Abstract
Pentamidine is a second-line agent in the treatment of leishmaniasis whose mode of action and resistance mechanism are not well understood. In this work, we show that the intracellular ABC protein PRP1 (pentamidine resistance protein 1) (ABCC7) can confer resistance to pentamidine in Leishmania sp. parasites in the intracellular stage.
Leishmaniasis is a significant cause of morbidity and mortality in several countries, and its clinical manifestation depends on species, host genetic factors, and immune response (19). Treatment depends on chemotherapy, where pentavalent antimonials are still the mainstay against all Leishmania species (12, 19). Antimonial drug resistance has increased the use of second-line drugs, including miltefosine, amphotericin B, and pentamidine, despite their associated adverse reactions (6, 19). The mechanism of action of pentamidine in Leishmania is unclear; however, the mitochondrion appears to be an important target of this drug (1, 5, 6). Different pentamidine-resistant Leishmania species exhibited reduced accumulations of the drug both in the mitochondrion and in the cytosol (1, 16). In the case of Leishmania mexicana, it was proposed that pentamidine would be excluded from the cell by an ATP-binding cassette (ABC) transporter (1), although the situation appears to be different for Leishmania donovani (16). The protein responsible for decreased accumulation in mitochondria is still unknown.
Several ABC proteins contain two transmembrane domains and two conserved nucleotide binding domains with three characteristic motifs: the Walker A and B motifs and the signature C motif of the ABC transporters (13). Eight different subfamilies of ABC transporters (ABCA to ABCH) have been described for eukaryotic cells (7), and representatives of all subfamilies are also present in Leishmania (15). Some members of ABC transporter subfamilies were characterized and shown to be associated with drug resistance in Leishmania. A homologue of the human P glycoprotein was found to confer resistance to a number of drugs, including miltefosine (reviewed in reference 20). The ABC transporter PRP1 (pentamidine resistance protein 1) (ABCC7) was shown to confer pentamidine resistance in the promastigote form of Leishmania major (3). This ABC transporter is specific to the genus Leishmania since no orthologues were found in the genome of the related parasites Trypanosoma cruzi and Trypanosoma brucei (15). PRP1 is part of the Leishmania ABCC subfamily, which also includes MRPA (ABCC3), a protein involved in antimony resistance in Leishmania (8, 14). PRP1 and MRPA are intracellular proteins and are possibly associated with the tubulovesicular element that is linked to exo- and endocytosis pathways (4, 11, 18). We hypothesized that PRP1 would transport pentamidine into an intracellular organelle that would be exocytosed by the cell throughout the flagellar pocket (4). These data are derived from work with promastigotes, and in this study, we have tested whether PRP1 can confer resistance to pentamidine in the more relevant amastigote stage of the parasite.
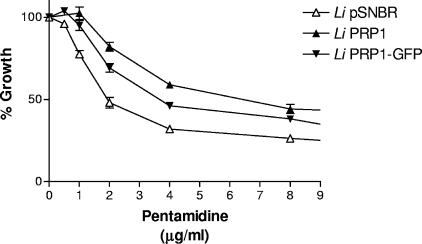
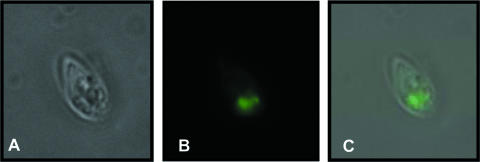
We first transfected the PRP1 gene (3) in Leishmania infantum (MHOM/MA/67/ITMAP-263) promastigotes, which by growth of cells at 37°C and an acidic pH can differentiate easily in axenic amastigotes (24). The PRP1 transfectants were ∼3-fold more resistant to pentamidine when grown as axenic amastigotes (Fig. 1). The amastigote nature of our cell culture was confirmed by monitoring the expression of the amastin gene that is known to be specifically expressed in amastigotes (27). Indeed, the expression of amastin was increased 20 times in L. infantum axenic amastigotes, as determined using established quantitative real-time reverse transcription-PCR protocols (10, 17) (data not shown). A PRP1-green fluorescent protein (GFP) fusion has allowed the localization of this fusion protein to an intracellular organelle in the promastigote stage of the parasite (4). Transfection of PRP1-GFP in L. infantum and differentiation of the parasite in amastigotes have permitted a demonstration that this fusion protein confers pentamidine resistance (Fig. 1) and is also located intracellularly (Fig. 2).
FIG. 1.
PRP1 confers pentamidine resistance in Leishmania infantum (Li) axenic amastigotes. A growth curve is shown for L. infantum axenic amastigotes in MAA/20 medium (24) in the presence of pentamidine. Promastigote parasites were transfected with the plasmids indicated (the control plasmid, pSNBR [22]; PRP1 [pSNBR/8kb SmaI-A] [3]; and PRP1-GFP [4]) and induced for differentiation to axenic amastigotes (24). Experiments were done in triplicate.
FIG. 2.
Cellular localization of the construct PRP1-GFP. The fusion protein was studied by fluorescence microscopy with L. infantum axenic amastigotes. Transfectant parasites were immobilized in 1% (wt/vol) low-melting-point agarose in phosphate-buffered saline and observed with a Nikon Eclipse TE300 microscope (100× objective) using appropriate GFP excitation/emission filters (HYQ filter cube; Nikon). A, phase contrast; B, GFP fluorescence; C, panels A and B merged.
PRP1 can confer pentamidine resistance in axenic amastigotes. We then attempted to show its role in resistance in intracellular parasites by using the THP-1 infection model and luciferase-expressing parasites (21, 26). This was not possible with the L. infantum strain used, possibly due to its higher intrinsic resistance to pentamidine inside macrophages (Table 1) . It is salient that the same L. infantum strain was also not useful for monitoring the role of MRPA in pentavalent antimony resistance inside macrophages (8).
TABLE 1.
Sensitivities to pentamidine, verapamil, and a combination of the two drugs verified for wild-type Leishmania amastigote parasitesa
| Species | Sensitivity (50% inhibitory concentration) for:
|
P | ||
|---|---|---|---|---|
| PEN (μg/ml) | VER (μM) | PEN-VER combinationb | ||
| L. major | 5.1 ± 1.5 | 40.3 ± 10.1 | 3.2 ± 0.3 μg/ml, 15 μM | <0.05 |
| L. amazonensis | 6.8 ± 1.2 | 19.1 ± 3.9 | 2.8 ± 0.3 μg/ml, 15 μM | <0.05 |
| L. infantum | 10.0 ± 1.7 | 43.2 ± 7.1 | 4.7 ± 1.7 μg/ml, 20 μM | <0.05 |
Promastigote parasites were transfected with the plasmid pSP1.2LUCαHYGα (21) and used to infect THP-1 cells (26). The 50% inhibitory concentrations were determined by luciferase activity (percent relative light units). The means ± standard deviations for at least three independent experiments done in triplicate are given. P values significantly different from those for the parasites treated with nontoxic concentrations of verapamil are given. PEN, pentamidine; VER, verapamil.
Values are 50% inhibitory concentrations for pentamidine followed by those for verapamil.
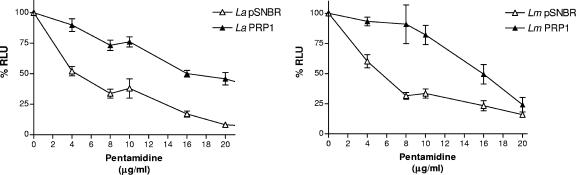
The PRP1 construct was then transfected in the promastigotes of Leishmania (Leishmania) amazonensis (MHOM/BR/1973/M2269) and L. (L.) major LV39 strains. These PRP1 transfectant promastigote cells were more resistant to pentamidine (data not shown). Recombinant promastigotes were used for infecting the human monocyte cell line THP-1. The transfectants of these two species transfected with PRP1 were three times more resistant to pentamidine than those of the control transfected line containing pSNBR (Fig. 3).
FIG. 3.
PRP1 mediates pentamidine resistance in intracellular Leishmania amastigotes. Luciferase-expressing amastigotes of Leishmania spp. were grown in a human leukemia monocyte cell line (THP-1 cells) as described previously (26). THP-1 cells were infected with stationary-phase L. amazonensis (La) and L. major (Lm) promastigotes in 24-microwell plates at a parasite/macrophage ratio of 15:1. Noninternalized parasites were removed by three washes, and pentamidine was added to the infected macrophages. After 4 days of infection with the drug, cells were washed and the luciferase activities of the luciferase gene-expressing recombinant parasites were determined as described elsewhere (21). Promastigote parasites were cotransfected with the plasmid pSNBR (22) or PRP1 (pSNBR/8kb SmaI-A) (3) and with the vector pSP1.2LUCαHYGα (27) to quantify intracellular parasites (21, 26). Experiments were done at least three times in triplicate. Values are represented as numbers of relative light units (RLU).
The calcium channel blocker verapamil, known to reverse multidrug resistance in mammalian cells (2, 23), was also shown to modulate the activities of Leishmania efflux ABC transporters (9). Pentamidine resistance could be reversed by the use of nontoxic concentrations of verapamil in L. mexicana promastigotes (1), although no reversal of resistance was observed in pentamidine-resistant L. mexicana axenic amastigotes treated with verapamil (25). Nontoxic concentrations of verapamil were able to increase the intrinsic pentamidine susceptibilities in the intracellular forms of three Leishmania species (Table 1). This prompted us to test whether verapamil had the ability to reverse PRP1-mediated pentamidine resistance. The use of subtoxic concentrations of verapamil has not, however, reversed PRP1-mediated resistance (results not shown).
This study has shown that the Leishmania ABC transporter PRP1 (ABCC7) can confer resistance to pentamidine in the amastigote stage and in intracellular parasites. It remains to be seen whether overexpression of PRP1, as recently shown for MRPA in an antimony-resistant field isolate (17), is observed in patients for whom pentamidine treatment has failed.
Acknowledgments
We thank J. J. Shaw for the L. amazonensis strain.
This work was supported by CNPq (473121-2), LIM-48, and FAPESP (06/04656-4) and by fellowships from CNPq (P.C.C.) and CAPES (A.C.C.). A.C.C. acknowledges the help of CAPES for a brief visit to Canada. Work in the M.O. laboratory is supported by CIHR Group and operating grants. M.O. is a Burroughs Wellcome Fund Scholar in molecular parasitology and the holder of a Canada Research Chair in antimicrobial resistance.
Footnotes
Published ahead of print on 23 April 2007.
REFERENCES
- 1.Basselin, M., H. Denise, G. H. Coombs, and M. P. Barrett. 2002. Resistance to pentamidine in Leishmania mexicana involves exclusion of the drug from the mitochondrion. Antimicrob. Agents Chemother. 46:3731-3738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bitonti, A. J., A. Sjoerdsma, P. P. McCann, D. E. Kyle, A. M. Oduola, R. N. Rossan, W. K. Milhous, and D. E. Davidson, Jr. 1988. Reversal of chloroquine resistance in malaria parasite Plasmodium falciparum by desipramine. Science 242:1301-1303. [DOI] [PubMed] [Google Scholar]
- 3.Coelho, A. C., S. M. Beverley, and P. C. Cotrim. 2003. Functional genetic identification of PRP1, an ABC transporter superfamily member conferring pentamidine resistance in Leishmania major. Mol. Biochem. Parasitol. 130:83-90. [DOI] [PubMed] [Google Scholar]
- 4.Coelho, A. C., E. H. Yamashiro-Kanashiro, S. F. Bastos, R. A. Mortara, and P. C. Cotrim. 2006. Intracellular location of the ABC transporter PRP1 related to pentamidine resistance in Leishmania major. Mol. Biochem. Parasitol. 150:378-383. [DOI] [PubMed] [Google Scholar]
- 5.Croft, S. L., and G. H. Coombs. 2003. Leishmaniasis—current chemotherapy and recent advances in the search for novel drugs. Trends Parasitol. 19:502-508. [DOI] [PubMed] [Google Scholar]
- 6.Croft, S. L., S. Sundar, and A. H. Fairlamb. 2006. Drug resistance in leishmaniasis. Clin. Microbiol. Rev. 19:111-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dean, M., A. Rzhetsky, and R. Allikmets. 2001. The human ATP-binding cassette (ABC) transporter superfamily. Genome Res. 11:1156-1166. [DOI] [PubMed] [Google Scholar]
- 8.El Fadili, K., N. Messier, P. Leprohon, G. Roy, C. Guimond, N. Trudel, N. G. Saravia, B. Papadopoulou, D. Legare, and M. Ouellette. 2005. Role of the ABC transporter MRPA (PGPA) in antimony resistance in Leishmania infantum axenic and intracellular amastigotes. Antimicrob. Agents Chemother. 49:1988-1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Essodaigui, M., F. Frezard, E. S. Moreira, F. Dagger, and A. Garnier-Suillerot. 1999. Energy-dependent efflux from Leishmania promastigotes of substrates of the mammalian multidrug resistance pumps. Mol. Biochem. Parasitol. 100:73-84. [DOI] [PubMed] [Google Scholar]
- 10.Gagnon, D., A. Foucher, I. Girard, and M. Ouellette. 2006. Stage specific gene expression and cellular localization of two isoforms of the serine hydroxymethyltransferase in the protozoan parasite Leishmania. Mol. Biochem. Parasitol. 150:63-71. [DOI] [PubMed] [Google Scholar]
- 11.Ghedin, E., A. Debrabant, J. C. Engel, and D. M. Dwyer. 2001. Secretory and endocytic pathways converge in a dynamic endosomal system in a primitive protozoan. Traffic 2:175-188. [DOI] [PubMed] [Google Scholar]
- 12.Herwaldt, B. L. 1999. Leishmaniasis. Lancet 354:1191-1199. [DOI] [PubMed] [Google Scholar]
- 13.Higgins, C. F. 1992. ABC transporters: from microorganisms to man. Annu. Rev. Cell Biol. 8:67-113. [DOI] [PubMed] [Google Scholar]
- 14.Legare, D., D. Richard, R. Mukhopadhyay, Y. D. Stierhof, B. P. Rosen, A. Haimeur, B. Papadopoulou, and M. Ouellette. 2001. The Leishmania ATP-binding cassette protein PGPA is an intracellular metal-thiol transporter ATPase. J. Biol. Chem. 276:26301-26307. [DOI] [PubMed] [Google Scholar]
- 15.Leprohon, P., D. Legare, I. Girard, B. Papadopoulou, and M. Ouellette. 2006. Modulation of Leishmania ABC protein gene expression through life stages and among drug-resistant parasites. Eukaryot. Cell 5:1713-1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mukherjee, A., P. K. Padmanabhan, M. H. Sahani, M. P. Barrett, and R. Madhubala. 2006. Roles for mitochondria in pentamidine susceptibility and resistance in Leishmania donovani. Mol. Biochem. Parasitol. 145:1-10. [DOI] [PubMed] [Google Scholar]
- 17.Mukherjee, A., P. K. Padmanabhan, S. Singh, G. Roy, I. Girard, M. Chatterjee, M. Ouellette, and R. Madhubala. 2007. Role of ABC transporter MRPA, gamma-glutamylcysteine synthetase and ornithine decarboxylase in natural antimony-resistant isolates of Leishmania donovani. J. Antimicrob. Chemother. 59:204-211. [DOI] [PubMed] [Google Scholar]
- 18.Mullin, K. A., B. J. Foth, S. C. Ilgoutz, J. M. Callaghan, J. L. Zawadzki, G. I. McFadden, and M. J. McConville. 2001. Regulated degradation of an endoplasmic reticulum membrane protein in a tubular lysosome in Leishmania mexicana. Mol. Biol. Cell 12:2364-2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Murray, H. W., J. D. Berman, C. R. Davies, and N. G. Saravia. 2005. Advances in leishmaniasis. Lancet 366:1561-1577. [DOI] [PubMed] [Google Scholar]
- 20.Perez-Victoria, J. M., A. Di Pietro, D. Barron, A. G. Ravelo, S. Castanys, and F. Gamarro. 2002. Multidrug resistance phenotype mediated by the P-glycoprotein-like transporter in Leishmania: a search for reversal agents. Curr. Drug Targets 3:311-333. [DOI] [PubMed] [Google Scholar]
- 21.Roy, G., C. Dumas, D. Sereno, Y. Wu, A. K. Singh, M. J. Tremblay, M. Ouellette, M. Olivier, and B. Papadopoulou. 2000. Episomal and stable expression of the luciferase reporter gene for quantifying Leishmania spp. infections in macrophages and in animal models. Mol. Biochem. Parasitol. 110:195-206. [DOI] [PubMed] [Google Scholar]
- 22.Ryan, K. A., S. Dasgupta, and S. M. Beverley. 1993. Shuttle cosmid vectors for the trypanosomatid parasite Leishmania. Gene 131:145-150. [DOI] [PubMed] [Google Scholar]
- 23.Safa, A. R., C. J. Glover, J. L. Sewell, M. B. Meyers, J. L. Biedler, and R. L. Felsted. 1987. Identification of the multidrug resistance-related membrane glycoprotein as an acceptor for calcium channel blockers. J. Biol. Chem. 262:7884-7888. [PubMed] [Google Scholar]
- 24.Sereno, D., and J. L. Lemesre. 1997. Axenically cultured amastigote forms as an in vitro model for investigation of antileishmanial agents. Antimicrob. Agents Chemother. 41:972-976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sereno, D., and J. L. Lemesre. 1997. In vitro life cycle of pentamidine-resistant amastigotes: stability of the chemoresistant phenotypes is dependent on the level of resistance induced. Antimicrob. Agents Chemother. 41:1898-1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sereno, D., G. Roy, J. L. Lemesre, B. Papadopoulou, and M. Ouellette. 2001. DNA transformation of Leishmania infantum axenic amastigotes and their use in drug screening. Antimicrob. Agents Chemother. 45:1168-1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu, Y., Y. El Fakhry, D. Sereno, S. Tamar, and B. Papadopoulou. 2000. A new developmentally regulated gene family in Leishmania amastigotes encoding a homolog of amastin surface proteins. Mol. Biochem. Parasitol. 110:345-357. [DOI] [PubMed] [Google Scholar]