Extended Data Fig. 1. CheckMate 816 analysis population.
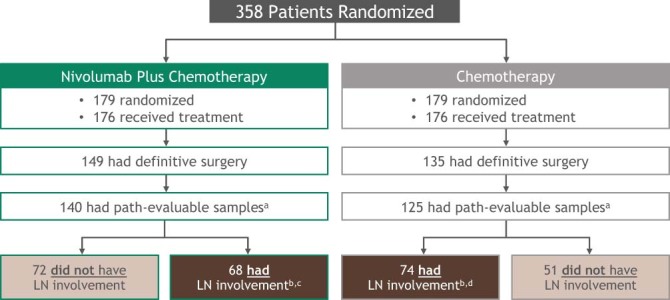
Database lock: October 20, 2021; minimum follow-up: 21 months; median follow-up, 29.5 months. LN denotes lymph node; RVT, residual viable tumor. aRepresents patients with path-evaluable samples from both the primary tumor and LN; 141 and 126 patients in the nivolumab plus chemotherapy arm and the chemotherapy arm, respectively, had path-evaluable samples from the primary tumor only. bPathologic evidence of ≥0% RVT in LNs. c94 patients and d71 patients had LN involvement based on baseline radiographic imaging in the path-evaluable population; LN involvement refers to pathologic evidence of LN disease at resection that had or had not fully regressed after neoadjuvant treatment (0% or >0% RVT in the resected LN).
