Abstract
Background:
We conducted a randomized, double-blind, phase 3 study to evaluate perioperative pembrolizumab in early-stage NSCLC.
Methods:
Participants with resectable stage II, IIIA, or IIIB (N2) NSCLC were randomized (1:1) to neoadjuvant pembrolizumab 200mg or placebo once every 3 weeks plus cisplatin-based chemotherapy for 4 cycles followed by surgery and adjuvant pembrolizumab 200mg or placebo once every 3 weeks for ≤13 cycles. Dual primary endpoints were event-free survival and overall survival. Secondary endpoints included major pathological response, pathological complete response, and safety.
Results:
797 participants were randomized to the pembrolizumab (n=397) or placebo (n=400) group. At the prespecified first interim analysis, median follow-up was 25.2 months. 24-month event-free survival rates were 62.4% in the pembrolizumab group versus 40.6% in the placebo group (HR, 0.58; 95% CI, 0.46–0.72; P<0.00001). 24-month overall survival estimates were 80.9% versus 77.6% (P=0.02124). Major pathologic response rates were 30.2% versus 11.0% (difference, 19.2; 95% CI, 13.9–24.7; P<0.00001). Pathologic complete response rates were 18.1% versus 4.0% (difference, 14.2; 95% CI, 10.1–18.7; P<0.00001). Across all treatment phases, 44.9% of participants in the pembrolizumab group and 37.3% in the placebo group had grade ≥3 treatment-related adverse events, including 1.0% and 0.8% who had grade 5 events.
Conclusions:
Neoadjuvant pembrolizumab plus chemotherapy followed by resection and adjuvant pembrolizumab significantly improved event-free survival, major pathologic response, and pathologic complete response compared with neoadjuvant chemotherapy alone in resectable, early-stage NSCLC. Overall survival was not significantly different at this analysis. (Funded by Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA; ClinicalTrials.gov number, NCT03425643.)
Introduction
Programmed cell death protein 1 (PD-1) and programmed death ligand 1 (PD-L1) immune checkpoint inhibitor-based regimens are standard treatments for advanced or metastatic non-small cell lung cancer (NSCLC) without targetable molecular drivers.1–5 The benefit of these drugs in earlier disease stages was first seen in the PACIFIC trial, in which the PD-L1 inhibitor durvalumab improved progression-free survival and overall survival when given after concurrent chemoradiotherapy for unresectable stage III NSCLC.6,7 Results of several phase 2 studies suggested a benefit for PD-1/PD-L1 inhibitors given as monotherapy or in combination with chemotherapy in the neoadjuvant NSCLC setting.8–10 This benefit was confirmed in the phase 3 CheckMate 816 trial in which neoadjuvant nivolumab plus chemotherapy improved event-free survival compared with neoadjuvant chemotherapy alone (hazard ratio, 0.63 [97.38% CI, 0.43–0.91], P=0.005).11
The IMpower010 study provided the first evidence of benefit for adjuvant checkpoint inhibition, showing that the PD-L1 inhibitor atezolizumab improved disease-free survival versus placebo when given after complete resection and adjuvant chemotherapy in patients with PD-L1-expressing, stage II-IIIA NSCLC (hazard ratio, 0.66 [95% CI, 0.50–0.88], P=0.0039).12 The PEARLS/KEYNOTE-091 study similarly demonstrated a disease-free survival benefit for adjuvant therapy with the PD-1 inhibitor pembrolizumab given after complete resection and, when recommended by guidelines, adjuvant chemotherapy in a PD-L1-unselected population of patients with stage IB-IIIA NSCLC (hazard ratio, 0.76 [95% CI, 0.63–0.91], P=0.0014).13
Neoadjuvant nivolumab plus chemotherapy and single-agent adjuvant atezolizumab and pembrolizumab are all approved by the US FDA and have various global indications; however, either approach alone leaves many patients at risk of relapse and eventual death from NSCLC. In the placebo-controlled, phase 3 KEYNOTE-671 trial, we assessed whether a perioperative approach of combined neoadjuvant pembrolizumab plus cisplatin-based chemotherapy followed by surgical resection and adjuvant pembrolizumab improves efficacy compared with neoadjuvant cisplatin-based chemotherapy and resection alone in resectable stage II-III NSCLC. We report efficacy and safety data from the prespecified first interim analysis.
Methods
Participants
Participants were eligible for enrollment if they provided written, informed consent; were aged ≥18 years; had previously untreated, pathologically confirmed, stage II, IIIA, or IIIB (N2) NSCLC assessed per the American Joint Committee on Cancer staging system (8th edition14; details available in the Supplementary Methods and Table S1) considered resectable after surgical consultation and investigator assessment; had Eastern Cooperative Oncology Group performance-status score of 0 or 1 (on a 5-point scale, with 0 indicating no symptoms and higher scores indicating increasing disability15) within 10 days of randomization; and were able to provide a tumor sample for PD-L1 assessment at a central laboratory. Full eligibility criteria are available in Section 6 of the protocol, available with the full text of this article at NEJM.org.
Trial Design and Treatments
In this double-blind, placebo-controlled, phase 3 study, randomization was performed centrally using an interactive response system. Participants were stratified by stage (II vs III), PD-L1 tumor proportion score (<50% vs ≥50% based on PD-L1 IHC 22C3 pharmDx [Agilent Technologies]), histology (squamous vs nonsquamous), and geographic region (east Asia vs not east Asia) and randomized (1:1) to receive pembrolizumab or placebo. Neoadjuvant therapy comprised four cycles of pembrolizumab (200 mg) or placebo given intravenously once every 3 weeks plus cisplatin and gemcitabine (squamous histology) or cisplatin and pemetrexed (nonsquamous histology); 4 cycles of neoadjuvant therapy were used in accordance with guideline recommendations for neoadjuvant therapy at the time of study design in 2017. Surgery was to be performed per local standards no later than 20 weeks after the first neoadjuvant therapy dose; radiotherapy was administered in select circumstances. Adjuvant therapy was to be initiated no sooner than 4 weeks and no later than 12 weeks after surgery and comprised pembrolizumab (200 mg) or placebo given intravenously once every 3 weeks for up to 13 additional cycles. Treatment was continued until disease progression or recurrence, intolerable toxicity, investigator decision, withdrawal of consent, or other reasons (summarized in the Supplementary Appendix). Additional treatment information, including chemotherapy regimen and lymphadenectomy details and circumstances in which radiotherapy was to be administered, is available in the Supplementary Appendix.
Assessments and Endpoints
Pathological response following neoadjuvant therapy was assessed by examination of hematoxylin-and-eosin-stained slides of resected lung tissue and lymph nodes. Definitions of R0, R1, and R2 resection are outlined in the Supplementary Appendix. Computed tomography (strongly preferred) or magnetic resonance imaging of the chest and abdomen was performed during screening, throughout all treatment phases, and during follow-up according to the schedule outlined in the Supplementary Appendix. While imaging was performed after neoadjuvant therapy but before surgery, tumors were not formally restaged before surgery. Participants were contacted for survival every 12 weeks. Adverse events and laboratory abnormalities were assessed regularly throughout all treatment phases and for 30 days after discontinuation (up to 90 days for serious events in the absence of new anticancer therapy) and graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.03. Potentially immune-mediated adverse events and infusion reactions were based on a list of preferred terms prepared by the sponsor and considered regardless of attribution to treatment by the investigator. EGFR mutation and ALK translocation status were tested locally per investigator discretion.
Dual primary endpoints were event-free survival (time from randomization to first occurrence of local progression precluding planned surgery, unresectable tumor, progression or recurrence per RECIST 1.1 by investigator assessment, or death from any cause) and overall survival (time from randomization to death from any cause). Key secondary endpoints included major pathological response (≤10% viable tumor cells in resected primary tumor and lymph nodes) and pathological complete response (absence of residual invasive cancer in resected primary tumor and lymph nodes [ypT0/Tis ypN0]) assessed by blinded, central pathologist examination and safety.
Trial Oversight
A panel of academic advisors and sponsor employees designed the study. An external, independent data and safety monitoring committee oversees the trial, assessing safety regularly and efficacy at prespecified interim analyses. The protocol and all amendments were approved by the appropriate ethics body for each participating center. All authors vouch for data accuracy and completeness and study conduct in accordance with the protocol, its amendments, and Good Clinical Practice guidelines. All authors attest they participated in writing or reviewing and editing the manuscript. A medical writer employed by the sponsor assisted with manuscript preparation.
Statistical Analysis
Efficacy was assessed in the intention-to-treat population (all randomized participants). Safety was assessed in the as-treated population (all randomized participants who received ≥1 dose of study treatment). Event-free survival and overall survival were estimated using the Kaplan-Meier method. The magnitude of the treatment differences (i.e., hazard ratios and associated 95% confidence intervals [CIs]) was calculated using a stratified Cox regression model with treatment as a covariate and Efron’s method of handling ties; between-group differences were assessed using the stratified log-rank test. If the proportional hazards assumption was not valid, the restricted mean survival time method16 was performed as a sensitivity analysis. Between-group comparisons of the percentage of participants with major pathological response and the percentage with pathological complete response were performed using the stratified Miettinen and Nurminen method with strata weighting by sample size. The randomization stratification factors were applied to all stratified analyses.
The statistical analysis plan is available in protocol Section 10. The graphical method of Maurer and Bretz17 was used to strictly control the family-wise type I error rate at one-sided α=0.025 across the event-free survival, overall survival, major pathological response, and pathological complete response hypotheses among the interim and final analyses (Fig. S1). The Lan-DeMets O’Brien-Fleming spending function will be used to control type I error for event-free survival and overall survival in the interim and final analyses. Power statements for each hypothesis are in the Supplementary Appendix. The study is considered positive if at least one of the primary endpoints is significantly improved in the pembrolizumab group.
The data reported herein are from the first interim analysis (data cutoff, July 29, 2022), which was to be performed ~5 months after the last participant was randomized and after ~326 event-free survival events occurred. On the basis of the observed number of events, the multiplicity-adjusted one-sided alpha levels at this analysis were 0.00462 for event-free survival, 0.0001 for major pathological response, and 0.0001 for pathological complete response.
Results
Participants and Treatment
Between April 2018 and December 2021, 1364 participants were screened and 797 were randomized to treatment with neoadjuvant pembrolizumab plus chemotherapy followed by surgery and adjuvant pembrolizumab (pembrolizumab group; n = 397) or with neoadjuvant placebo plus chemotherapy followed by surgery and adjuvant placebo (placebo group; n = 400) (Fig. S2). Baseline demographics and disease characteristics were balanced between groups (Table 1) and generally representative of the broader lung cancer population (Table S2). Although Black or African American participants were underrepresented in the overall global population, they accounted for 8 (10.3%) of the 78 participants enrolled in the United States.
Table 1.
Baseline Demographics and Disease Characteristics (Intention-to-Treat Population)
| Pembrolizumab Group (N = 397) | Placebo Group (N = 400) | |
|---|---|---|
| Age | ||
| Median (range) — yr | 63 (26–83) | 64 (35–81) |
| ≥65 yr — no. (%) | 176 (44.3) | 186 (46.5) |
| Sex — no. (%) | ||
| Female | 118 (29.7) | 116 (29.0) |
| Male | 279 (70.3) | 284 (71.0) |
| Race or ethnic group — no. (%) | ||
| American Indian or Alaska Native | 1 (0.3) | 0 |
| Asian | 124 (31.2) | 125 (31.3) |
| Black | 6 (1.5) | 10 (2.5) |
| Multiple | 3 (0.8) | 10 (2.5) |
| White | 250 (63.0) | 239 (59.8) |
| Missing data | 13 (3.3) | 16 (4.0) |
| Geographic region — no. (%) | ||
| East Asia | 123 (31.0) | 121 (30.3) |
| Other | 274 (69.0) | 279 (69.8) |
| ECOG performance-status score* — no. (%) | ||
| 0 | 253 (63.7) | 246 (61.5) |
| 1 | 144 (36.3) | 154 (38.5) |
| Smoking status — no. (%) | ||
| Current smoker | 96 (24.2) | 103 (25.8) |
| Former smoker | 247 (62.2) | 250 (62.5) |
| Never smoked | 54 (13.6) | 47 (11.8) |
| Pathological stage at baseline — no. (%) | ||
| II | 118 (29.7) | 121 (30.3) |
| III | 279 (70.3) | 279 (69.8) |
| IIIA | 217 (54.7) | 225 (56.3) |
| IIIB | 62 (15.6) | 54 (13.5) |
| Tumor stage — no. (%) | ||
| T1 | 55 (13.9) | 61 (15.3) |
| T2 | 106 (26.7) | 126 (31.5) |
| T3 | 121 (30.5) | 109 (27.3) |
| T4 | 115 (29.0) | 104 (26.0) |
| Node stage — no. (%) | ||
| N0 | 148 (37.3) | 142 (35.5) |
| N1 | 81 (20.4) | 71 (17.8) |
| N2 | 168 (42.3) | 187 (46.8) |
| Histologic features — no. (%) | ||
| Nonsquamous | 226 (56.9) | 227 (56.8) |
| Squamous | 171 (43.1) | 173 (43.3) |
| PD-L1 tumor proportion score — no. (%) | ||
| ≥50% | 132 (33.2) | 134 (33.5) |
| <50% | 265 (66.8) | 266 (66.5) |
| 1–49% | 127 (32.0) | 115 (28.8) |
| <1% | 138 (34.8) | 151 (37.8) |
| EGFR mutation status — no. (%) | ||
| No | 111 (28.0) | 127 (31.8) |
| Yes | 14 (3.5) | 19 (4.8) |
| Unknown | 272 (68.5) | 254 (63.5) |
| ALK translocation status — no. (%) | ||
| No | 104 (26.2) | 133 (33.3) |
| Yes | 12 (3.0) | 9 (2.3) |
| Unknown | 281 (70.8) | 258 (64.5) |
The intention-to-treat population included all the participants who had undergone randomization. Percentages may not total 100 because of rounding. PD-L1 denotes programmed death ligand 1.
Race and ethnic group were reported by the participant
Eastern Cooperative Oncology Group (ECOG) performance-status scores range from 0 to 5, with 0 indicating no symptoms and higher scores indicating greater disability.
Median time from randomization to data cutoff was 25.2 months (range, 7.5–50.6). In the pembrolizumab group, 396 participants received ≥1 dose of neoadjuvant therapy for a median of 4 cycles; of these, 325 (82.1%) underwent in-study surgery and 290 (73.2%) received ≥1 dose of adjuvant therapy (Fig. S2). In the placebo group, 399 participants received ≥1 dose of neoadjuvant therapy for a median of 4 cycles; of these, 317 (79.4%) underwent in-study surgery and 267 (66.9%) received ≥1 dose of adjuvant therapy (Fig. S2). Table S3 summarizes the reasons for not undergoing in-study surgery. The most common surgical procedure was lobectomy (Table S4). Among participants who underwent in-study surgery, 92.0% in the pembrolizumab group versus 84.2% in the placebo group had complete (R0) resection, 5.2% versus 9.8% had incomplete (R1) resection, 1.2% versus 1.3% had incomplete (R2) resection, and 1.5% versus 4.7% had unresectable tumors; median duration of the surgical hospital stay was 8.0 days (range, 1–50) versus 7.5 days (range, 1–65). Table S5 summarizes treatment exposure. In the intention-to-treat population, 17.1% of participants in the pembrolizumab group and 37.3% of participants in the placebo group received ≥1 subsequent systemic anticancer therapy, including 5.0% and 21.3%, respectively, who received subsequent immunotherapy.
Efficacy
344 (43.2%) participants had an event-free survival event, most commonly disease progression or recurrence (Table S6). The estimated percentage of participants alive without an event at 24 months was 62.4% (95% CI, 56.8–67.5) in the pembrolizumab group and 40.6% (95% CI, 34.8–46.3) in the placebo group. Median event-free survival was not reached (95% CI, 34.1 months to not reached) in the pembrolizumab group and was 17.0 months (95% CI, 14.3–22.0) in the placebo group (hazard ratio, 0.58; 95% CI, 0.46–0.72; P<0.00001) (Fig. 1A). The event-free survival benefit for pembrolizumab was generally consistent across all subgroups examined but some subgroups were small with a low number of events (Fig. 1B).
Figure 1. Event-Free Survival as Assessed According to Investigator Review (Intention-to-Treat Population).
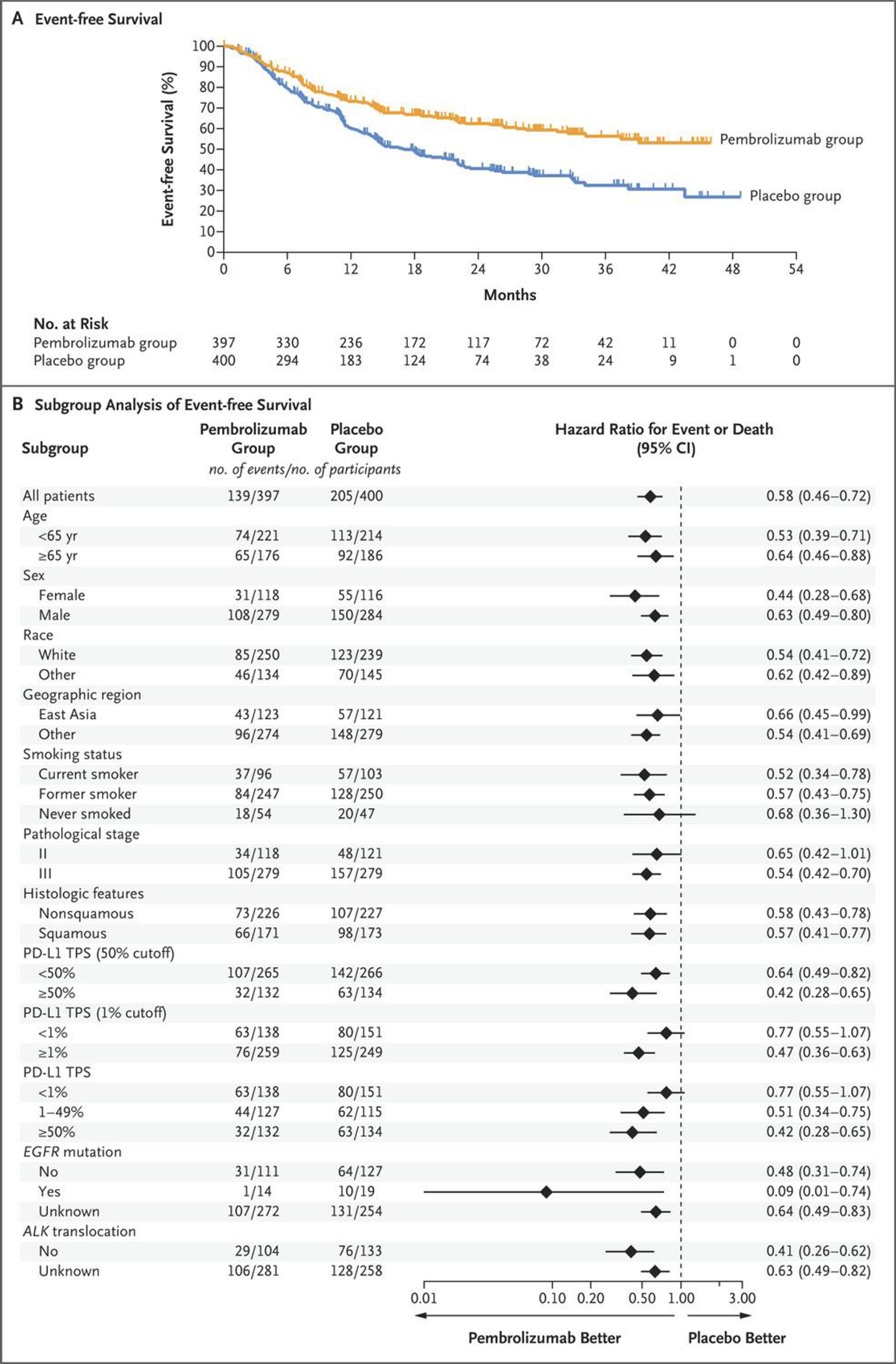
Panel A shows Kaplan–Meier estimates of event-free survival. Event-free survival was defined as the time from randomization to the first occurrence of local progression that precluded the planned surgery, unresectable tumor, progression or recurrence (according to the Response Evaluation Criteria in Solid Tumors, version 1.1) by the investigator’s assessment, or death from any cause. The intention-to-treat population included all the participants who had undergone randomization. Tick marks indicate censored data. Panel B shows event-free survival in subgroups. The magnitude of the event-free survival treatment effect in subgroups was calculated with the use of an unstratified Cox regression model with trial group as a covariate and Efron’s method of handling ties. Race was reported by the participant. The subgroup of participants with ALK translocation (21 participants) was excluded from the forest plot because the statistical analysis plan specified that subgroups with less than 30 participants were to be excluded from the forest plot. PD-L1 denotes programmed death ligand 1, and TPS tumor proportion score.
With 177 (22.2%) deaths, the estimated percentage of participants alive at 24 months was 80.9% (95% CI, 76.2–84.7) in the pembrolizumab group and 77.6% (95% CI, 72.5–81.9) in the placebo group (Fig. 2). Median overall survival was not reached in the pembrolizumab group and was 45.5 months in the placebo group. At this first interim analysis, the significance boundary was not crossed (P=0.02124). Restricted mean survival time at 48 months was 39.7 months and 36.6 months, respectively (difference, 3.1 months; 95% CI, 0.6–5.6). The between-group difference in overall survival measured by the hazard ratio is available in the Supplementary Results.
Figure 2. Overall Survival (Intention-to-Treat Population).
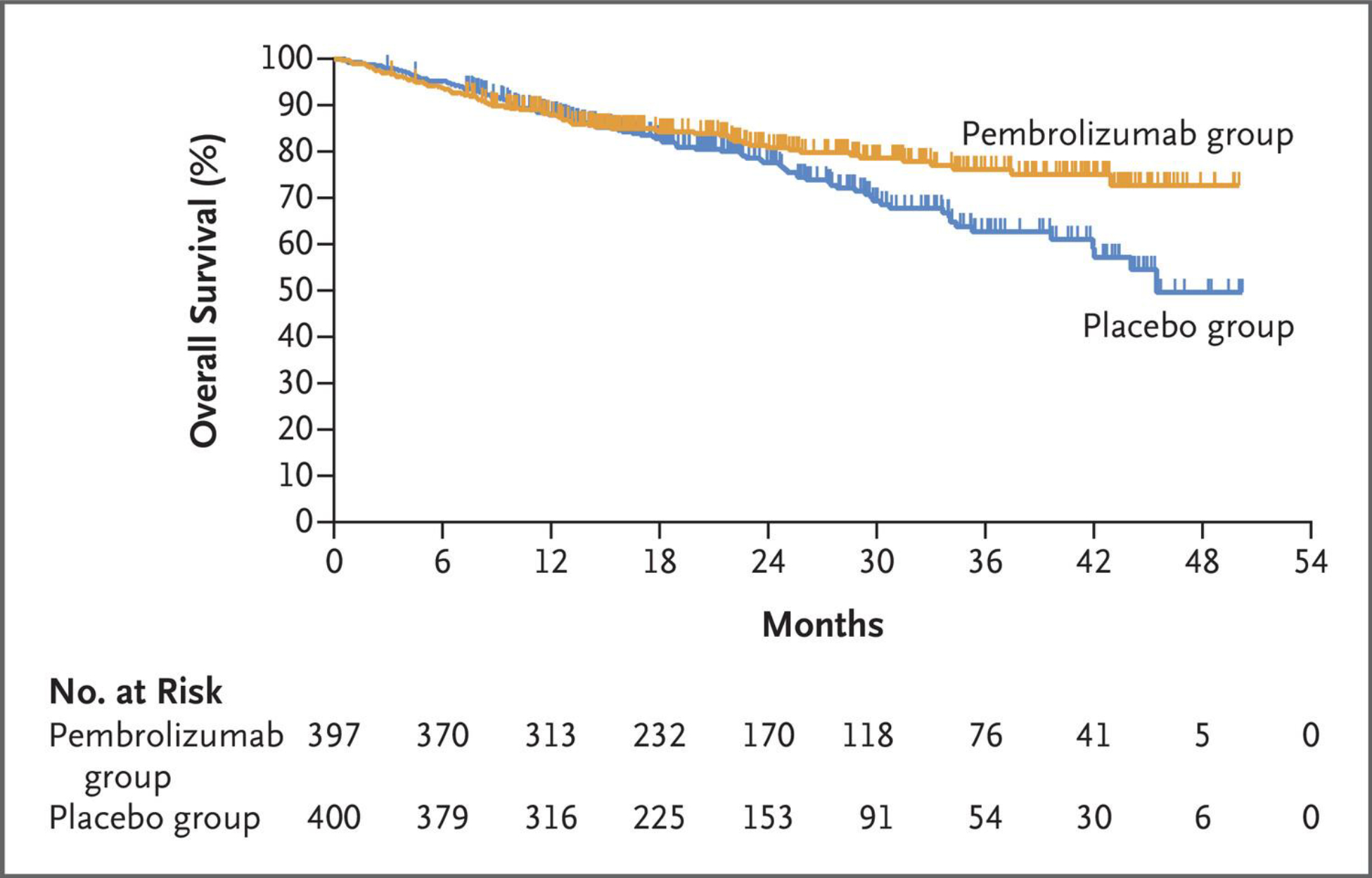
Tick marks indicate censored data.
Major pathological response occurred in 120 (30.2%; 95% CI, 25.7–35.0) participants in the pembrolizumab group and 44 (11.0%; 95% CI, 8.1–14.5) participants in the placebo group (difference, 19.2; 95% CI, 13.9–24.7; P<0.00001). Pathological complete response occurred in 72 (18.1%; 95% CI, 14.5–22.3) participants in the pembrolizumab group and 16 (4.0%; 95% CI, 2.3–6.4) participants in the placebo group (difference, 14.2; 95% CI, 10.1–18.7; P<0.00001). An exploratory analysis showed an event-free survival benefit for the pembrolizumab group regardless of whether participants had major pathological response (Fig. 3A) or pathological complete response (Fig. 3B).
Figure 3. Exploratory Analysis of Event-Free Survival According to Major Pathological Response and Pathological Complete Response (Intention-to-Treat Population).
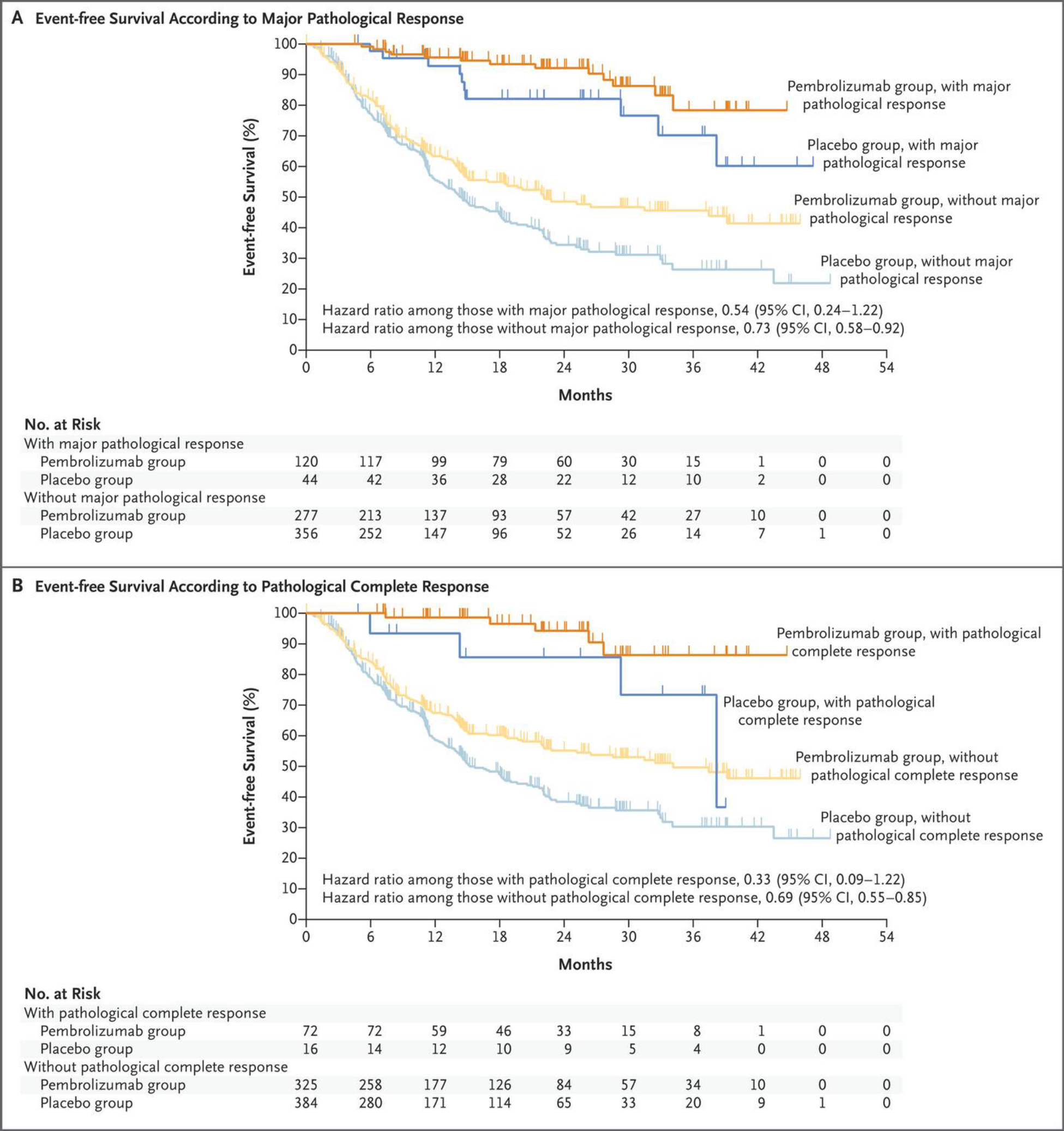
Event-free survival was assessed according to investigator review. The hazard ratios for disease progression, disease recurrence, or death, along with the 95% confidence intervals, were calculated with the use of an unstratified Cox regression model with treatment as a covariate and Efron’s method of handling ties. A major pathological response was defined as no more than 10% viable tumor cells in resected primary tumor and lymph nodes, and a pathological complete response as the absence of residual invasive cancer in resected primary tumor and lymph nodes (ypT0/Tis ypN0) as assessed on the basis of blinded, central examination by a pathologist. Tick marks indicate censored data.
Safety
Across all treatment phases in the as-treated population, treatment-related adverse events occurred in 96.7% of 396 participants in the pembrolizumab group and in 95.0% of 399 participants in the placebo group, including 44.9% and 37.3%, respectively, with grade ≥3 treatment-related adverse events and 17.7% and 14.3%, respectively, with serious treatment-related adverse events. The most common treatment-related adverse events in both groups were nausea, neutrophil count decreased, and anemia; the most common grade ≥3 treatment-related events were neutrophil count decreased, anemia, white blood cell count decreased, and platelet count decreased (Table 2). Treatment-related adverse events are summarized by treatment phase in Tables S7 and S8. Treatment-related adverse events led to death in 4 (1.0%) participants in the pembrolizumab group (n=1 each from immune-mediated lung disease, pneumonia, and sudden cardiac death during the neoadjuvant/surgery phase, n=1 from atrial fibrillation during the adjuvant phase) and in 3 (0.8%) participants in the placebo group (n=1 each from acute coronary syndrome, pneumonia, and pulmonary hemorrhage during the neoadjuvant/surgery phase). Treatment-related adverse events led to discontinuation of all study treatment in 12.6% of participants in the pembrolizumab group and 5.3% of participants in the placebo group. Among participants who underwent surgery, 71.1% in the pembrolizumab group and 71.3% in the placebo group experienced ≥1 adverse event of any cause during the surgical treatment phase, most commonly procedural pain (Table S9). Six (1.8%) participants in the pembrolizumab group and 2 (0.6%) in the placebo group died of any cause within 30 days after surgery; an additional 7 (2.2%) and 3 (0.9%) participants, respectively, died of any cause within 31–90 days after surgery (Table S10).
Table 2.
Treatment-Related Adverse Events Across Treatment Phases (As-Treated Population)
| Event | Pembrolizumab Group (N = 396) | Placebo Group (N = 399) |
|---|---|---|
| number of participants (percent) | ||
| Any treatment-related adverse event | 383 (96.7) | 379 (95.0) |
| Grade 3–5 treatment-related adverse event | 178 (44.9) | 149 (37.3) |
| Serious treatment-related adverse event | 70 (17.7) | 57 (14.3) |
| Treatment-related adverse event that led to death | 4 (1.0)* | 3 (0.8)† |
| Treatment-related adverse event that led to discontinuation of all trial treatment | 50 (12.6) | 21 (5.3) |
The as-treated population included all the participants who underwent randomization and received at least one dose of pembrolizumab or placebo plus chemotherapy. Treatment-related adverse events were adverse events considered by the investigator to be related to chemotherapy, pembrolizumab, or placebo.
The causes of death were atrial fibrillation (in one participant), immune-mediated lung disease (in one), pneumonia (in one), and sudden cardiac death (in one); All deaths occurred during the neoadjuvant/surgery phase except for the death due to atrial fibrillation, which occurred during the adjuvant phase.
The causes of death were acute coronary syndrome (in one participant), pneumonia (in one), and pulmonary hemorrhage (in one); All deaths occurred during the neoadjuvant/surgery phase.
Potentially immune-mediated adverse events and infusion reactions occurred in 25.3% of participants in the pembrolizumab group and in 10.5% of participants in the placebo group(Table S11). These events were of grade ≥3 in 5.8% and 1.5% of participants, respectively. The most common potentially immune-mediated adverse events were hypothyroidism, hyperthyroidism, and pneumonitis in both the neoadjuvant/surgery and adjuvant treatment phases (Table S11). One participant died from a potentially immune-mediated adverse event (pneumonitis [recorded in the database as the aforementioned immune-mediated lung disease] in the pembrolizumab group).
Discussion
The randomized, placebo-controlled, phase 3 KEYNOTE-671 trial showed a significant improvement in event-free survival, major pathologic response, and complete pathologic response for neoadjuvant pembrolizumab plus cisplatin-based chemotherapy followed by surgical resection and adjuvant pembrolizumab compared with neoadjuvant chemotherapy and surgery alone. The overall survival benefit was not significant at this first interim analysis. Neoadjuvant pembrolizumab did not impact exposure to neoadjuvant chemotherapy or the choice of surgical approach, compromise the ability to undergo surgery, or increase surgical complications.
The event-free survival curves separated in favor of the pembrolizumab group by four months, and the hazard ratio for event-free survival was 0.58 (95% CI, 0.46–0.72; P<0.00001). 24-month event-free survival estimates were 62.4% in the pembrolizumab group and 40.6% in the placebo group. The percentage of participants with major pathologic response was nearly tripled in the pembrolizumab group (30.2% vs 11.0% in the placebo group), and the percentage of participants with pathologic complete response was nearly quadrupled (18.1% vs 4.0%). Exploratory analysis showed an event-free survival benefit for the pembrolizumab group in participants with and without major pathologic response and in participants with and without pathologic complete response, suggesting that the adjuvant component of the regimen may provide benefit beyond that of neoadjuvant therapy and surgery alone. Additional analysis of KEYNOTE-671 and other trials, as well as future studies designed to directly answer the question, will be necessary to rule out other potential explanations and make definitive conclusions regarding the benefit of adjuvant immunotherapy after neoadjuvant chemoimmunotherapy, particularly in subsets defined by response to neoadjuvant treatment. Although cross-study comparisons should be done with caution given different designs and chemotherapy regimens, it is interesting to note that the hazard ratio for event-free survival in participants without pathological complete response was 0.84 (95% CI, 0.61–1.17) in CheckMate 816 and 0.69 (95% CI, 0.55–0.85) in KEYNOTE-671. Long-term data will help determine the relative benefit of perioperative versus neoadjuvant checkpoint inhibition.
The event-free survival benefit of pembrolizumab was generally consistent across all subgroups analyzed. Although participants with stage II disease and those who never smoked appeared to have less benefit with pembrolizumab, these subgroups were small with low event rates, leading to wide and overlapping confidence intervals. The benefit of pembrolizumab appeared similar in participants with squamous and nonsquamous histology. This is notable because several trials of checkpoint inhibitor-based regimens have shown that participants with nonsquamous histology have better outcomes than those with squamous histology.2,3,6,11–13 Molecular testing was not mandated in KEYNOTE-671, and very few participants with EGFR mutations or ALK translocations were identified, limiting any insights in these subgroups. The relative benefit in the pembrolizumab group increased with increasing PD-L1 expression (hazard ratio for event-free survival of 0.42 for PD-L1 tumor proportion score ≥50%, 0.51 for tumor proportion score 1–49%, and 0.77 for tumor proportion score <1%), but in all cases, the hazard ratio favored the pembrolizumab group and the 95% confidence intervals overlapped one another.
Results of the first interim analyses of two other placebo-controlled, phase 3 trials of perioperative checkpoint inhibition. In the global AEGEAN study, addition of perioperative durvalumab significantly improved event-free survival, major pathologic response, and pathologic complete response compared with neoadjuvant chemotherapy and surgery alone in resectable stage II-III NSCLC.18 In the Neotorch study conducted in China, addition of perioperative toripalimab significantly improved event-free survival, major pathologic response, and pathologic complete response compared with neoadjuvant chemotherapy and surgery alone in resectable stage III NSCLC.19 Although some differences are noted between the enrolled populations and designs of KEYNOTE-671, AEGEAN, and Neotorch, their findings taken together support the benefit of perioperative immune checkpoint inhibitor for resectable stage II-III NSCLC.
The safety profile of the combined regimen of pembrolizumab plus chemotherapy followed by surgery and adjuvant pembrolizumab was consistent with safety profiles of the individual medications, and no new safety signals were identified. The frequency of treatment-related serious adverse events was similar to that in previously reported studies of chemotherapy combined with checkpoint inhibitors,2,3 and the majority of reported adverse events are those associated with chemotherapy (e.g., anemia and nausea). The incidence and nature of immune-mediated adverse events in the pembrolizumab group was likewise consistent with prior reports. A low and comparable rate of deaths due to adverse events was seen in both treatment groups.
A limitation of the KEYNOTE-671 study design is that it does not permit direct analysis of the relative contributions of the neoadjuvant and adjuvant components of the treatment regimen. Such an analysis would have required a much larger sample size to accommodate two additional treatment groups—neoadjuvant pembrolizumab plus chemotherapy with adjuvant placebo and neoadjuvant placebo plus chemotherapy with adjuvant placebo. Similar to the other reported studies of perioperative18–20 and neoadjuvant11 checkpoint inhibition, the follow-up duration is relatively short, limiting interpretation of long-term outcomes at this first interim analysis. While these other perioperative and neoadjuvant studies allowed carboplatin-based regimens, KEYNOTE-671 limited neoadjuvant therapy to cisplatin-based regimens only.
In summary, the phase 3 KEYNOTE-671 trial demonstrated that the addition of pembrolizumab to neoadjuvant cisplatin-based chemotherapy followed by surgical resection and adjuvant pembrolizumab led to a significant improvement in event-free survival, major pathologic response, and pathologic complete response for participants with resectable stage II/III NSCLC.
Supplementary Material
Table 3.
Treatment-Related Adverse Events with Incidence of 10% or Greater in Either Trial Group (As-Treated Population)
| Event | Pembrolizumab Group (N=396) | Placebo Group (N=399) | ||
|---|---|---|---|---|
| Any Grade | Grade 3–4 | Any Grade | Grade 3–4 | |
| Nausea | 215 (54.3) | 8 (2.0) | 204 (51.1) | 6 (1.5) |
| Neutrophil count decreased | 167 (42.2) | 82 (20.7) | 167 (41.9) | 78 (19.5) |
| Anemia | 143 (36.1) | 29 (7.3) | 135 (33.8) | 22 (5.5) |
| White blood cell count decreased | 111 (28.0) | 21 (5.3) | 98 (24.6) | 22 (5.5) |
| Fatigue | 108 (27.3) | 6 (1.5) | 94 (23.6) | 3 (0.8) |
| Constipation | 106 (26.8) | 3 (0.8) | 100 (25.1) | 0 |
| Decreased appetite | 91 (23.0) | 6 (1.5) | 88 (22.1) | 0 |
| Vomiting | 75 (18.9) | 4 (1.0) | 58 (14.5) | 1 (0.3) |
| Platelet count decreased | 74 (18.7) | 20 (5.1) | 74 (18.5) | 24 (6.0) |
| Blood creatinine increased | 56 (14.1) | 3 (0.8) | 48 (12.0) | 0 |
| Diarrhea | 52 (13.1) | 6 (1.5) | 56 (14.0) | 3 (0.8) |
| Alanine aminotransferase increased | 51 (12.9) | 7 (1.8) | 31 (7.8) | 4 (1.0) |
| Asthenia | 45 (11.4) | 4 (1.0) | 55 (13.8) | 2 (0.5) |
| Rash | 45 (11.4) | 3 (0.8) | 26 (6.5) | 0 |
| Alopecia | 40 (10.1) | 0 | 40 (10.0) | 1 (0.3) |
Acknowledgments
We thank the patients and their families and caregivers for participating in the study; the investigators and site personnel; the independent data and safety monitoring committee; and the following employees of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA: Geri Lyn Ferraro, Ann Marie Mantz, and Andrea Rybak-Feiglin for study support; Gregory M. Lubiniecki for input into study design and study oversight; M. Catherine Pietanza for input into study design, study oversight, and critical review of the manuscript; Jin Zhang and Xuan Deng for statistical support; and Melanie A. Leiby for medical writing and editorial assistance.
Support
Supported by Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA. Jamie Chaft was additionally supported by a grant from the National Institutes of Health (P30 CA008748). HW, ML, TK, MT, S-HL, SG, KC, CD, MM, EE, GLM, OB, DR-A, JC, SN, and JDS received study funding to the institution from Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA to support study conduct. HW, ML, TK, MT, S-HL, JH, SG, KC, CD, MM, EE, GLM, OB, DR-A, JC, SN, JY, SMK, AS, and JDS received medical writing and editorial support in preparation of this manuscript. JY, SMK, and AS report salary for full-time employment from Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA. SMK and AS additionally report stock ownership in Merck & Co., Inc., Rahway, NJ, USA.
References
- 1.Reck M, Rodriguez-Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med 2016;375:1823–33. [DOI] [PubMed] [Google Scholar]
- 2.Gandhi L, Rodriguez-Abreu D, Gadgeel S, et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med 2018;378:2078–92. [DOI] [PubMed] [Google Scholar]
- 3.Paz-Ares L, Luft A, Vicente D, et al. Pembrolizumab plus chemotherapy for squamous non-small-cell lung cancer. N Engl J Med 2018;379:2040–51. [DOI] [PubMed] [Google Scholar]
- 4.Hendriks LE, Kerr KM, Menis J, et al. Non-oncogene-addicted metastatic non-small-cell lung cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol 2023;34:358–76. [DOI] [PubMed] [Google Scholar]
- 5.NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®:) Non-Small Cell Lung Cancer. Version 3.2023. 2023. [Google Scholar]
- 6.Antonia SJ, Villegas A, Daniel D, et al. Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. N Engl J Med 2017;377:1919–29. [DOI] [PubMed] [Google Scholar]
- 7.Antonia SJ, Villegas A, Daniel D, et al. Overall survival with durvalumab after chemoradiotherapy in stage III NSCLC. N Engl J Med 2018;379:2342–50. [DOI] [PubMed] [Google Scholar]
- 8.Forde PM, Chaft JE, Smith KN, et al. Neoadjuvant PD-1 blockade in resectable lung cancer. N Engl J Med 2018;378:1976–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chaft JE, Oezkan F, Kris MG, et al. Neoadjuvant atezolizumab for resectable non-small cell lung cancer: an open-label, single-arm phase II trial. Nat Med 2022;28:2155–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Provencio M, Nadal E, Insa A, et al. Neoadjuvant chemotherapy and nivolumab in resectable non-small-cell lung cancer (NADIM): an open-label, multicentre, single-arm, phase 2 trial. Lancet Oncol 2020;21:1413–22. [DOI] [PubMed] [Google Scholar]
- 11.Forde PM, Spicer J, Lu S, et al. Neoadjuvant nivolumab plus chemotherapy in resectable lung cancer. N Engl J Med 2022;386:1973–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Felip E, Altorki N, Zhou C, et al. Adjuvant atezolizumab after adjuvant chemotherapy in resected stage IB-IIIA non-small-cell lung cancer (IMpower010): a randomised, multicentre, open-label, phase 3 trial. Lancet 2021;398:1344–57. [DOI] [PubMed] [Google Scholar]
- 13.O’Brien M, Paz-Ares L, Marreaud S, et al. Pembrolizumab versus placebo as adjuvant therapy for completely resected stage IB-IIIA non-small-cell lung cancer (PEARLS/KEYNOTE-091): an interim analysis of a randomised, triple-blind, phase 3 trial. Lancet Oncol 2022;23:1274–86. [DOI] [PubMed] [Google Scholar]
- 14.Brierly JD, Gospodarowicz MK, Wittekind C. TNM Classification of Malignant Tumors. Eighth Edition. Oxford, UK: John Wiley & Sons; 2016. [Google Scholar]
- 15.Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 1982;5:649–55. [PubMed] [Google Scholar]
- 16.Zhao L, Claggett B, Tian L, et al. On the restricted mean survival time curve in survival analysis. Biometrics 2016;72:215–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maurer W, Bretz F. Multiple testing in group sequential trials using graphical approaches. Stat Biopharm Res 2013;5:311–20. [Google Scholar]
- 18.Heymach JV, Harpole D, Mitsudomi T, et al. CT005: AEGEAN: a phase 3 trial of neoadjvuant durvalumab + chemotherpy followed by adjvuant durvalumab in patients with resectable NSCLC. Abstract presented at: AACR Annual Meeting 2023; April 14–19, 2023; Orlando, FL. [Google Scholar]
- 19.Lu S, Wu L, Zhang W, et al. Perioperative toripalimab + platinum-doublet chemotherapy vs chemotherapy in resectable stage II/III non-small cell lung cancer (NSCLC): interim event-free survival (EFS) analysis of the phase III Neotorch study. J Clin Oncol 2023;41:425126. [Google Scholar]
- 20.Provencio-Pulla M, Nadal E, Larriba JLG, et al. Nivolumab plus chemotherapy versus chemotherapy as neoadjuvant treatment for resectable stage IIIA NSCLC: Primary endpoint results of pathological complete response (pCR) from phase II NADIM II trial. J Clin Oncol 2022;40:8501. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


