate estimation of the number of TREC molecules per sample was performed for 175 individuals. Each data point represents the average value obtained from two simultaneous PCRs performed on two (i and ii) occasions for a single donor. Overall, the assay was shown to be highly reproducible as evidenced by the regression line's closeness to the diagonal and Pearson's correlation coefficient r = 0.96 (p < 0.01). However, as the number of TREC determined per reaction approached 10, the reproducibility of the assay was less. The vertical dashed line represents the average final value of WBLogTREC/ml, calculated according to Eq. (1), for samples in which 10 TREC were measured. Values to the left of the line thus represent samples with less than 10 TREC and those to the right those with more than 10 TREC.
The coefficient of variation for the real time reaction was calculated by running two standard samples of DNA in 25 separate real time experiments and was 1.93% and 1.49% for each standard sample respectively (data not shown). Thus, the overall experimental coefficient of variation from DNA extraction to TREC determination (variation in DNA extraction multiplied by variation in RQ-PCR of TREC) was 8.7%, range 7.6–9.8%.
3.3. Comparison of TREC determination from WB derived DNA with that from PBMC and CD4+ derived DNA
For a given quantity of total DNA analysed, selection of TREC-rich cells increases the probability of detecting a TREC in a PCR. Thus, in 200 ng DNA derived from selected T cells the absolute number of TREC will be higher than in 200 ng DNA from PBMC which in turn will be higher than in 200 ng DNA from WB. We have shown that our assay becomes less reproducible as TREC/reaction approaches 10 (Fig. 3). However, whereas the use of WB as a template for TREC measurement may reduce assay sensitivity, variable T cell recovery may alter the accuracy of analyses requiring cellular isolation. We therefore quantified TREC from DNA derived from each type of sample in patients known to have values around the limit of detection—elderly patients with rheumatoid arthritis taking disease modifying anti-rheumatic drugs (DMARDs) (Ponchel et al., 2002).
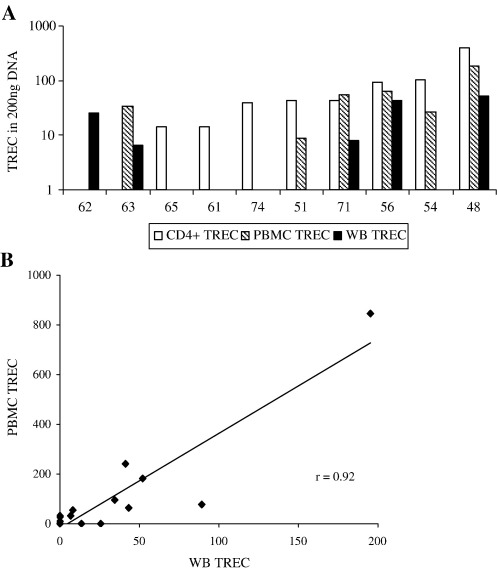
Samples were initially drawn from ten patients (mean age 60.5 yrs (range 48–74 years)) and TREC quantified for each using WB, isolated PBMC and CD4+ T cells. Fig. 4(A) shows that TREC were detected in 8 patients using CD4+ T cell DNA, 6 patients using PBMC DNA and 5 patients using WB DNA. As the frequency of TREC per sample increased (as judged by the TREC content of CD4+ T cells) the likelihood of detecting TREC using both of the other sample types increased, confirming the sensitivity advantage of selecting a TREC-rich subset. However it is notable that in two patients where insufficient CD4+ T cells were recovered, TREC were detected in both using WB DNA and from one using PBMC DNA, emphasising the potential cost of cell isolation in terms of accuracy. The detection of TREC in WB DNA but not in PBMC DNA from the same individual may also reflect stochastic factors as the limit of assay sensitivity is approached.
Fig. 4.
(A) Comparison of TREC values derived from WB DNA (filled bars), PBMC DNA (striped bars) and CD4+ T cell DNA (white bars) in blood drawn from ten patients with rheumatoid arthritis. The age (yrs) of the patients is given below each. TREC values are those derived from the assay standard curve to give raw data values for comparison. Where there are no bars for individual subsets TREC were not detected in the PCR reaction (or insufficient DNA extracted in the case of the two samples where CD4+ samples were tested). Each reaction was performed using 200 ng of DNA. (B) TREC values derived from the PCR reactions were compared in these ten patients plus a further six patients from DNA extracted from PBMC and WB DNA. The values derived are strongly correlated (r = 0.92; p < 0.01).
We then compared TREC values obtained from PBMC and WB DNA to confirm that results from both assays were consistent. We did not compare either with CD4+ T cell DNA since the former two assays will additionally include TREC present in CD8+ T cells. To increase the sample size for this analysis we isolated DNA from a further 6 RA patients. TREC were detected in 10/16 using WB and 11/16 using PBMC DNA. The correlation coefficient between WB TREC and PBMC TREC values was very strong (r = 0.92; r < 0.01) confirming equivalence of the data (Fig. 4B).
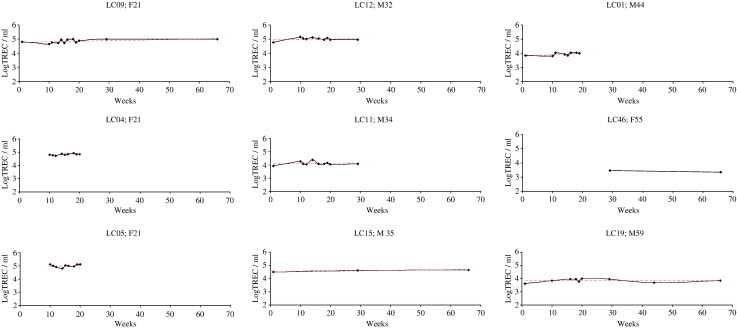
3.4. Whole blood TREC per millilitre (WBLogTREC/ml) values are biologically stable over time in healthy individuals
Despite a decline of approximately 1.5 logs over the age span tested, TREC values at any one age in normal healthy individuals are still highly variable (Zhang et al., 1999). We therefore sought to examine whether biological variation within an individual over time might contribute to this variation. Nine healthy male and female volunteers aged between 22 and 60 had their WBTREC/ml quantified serially over a three month period. We found that WBLogTREC/ml remained stable over this period (Fig. 5). Using the standard deviation about the mean as a summary measure (as described by Matthews et al., 1990) we showed that there was no biological variation above that which could be expected from the known inter-assay variation (p = 0.45), confirming that any biological variation in healthy individuals occurs at a level not detectable by this assay.
Fig. 5.
Biological variation in WBLogTREC/ml in HC over 3 months. Nine individuals had blood drawn on more than two occasions over a three month period. On each occasion DNA was extracted and stored. TREC values were determined for each individual in a single real time experiment. Age and gender for each individual is given. The average value for each individual was taken and plotted against age to check that the group was a representative one (Pearson's r for the correlation with age = − 0.904; p < 0.01). The standard deviation about the mean for each individual was independent of age (data not shown).
3.5. WBLogTREC/ml declines with age and varies with gender in healthy individuals
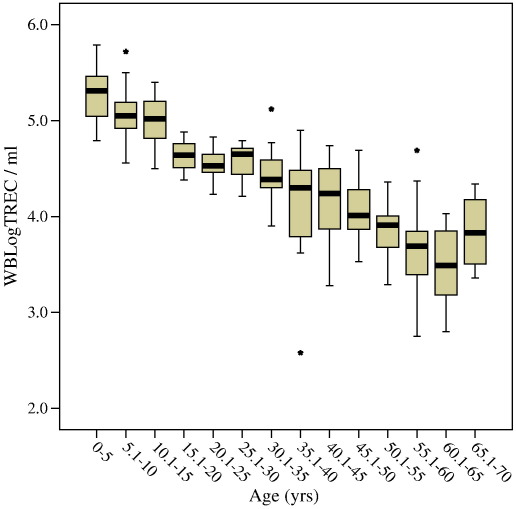
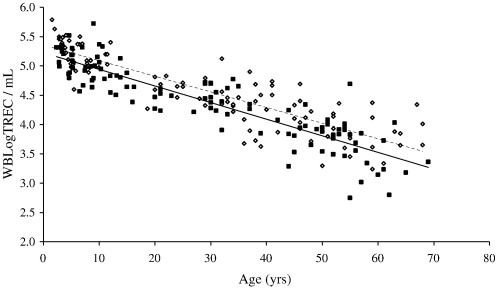
In healthy individuals thymic function is dependent on age. In a cohort of 221 healthy controls ranging in age from new born (cord blood) to 69 we confirmed a decline in the number of TREC per ml of approximately 1.5Log. As Zhang et al. (1999) found, there is a suggestion of an accelerated decline in thymic function at puberty but it was not possible to confirm this observation because our sample number in this age range was small (n = 4 for 15–20 years) (Fig. 6 and Table 1). Moreover, samples at this age were all from males. It has previously been shown that males have reduced thymic function compared to age-matched females (Pido-Lopez et al., 2001). Using the whole blood TREC assay we have confirmed this difference and demonstrated that it extends back into childhood (Fig. 7). The overall age-adjusted mean WBLogTREC/ml for males (n = 113) is 4.378 and for females (n = 106) is 4.573 (p = 0.043). Zhang et al. (1999) did not analyse their data on the basis of gender so comparisons cannot be made.
Fig. 6.
Healthy control samples were divided into 5 year groupings and WBLogTREC/ml determined. The mean (thick bar), inter-quartile range (boxes) and 95% confidence intervals (whiskers) are plotted. Outliers are plotted as stars (⁎). Age was negatively correlated with WBLogTREC/ml (Pearson's r = − 0.86; p < 0.01).
Table 1.
Mean WBLogTREC/ml for five year cohorts of healthy controls (male and female combined) with descriptive statistics for each cohort
| Age groups | n | Minimum | Maximum | Mean | Standard deviation |
|---|---|---|---|---|---|
| 0–5 | 32 | 4.79 | 5.79 | 5.26 | 0.26 |
| 5.1–10 | 33 | 4.56 | 5.72 | 5.05 | 0.26 |
| 10.1–15 | 15 | 4.50 | 5.4 | 5 | 0.28 |
| 15.1–20 | 4 | 4.38 | 4.88 | 4.64 | 0.20 |
| 20.1–25 | 14 | 4.23 | 4.83 | 4.53 | 0.19 |
| 25.1–30 | 9 | 4.21 | 4.79 | 4.55 | 0.20 |
| 30.1–35 | 20 | 3.90 | 5.12 | 4.45 | 0.26 |
| 35.1–40 | 16 | 2.58 | 4.9 | 4.12 | 0.56 |
| 40.1–45 | 9 | 3.28 | 4.74 | 4.16 | 0.46 |
| 45.1–50 | 16 | 3.53 | 4.69 | 4.05 | 0.30 |
| 50.1–55 | 23 | 3.29 | 4.36 | 3.84 | 0.26 |
| 55.1–60 | 15 | 2.75 | 4.69 | 3.68 | 0.50 |
| 60.1–65 | 10 | 2.80 | 4.03 | 3.48 | 0.40 |
| 65.1–70 | 4 | 3.36 | 4.34 | 3.84 | 0.43 |
Data corresponds to values presented in Fig. 5.
Fig. 7.
Gender differences are detectable in WBLogTREC/ml when females (open circles/ dashed regression line) are compared with males (closed circles—solid regression line) at any age. There is a statistically significant difference between the two groups after correcting for age (p = 0.043). The negative correlation with age is maintained in each population. (A single sample (male; TREC/ml—2.58) was removed from this analysis because it was an outlier exerting significant influence on the result when included).
4. Discussion
We present here a novel modification to the well established and widely used method of measuring thymic function – the ‘TREC Assay’ (Douek et al., 1998) – which quantifies TREC per ml directly from very small volumes of peripheral whole blood. As discussed earlier, we and others have concluded that TREC/ml provides a better estimate of the thymic contribution to the peripheral T cell compartment in cross-sectional analyses than TREC per cell, the interpretation of which is complicated by the effects of peripheral cellular division. Whereas under steady state conditions TREC/ml and TREC per 106 PBMC (a frequently used unit of TREC per cell) are likely to give comparable results, this is not true where there is significant and rapid T cell turnover as, for example, in HIV/AIDS after highly active anti-retroviral therapy or following HSCT for haematological malignancy or autoimmune disease (Ye and Kirschner, 2002). Such clinical situations are, however, of considerable clinical interest.
The thymus is crucial to reconstitution of the T cell compartment following lymphodepletion and also in establishing a normal, diverse T cell repertoire capable of mounting an adaptive immune response to neo-antigens, inhibiting auto-reactive processes (by the generation of thymus derived regulatory T cells) and detecting early malignant expansions (Dumont-Girard et al., 1998). Of note, recent evidence suggests that early lymphocyte reconstitution following allogeneic or autologous stem cell transplant (SCT) for haematological malignancy is positively correlated with a better clinical outcome and decreased risk of relapse (Joao et al., 2006; Parkman et al., 2006; Svaldi et al., 2003). It is known that adults reconstitute poorly in comparison with children, that impaired reconstitution is correlated with risk of life threatening infection post transplant (Small et al., 1999), and that this is due to age related thymic involution (Fallen et al., 2003). Therefore, investigators are now developing strategies which aim to restore thymic function and reverse thymic involution in adults (van den Brink et al., 2004) and thymic adjuvants such as Keratinocyte Growth Factor, IL-7, Lupron (chemical sex-steroid ablative agent) and in vitro expanded T/NK precursor cells are currently being evaluated in early clinical trials (for recent reviews see Goldberg et al., 2007; Legrand et al., 2007). The impact of these agents on thymic function will therefore be evaluated in larger clinical studies in the near future.
We have devised and validated a ‘whole blood TREC assay’ using a large group of healthy male and female individuals over a seven decade age range for use in both cross-sectional and longitudinal studies. Using this assay we have confirmed the described 1.5Log fold decline in TREC values with normal aging (Zhang et al., 1999) and the small, previously described, increase in thymic function in females when compared with age-matched males (Pido-Lopez et al., 2001). We have shown that, in individual adults, thymic function is stable in the short to medium term, irrespective of age, thus demonstrating that this assay can be used to monitor thymic function longitudinally and, furthermore, that cross-sectional analyses can be considered representative of recent thymic function, at least in healthy individuals. The assay is cheap, reproducible, stable over time and robust to use in longitudinal studies. Our modification to the established TREC assay allows thymic function to be measured rapidly from very small volumes of blood and thus particularly lends itself to use in clinical settings where limited tissue is available, for example in paediatric studies, and/or where longitudinal information is required. The lower limit of detection of our assay is higher than that for assays quantifying TREC using selected T cell subsets but is directly comparable with assays using PBMC derived DNA, with the advantage of significantly lower tissue volume requirement and sample processing time. When working at the limit of assay sensitivity the probability of TREC detection could be increased by quantifying TREC from replicate aliquots of individual samples and adjusting the derived values accordingly.
Our assay cannot distinguish between CD4+ and CD8+ contributions to the recent thymic emigrant subset. However, this limitation is common to most thymic function measurements. Sorting peripheral T cell subsets and quantifying TREC within them allows both maturation pathways of recent thymic emigrants to be explored (as we have previously shown Ponchel et al., 2002) and, where the total T cell count is known, TREC/ml to be calculated. However, this requires large volumes of blood and is less acceptable to patients and treating physicians when there are numerous other clinical and experimental requirements for peripheral blood. Reflecting this problem, several clinical studies have moved to quantify recent thymic emigrants using the phenotype CD45RA+CD31+. Whilst this subset is certainly TREC-rich (Kimmig et al., 2002; Junge et al., 2007) it does not always correlate strongly with TREC values and may be capable of self-renewal in the absence of thymic output quantified by TREC/ml (Kilpatrick et al., 2008; Lorenzi et al., 2008).
Eyrich et al. have recently employed a novel approach to the measurement of thymic function in the post-SCT context. They showed that two specific time points post transplant were of particular clinical interest—Tmin (days until first detectable TREC) and Tplateau (days until stable TREC values). These two time points were independently regulated by age and grafted stem cell number respectively (Eyrich et al., 2005). The effects of differing conditioning regimes, graft T cell depletion, use of ex vivo thymocyte precursor expansion protocols and thymic adjuvants are all currently being investigated in clinical trials to determine their impact on thymic output. Our assay will provide a useful clinical tool for the study of these strategies by allowing, for example, Tmin and Tplateau to be readily quantified from small clinical samples in large clinical cohorts. Time to detection of first TREC may be delayed using our assay in such a setting but this disadvantage should be outweighed by the significantly reduced sample volume and manipulation required to conduct the assay—a consideration of particular relevance in a paediatric context. Since early reconstitution appears to be associated with better prognosis following HSCT in haematological malignancy, our assay could also be validated as a prognostic marker.
In summary, our group has previously validated and published an assay that is designed specifically for the determination of TREC per cell (Ponchel et al., 2003). We now present an assay that directly quantifies TREC/ml from whole blood. The combination of the two assays, alongside appropriate cell surface labelling contributes a set of tools with which thymic reconstitution can be investigated following lymphoablative treatments and in the clinical evaluation of thymic adjuvant therapies.
Acknowledgement
The authors are grateful to D. Douek for the generous gift of the sjTREC plasmid used in this work.
Footnotes
This work was funded by a Clinical Research Fellowship (AR Lorenzi) from the Arthritis Research Campaign (UK) and a PhD Studentship (M Jefferson) from the Oliver Bird Foundation.
References
- Arstila T.P., Casrouge A., Baron V., Even J., Kanellopoulos J., Kourilsky P. A direct estimate of the human alphabeta T cell receptor diversity. Science. 1999;286(5441):958. doi: 10.1126/science.286.5441.958. [DOI] [PubMed] [Google Scholar]
- Bland M. 3 ed. Oxford University Press; 2000. An Introduction to Medical Statistics. [Google Scholar]
- de Villartay J.P., Hockett R.D., Coran D., Korsmeyer S.J., Cohen D.I. Deletion of the human T-cell receptor delta-gene by a site-specific recombination. Nature. 1988;335(6186):170. doi: 10.1038/335170a0. [DOI] [PubMed] [Google Scholar]
- Douek D.C., McFarland R.D., Keiser P.H., Gage E.A., Massey J.M., Haynes B.F. Changes in thymic function with age and during the treatment of HIV infection. Nature. 1998;396(6712):690. doi: 10.1038/25374. [DOI] [PubMed] [Google Scholar]
- Douek D.C., Vescio R.A., Betts M.R., Brenchley J.M., Hill B.J., Zhang L. Assessment of thymic output in adults after haematopoietic stem-cell transplantation and prediction of T-cell reconstitution. Lancet. 2000;355(9218):1875. doi: 10.1016/S0140-6736(00)02293-5. [DOI] [PubMed] [Google Scholar]
- Douek D.C., Betts M.R., Hill B.J., Little S.J., Lempicki R., Metcalf J.A. Evidence for increased T cell turnover and decreased thymic output in HIV infection. J. Immunol. 2001;167(11):6663. doi: 10.4049/jimmunol.167.11.6663. [DOI] [PubMed] [Google Scholar]
- Dumont-Girard F., Roux E., van Lier R.A., Hale G., Helg C., Chapuis B. Reconstitution of the T-cell compartment after bone marrow transplantation: restoration of the repertoire by thymic emigrants. Blood. 1998;92(11):4464. [PubMed] [Google Scholar]
- Eyrich M., Wollny G., Tzaribaschev N., Dietz K., Brugger D., Bader P. Onset of thymic recovery and plateau of thymic output are differentially regulated after stem cell transplantation in children. Biol. Blood Marrow Transplant. 2005;11(3):194. doi: 10.1016/j.bbmt.2004.12.001. [DOI] [PubMed] [Google Scholar]
- Fallen P.R., McGreavey L., Madrigal J.A., Potter M., Ethell M., Prentice H.G. Factors affecting reconstitution of the T cell compartment in allogeneic haematopoietic cell transplant recipients. Bone Marrow Transplant. 2003;32(10):1001. doi: 10.1038/sj.bmt.1704235. [DOI] [PubMed] [Google Scholar]
- Ginzinger D.G. Gene quantification using real-time quantitative PCR: an emerging technology hits the mainstream. Exp. Hematol. 2002;30(6):503. doi: 10.1016/s0301-472x(02)00806-8. [DOI] [PubMed] [Google Scholar]
- Goldberg G.L., Zakrzewski J.L., Perales M.A., van den Brink M.R. Clinical strategies to enhance T cell reconstitution. Semin. Immunol. 2007 doi: 10.1016/j.smim.2007.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazenberg M.D., Otto S.A., Cohen Stuart J.W., Verschuren M.C., Borleffs J.C., Boucher C.A. Increased cell division but not thymic dysfunction rapidly affects the T-cell receptor excision circle content of the naive T cell population in HIV-1 infection. Nat. Med. 2000;6(9):1036. doi: 10.1038/79549. [DOI] [PubMed] [Google Scholar]
- Hug A., Korporal M., Schroder I., Haas J., Glatz K., Storch-Hagenlocher B. Thymic export function and T cell homeostasis in patients with relapsing remitting multiple sclerosis. J. Immunol. 2003;171(1):432. doi: 10.4049/jimmunol.171.1.432. [DOI] [PubMed] [Google Scholar]
- Joao C., Porrata L.F., Inwards D.J., Ansell S.M., Micallef I.N., Johnston P.B. Early lymphocyte recovery after autologous stem cell transplantation predicts superior survival in mantle-cell lymphoma. Bone Marrow Transplant. 2006;37(9):865. doi: 10.1038/sj.bmt.1705342. [DOI] [PubMed] [Google Scholar]
- Junge S., Kloeckener-Gruissem B., Zufferey R., Keisker A., Salgo B., Fauchere J.C. Correlation between recent thymic emigrants and CD31(+) (PECAM-1) CD4(+) T cells in normal individuals during aging and in lymphopenic children. Eur. J. Immunol. 2007 doi: 10.1002/eji.200636976. [DOI] [PubMed] [Google Scholar]
- Kilpatrick R.D., Rickabaugh T., Hultin L.E., Hultin P., Hausner M.A., Detels R. Homeostasis of the naive CD4+ T cell compartment during aging. J. Immunol. 2008;180(3):1499. doi: 10.4049/jimmunol.180.3.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmig S., Przybylski G.K., Schmidt C.A., Laurisch K., Mowes B., Radbruch A. Two subsets of naive T helper cells with distinct T cell receptor excision circle content in human adult peripheral blood. J. Exp. Med. 2002;195(6):789. doi: 10.1084/jem.20011756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krenger W., Schmidlin H., Cavadini G., Hollander G.A. On the relevance of TCR rearrangement circles as molecular markers for thymic output during experimental graft-versus-host disease. J. Immunol. 2004;172(12):7359. doi: 10.4049/jimmunol.172.12.7359. [DOI] [PubMed] [Google Scholar]
- Le Douarin N., Corbel C., Bandeira A., Thomas-Vaslin V., Modigliani Y., Coutinho A. Evidence for a thymus-dependent form of tolerance that is not based on elimination or anergy of reactive T cells. Immunol. Rev. 1996;149:35. doi: 10.1111/j.1600-065x.1996.tb00898.x. [DOI] [PubMed] [Google Scholar]
- Legrand N., Dontje W., van Lent A.U., Spits H., Blom B. Human thymus regeneration and T cell reconstitution. Semin. Immunol. 2007 doi: 10.1016/j.smim.2007.10.001. [DOI] [PubMed] [Google Scholar]
- Lewin S.R., Ribeiro R.M., Kaufmann G.R., Smith D., Zaunders J., Law M. Dynamics of T cells and TCR excision circles differ after treatment of acute and chronic HIV infection. J. Immunol. 2002;169(8):4657. doi: 10.4049/jimmunol.169.8.4657. [DOI] [PubMed] [Google Scholar]
- Livak F., Schatz D.G. T-cell receptor alpha locus V(D)J recombination by-products are abundant in thymocytes and mature T cells. Mol. Cell. Biol. 1996;16(2):609. doi: 10.1128/mcb.16.2.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzi A.R., Morgan T.A., Anderson A.E., Catterall J.B., Patterson A.M., Foster H. Thymic function in juvenile idiopathic arthritis. Ann. Rheum. Dis. 2008 doi: 10.1136/ard.2008.088112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackall C.L., Fleisher T.A., Brown M.R., Andrich M.P., Chen C.C., Feuerstein I.M. Age, thymopoiesis, and CD4+ T-lymphocyte regeneration after intensive chemotherapy. N. Engl. J. Med. 1995;332(3):143. doi: 10.1056/NEJM199501193320303. [DOI] [PubMed] [Google Scholar]
- Matthews J.N., Altman D.G., Campbell M.J., Royston P. Analysis of serial measurements in medical research. Bmj. 1990;300(6719):230. doi: 10.1136/bmj.300.6719.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray J.M., Kaufmann G.R., Hodgkin P.D., Lewin S.R., Kelleher A.D., Davenport M.P. Naive T cells are maintained by thymic output in early ages but by proliferation without phenotypic change after age twenty. Immunol. Cell Biol. 2003;81(6):487. doi: 10.1046/j.1440-1711.2003.01191.x. [DOI] [PubMed] [Google Scholar]
- Nobile M., Correa R., Borghans J.A., D'Agostino C., Schneider P., De Boer R.J. De novo T-cell generation in patients at different ages and stages of HIV-1 disease. Blood. 2004;104(2):470. doi: 10.1182/blood-2003-12-4265. [DOI] [PubMed] [Google Scholar]
- Parkman R., Cohen G., Carter S.L., Weinberg K.I., Masinsin B., Guinan E. Successful immune reconstitution decreases leukemic relapse and improves survival in recipients of unrelated cord blood transplantation. Biol. Blood Marrow Transplant. 2006;12(9):919. doi: 10.1016/j.bbmt.2006.05.008. [DOI] [PubMed] [Google Scholar]
- Petrie H.T., Livak F., Burtrum D., Mazel S. T cell receptor gene recombination patterns and mechanisms: cell death, rescue, and T cell production. J. Exp. Med. 1995;182(1):121. doi: 10.1084/jem.182.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pido-Lopez J., Imami N., Aspinall R. Both age and gender affect thymic output: more recent thymic migrants in females than males as they age. Clin. Exp. Immunol. 2001;125(3):409. doi: 10.1046/j.1365-2249.2001.01640.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponchel F., Morgan A.W., Bingham S.J., Quinn M., Buch M., Verburg R.J. Dysregulated lymphocyte proliferation and differentiation in patients with rheumatoid arthritis. Blood. 2002;100(13):4550. doi: 10.1182/blood-2002-03-0671. [DOI] [PubMed] [Google Scholar]
- Ponchel F., Toomes C., Bransfield K., Leong F.T., Douglas S.H., Field S.L. Real-time PCR based on SYBR-Green I fluorescence: an alternative to the TaqMan assay for a relative quantification of gene rearrangements, gene amplifications and micro gene deletions. BMC Biotechnol. 2003;3:18. doi: 10.1186/1472-6750-3-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro R.M., Perelson A.S. Determining thymic output quantitatively: using models to interpret experimental T-cell receptor excision circle (TREC) data. Immunol. Rev. 2007;216:21. doi: 10.1111/j.1600-065X.2006.00493.x. [DOI] [PubMed] [Google Scholar]
- Schlissel M.S. Regulating antigen-receptor gene assembly. Nat. Rev. Immunol. 2003;3(11):890. doi: 10.1038/nri1225. [DOI] [PubMed] [Google Scholar]
- Small T.N., Papadopoulos E.B., Boulad F., Black P., Castro-Malaspina H., Childs B.H. Comparison of immune reconstitution after unrelated and related T-cell-depleted bone marrow transplantation: effect of patient age and donor leukocyte infusions. Blood. 1999;93(2):467. [PubMed] [Google Scholar]
- Svaldi M., Lanthaler A.J., Dugas M., Lohse P., Pescosta N., Straka C. T-cell receptor excision circles: a novel prognostic parameter for the outcome of transplantation in multiple myeloma patients. Br. J. Haematol. 2003;122(5):795. doi: 10.1046/j.1365-2141.2003.04482.x. [DOI] [PubMed] [Google Scholar]
- Takeshita S., Toda M., Yamagishi H. Excision products of the T cell receptor gene support a progressive rearrangement model of the alpha/delta locus. Embo J. 1989;8(11):3261. doi: 10.1002/j.1460-2075.1989.tb08486.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Brink M.R., Alpdogan O., Boyd R.L. Strategies to enhance T-cell reconstitution in immunocompromised patients. Nat. Rev. Immunol. 2004;4(11):856. doi: 10.1038/nri1484. [DOI] [PubMed] [Google Scholar]
- Verschuren M.C., Wolvers-Tettero I.L., Breit T.M., Noordzij J., van Wering E.R., van Dongen J.J. Preferential rearrangements of the T cell receptor-delta-deleting elements in human T cells. J. Immunol. 1997;158(3):1208. [PubMed] [Google Scholar]
- Ye P., Kirschner D.E. Reevaluation of T cell receptor excision circles as a measure of human recent thymic emigrants. J. Immunol. 2002;168(10):4968. doi: 10.4049/jimmunol.168.10.4968. [DOI] [PubMed] [Google Scholar]
- Zhang L., Lewin S.R., Markowitz M., Lin H.H., Skulsky E., Karanicolas R. Measuring recent thymic emigrants in blood of normal and HIV-1-infected individuals before and after effective therapy. J. Exp. Med. 1999;190(5):725. doi: 10.1084/jem.190.5.725. [DOI] [PMC free article] [PubMed] [Google Scholar]