Abstract
End-stage kidney disease (ESKD) is fatal without treatment by kidney replacement therapies (KRTs). However, access to these treatment modalities can be problematic given the high costs. This systematic review (SR) aims to provide an updated economic evaluation of pairwise comparisons of KRTs and the implications for the proportion of patients with access to the KRT modalities, i.e., kidney transplantation (KT), hemodialysis (HD), and peritoneal dialysis (PD). This SR was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) 2020. We searched studies in PubMed, Embase, Scopus, and Cost Effectiveness Analysis (CEA) registry, from inception to March 2023. Thirteen studies were included with pairwise comparisons among three KRTs, with varying proportions of patients for each modality. Seven studies were from high-income countries, including five from Europe. Summary findings are presented on a cost-effectiveness plane and incremental net benefit (INB). KT was the most cost-effective intervention across the pairwise comparisons. KT and PD were both more cost-effective alternatives to HD. HD was more costly and less effective than PD in all studies except one. Concurrent efforts to increase both KT and PD represented the best scenario to improve treatment options for ESKD patients.
Similar content being viewed by others
Introduction
End-stage kidney disease (ESKD) is characterized by a progressive irreversible decline in kidney function that requires dialysis or transplantation and it is associated with premature mortality1,2. The burden of ESKD continues to increase, affecting approximately 10 million people annually3, with a reported global incidence of 144 individuals per million population4. The ESKD prevalence is higher in high- and upper-middle-income countries (HIC, upper-MICs), at 0.2% and 0.1%, in contrast to lower-middle and low-income countries (lower-MICs, LICs) at 0.07% and 0.05% respectively5.
ESKD prevalence is expected to increase further given the increase in non-communicable diseases (NCDs), e.g., hypertension, diabetes mellitus, cardiovascular diseases, and obesity, all of which are considered risk factors for ESKD, in addition to the increasing age of global populations3,6,7,8. An estimated 14.5 million people are predicted to succumb to ESKD globally by 2030, but only 37% (ca. 5.4 million) are likely to have accessibility to appropriate treatments9.
ESKD is managed through the provision of kidney replacement therapies (KRT) including kidney transplantation (KT), peritoneal dialysis (PD) and hemodialysis (HD)10,11. There is significant geographical and income-related variation in the provision of KRT, with 96% of ESKD patients from LICs unable to access KRT, compared to 40% of patients in HICs9. Although > 80% of dialysis patients in many countries receive HD, PD is still the principal KRT in some countries, (e.g., Hong Kong and Mexico), accounting for about 71% and 61% of dialysis patients, respectively.
KT remains the optimum treatment option for all ages regardless of the underlying cause of ESKD12,13, providing improved quality of life and survival length14. In the US, adjusted mortality rate was lowest for KT, followed by PD and HD with rates of 29, 154 and 166 per 1000 patient-years, respectively15. However, limited access to donor organs, high costs, lack of infrastructure and a shortage of skilled healthcare resources have restricted the use of KT as a therapeutic option; therefore, dialysis is more commonly prescribed6,16.
Despite the proven efficacy of all KRTs, patient accessibility across many countries remains problematic9. These barriers to access are mostly attributable to costs17 with significant implications for health coverage schemes3. Evidence from a systematic review to assess the cost-effectiveness of KRTs, particularly in resource limited settings, is required to provide support for health policy decisions. Previous reports were varied in terms of the cost-effectiveness of KRTs across regions and national income levels; for instance, a ‘PD-First’ policy was suggested to be most cost-effective in some studies18,19, in contrast to KT in others10,20. Most previous SRs focused on economic considerations related to the cost of illness or quality of life21,22,23. Other economic evaluations only focused on one or two KRT modalities6,24,25,26, with only a single study addressing the cost-effectiveness of all three KRTs27, albeit with limited ability to combine data.
Therefore, this study was conducted to provide an updated review of the cost-effectiveness of KRT modalities to determine the most cost-effective treatment using incremental net benefits (INB) and incremental cost-effectiveness ratios (ICER)28. Negative ICER may imply higher cost and lower intervention effectiveness or lower cost and higher intervention effectiveness leading to some ambiguity in interpretation, in contrast to the INB, which is more informative for the cost-effectiveness of treatment options29. In addition, ICER requires a specific threshold for interpretation, unlike the INB which incorporates a threshold within its estimation, providing more robust cost-effectiveness evaluations across studies30,31.
Methods
This study was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) 2020 statement32, and the review protocol was registered at PROSPERO (ID CRD42022358152).
Data sources and search strategy
The following online databases were systematically searched: Medline through PubMed, Embase, Scopus, Cost Effectiveness Analysis (CEA) registry by Tufts Medical Centre, National Health Service-Economic Evaluation Database (NHS-EED), Database of Abstracts of Reviews of Effect (DARE) and International Health Technologies Assessment (HTA) Database from inception to March 2023. The search terms are provided in Supplementary Table S1 and there were no restrictions on countries, regions or language.
Studies were selected if they met the following criteria: included an empirical cost-effectiveness and/or cost-utility analysis (CEA, CUA) in adult ESKD patients, considered any comparison of three KRTs (i.e., KT, HD and PD), and reported any of the following outcomes: ICER, INB, cost per quality-adjusted life year (QALY), cost per life year (LY) gained, incremental cost or incremental effectiveness. Studies were ineligible if they were partial economic evaluation studies, conference abstracts, commentaries, or review studies.
Selection of studies and data extraction
Two authors (PN and TO) independently selected studies by initially screening titles and abstracts. Full papers were then analyzed if a decision could not be made based on the abstract alone. Extracted data included study characteristics (i.e., study setting, economic evaluation type and design, willingness-to-pay (WTP) threshold, perspective, currency, discount rate, and time horizon), interventions and comparators, the study findings, including costs and utilities of interventions versus comparators, ICER, sensitivity analysis and study conclusions.
Any missing relevant data was sought on at least two occasions from the study’s corresponding authors. If the author did not respond, the article was excluded from the review. As a result, one article was disregarded. Any disagreements were resolved through discussion and consensus with the other authors (BSB, SY, UC, AT).
Quality assessment
A risk of bias assessment for all included studies was evaluated using the economic evaluation bias (ECOBIAS) checklist33 which has two parts: a general bias consisting of eleven items, and a model specification bias which included model structure, data, and consistency. Each item was graded as yes (high risk of bias), no (low risk of bias), partly, unclear or not applicable. Additionally, the completeness of reporting was assessed using the Consolidated Health Economic Evaluation Reporting Standards (CHEERS) checklist34 with 28 reference points. PN and TO carried out the quality assessment.
Data analysis
Cost-effectiveness analysis of KRT were described based on the study ICERs relative to the corresponding WTP threshold for that country. These results were collated as INB, an increment in the number of effectiveness units multiplied by the corresponding country threshold providing a beneficial increase in effectiveness expressed in monetary terms minus an incremental cost35,36. An intervention is considered cost-effective when the INB value is positive, i.e., greater than zero37. The WTP threshold was set according to the original included study; where not provided, it was calculated as three times the gross domestic product (GDP) per capita for each country, as reported by the World Health Organization (WHO)38, for the year in which the study was undertaken, with subsequent adjustment for consumer price index (CPI) and purchasing power parity (PPP)39. Studies that only provided standard country-specific thresholds were adjusted for PPP. Additionally, all currency data was harmonized to US$ 2022 using both CPI and PPP40; the currency conversion factors are provided in Supplementary Table S4.
Cost-effectiveness findings were summarized in a cost-effectiveness plane represented by incremental QALY on the x-axis and incremental cost on the y-axis. It has four quadrants; points that fell within the upper quadrants were considered more costly, while those in the right quadrants were considered more effective41.
Studies considered various KRT scenarios. Therefore, re-classification was undertaken to provide uniformity of KRT modalities to enable comparisons across studies. As a result, 5 scenarios were constructed as follows: Scenario 1 was classified to represent the current KRT status in each country and was used as the comparator. Scenario 2 included an increase in PD coverage with a corresponding decrease in HD, while KT was held constant. Scenario 3 included an increase in KT coverage with a corresponding decrease in HD, while PD was held constant. Scenario 4 included an increase in both PD and KT, with a decrease in HD, and Scenario 5 was any other approach reported and not captured under any of the aforementioned scenarios.
Results
Study selection and characteristics
There were 1464 articles identified from the systematic search of online databases. Of these, 13 met the inclusion criteria after duplicates and ineligible studies were removed as shown in the PRISMA flow diagram in Fig. 1.
PRISMA flow diagram. CEA, Cost-Effectiveness Analysis; CUA, Cost-Utility Analysis; DARE & HTA, Database of Abstracts of Reviews of Effects and Health Technology Assessment Database; EE, Economic Evaluation, HD, Haemodialysis; HIC, High Income Country; KRT, Kidney Replacement Therapy; KT, Kidney Transplantation; MIC, Middle Income Country; NHS-EED, National Institute of Health Research-Economic Evaluation Database; PD, Peritoneal Dialysis.
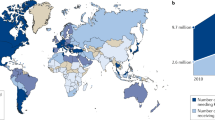
Table 1 presents the general characteristics of the 13 studies included. Of these, 7, 4 and 2 were conducted in HIC, upper-MICs, and lower-MICs, respectively as per the World Bank classification42, none were from low-income countries (LICs). Five studies were European19,43,44,45,46, three each from South-America47,48,49 and Western Pacific regions10,20,50, and a single study each from South-East Asia18 and the Eastern Mediterranean11, as presented in Fig. 2. Seven studies included CUAs10,11,18,19,43,44,50, five were both CUAs & CEAs20,45,46,47,48, and a single study was a CEA that reported outcomes in LYs gained49. All studies were in English except one reported in Spanish48.
A map showing the different approaches for the 13 economic evaluation studies included in this review across regions. HIC, High Income Country; MIC, Middle Income Country. This figure was generated using the free editable map available at this link: https://www.pptmaps.com/Editable-map-of-the-world-with-country-borders.html52, and then used PowerPoint software Version 2019.
Ten studies performed model-based analyses; two were cross-sectional, while one was a retrospective cohort that included an economic evaluation. Most studies reported a public-health provider perspective (N = 7); of the four studies that had a societal perspective, all included costs related to transportation while three reported indirect costs related to productivity loss. The time horizon in the studies ranged between 1 and lifetime, with most using a 5-year time horizon (N = 6). All but one study49 discounted for both health and outcomes, with the majority (N = 5) applying a 5% discount rate. Seven studies stated no conflict of interest10,11,18,43,46,47,50, and all but two44,48 declared their sources of funding. The information for country-specific ESKD prevalence was identified from independent reviews as it was not available for extraction from all of the thirteen studies included, which ranged from 0.03% in Columbia to 0.4% in Brazil.
Model design
All 10 model-based designs were applied using Markov models, with one also incorporating decision tree analysis43. The states ranged from 4 to 36, with the majority having four states (N = 5). Four studies had the same four states i.e., HD, PD, KT and death10,11,19,43, whereas another study had a pre-emptive transplant state instead of PD20. Two studies had 8 and 12 states respectively with 1-month cycle lengths46,47. Only one18 study considered complications associated with HD and PD in its model states, after adoption from a previous study by Yot et al.51.
Individual studies generally included pairwise comparisons of KRTs, i.e., HD-PD, HD-KT and PD-KT10,11,43,44,47,48,49 to determine the most cost-effective modality or alternatively provided comparisons of various scenarios by varying proportions of patients receiving KRT10,18,19,20,45,46,50 that reflected the current KRT status in their respective countries. One study considered both approaches10. There were 24 comparison pairs in the pairwise approach and 36 in the scenario specific analysis resulting in 60 evaluations in total.
Risk of bias assessment
Of the 13 studies, 7 addressed 60% of the ECOBIAS checklist items and were graded as low risk of bias. All studies measured resource use continuously, identified all costs relevant to ESKD and intervention, had an adequate comparator (i.e., usual care) and had transparent methods and process of data identification and data incorporation. Compliance with completeness of reporting according to the CHEERS checklist was 87% on average for all studies. No studies provided 100% reporting for all 28 items on the CHEERS checklist. The least reported items included characterizing distributional effects (23%), heterogeneity (31%) and conflict of interest (54%). These results are provided in Supplementary Tables S2 and S3 respectively.
Cost-effectiveness results
Incremental cost effectiveness ratio
In the pairwise comparison approach, all studies reported KT as the most cost-effective option10,11,15,43,44,47,48,49. HD was considered to be more costly and less effective than PD in all studies except one49, where continuous ambulatory peritoneal dialysis (CAPD) was considered less cost-effective than HD.
In the scenario specific approach, Scenario 2, an increase in PD with a corresponding decrease in HD, was found to be most cost-effective in two studies18,45, while scenario 3, an increase in KT with a corresponding decrease in HD was found to be most cost-effective in two studies20,50. Scenario 4, an increase in both PD and KT with a decrease in HD, was found to be most cost-effective in two studies10,46 and lastly Scenario 5 was found to be most cost-effective in a single study19. These results are presented in Table 2.
Some studies also explored the various forms of KT (i.e., cadaver, and living donor transplant46,49,50, PD (i.e., CAPD, Automated PD; APD, or continuous cycling PD; CCPD)45; and HD (i.e., Center HD, Home HD, and limited care HD)20,45. In the comparison between the various forms of PD versus other HD modalities, CAPD was found to be the most cost-effective treatment modality, followed by home HD, then CCPD or APD; the least cost-effective was limited care HD i.e., in a non-hospital facility45.
Seven studies did not report WTP threshold; of those that did, three reported GDP-based thresholds11,27,47 and three country-specific thresholds18,19,50. Upon standardization to US$ 2022, the threshold value in the studies ranged from US$ 7720 to US$ 144,283.
Cost-effectiveness (CE) plane
A CE plane was constructed for each comparison point with incremental QALYs on the X-axis and incremental costs on the Y-axis. This was presented separately for both approaches i.e., pairwise comparison of KRTs and scenario analysis with different population proportions under each modality.
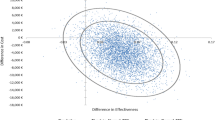
The CE-plane of pairwise KRT comparisons demonstrated in Fig. 3 shows interventions were more effective than the comparators if they were presented in the right quadrant. Points located within the right-lower quadrant indicate that the intervention was dominant or preferential to the comparator, suggesting that most studies that had KT as the intervention were more likely to be cost-effective compared to either HD or PD, and these mostly originated from HICs and upper-MICs43,44,48. Those points within the right-upper quadrant (which means the intervention was more costly and also more effective than the comparator) all had KT as the intervention and either HD or PD as the comparator and originated from upper- and lower-MICs10,11,47. Two points located within the left-lower quadrant indicated that PD was less costly and less effective than HD representing two studies (one each from lower MIC11 and HIC44).
Cost-effectiveness plane for pairwise comparison approach. H, High income country; KT, Kidney Transplantation; L, Lower-middle income country; U, Upper-middle income country; HD, Haemodialysis; PD, Peritoneal Dialysis; QALY, Quality-Adjusted Life Year. The blue square-shape dots symbolize HD comparator & PD intervention; red diamond-shape dots, HD comparator & KT intervention and green round-shape dots, PD comparator & KT intervention.
In the CE-plane represented by the individual scenario analyses Fig. 4, all points were located in the right quadrant indicating that Scenarios 2–4 were more effective than Scenario 1 for each study. The figure also suggests that increased PD coverage and decreased HD coverage, with KT remaining constant (Scenario 2), was less costly and as effective or more effective than the current status10,18,19,20,45,46,50. Increased KT and decreased HD with PD remaining constant (Scenario 3), was more costly in upper-MICs10 in contrast to those from HICs20,45,50 which were less costly and cost-effective compared to the current status for that specific scenario. For Scenario 4, increased PD and KT/decreased HD, tended to be comparable or more costly than Scenario 110,18,46; these would be cost-effective or not depending on country-specific WTP thresholds.
Cost-effectiveness plane for scenario analysis. H, High income country; L, Lower-middle income country; U, Upper-middle income country; QALY, Quality-Adjusted Life Year. The blue square-shape dots symbolize scenario 2; red diamond-shape dots scenario 3 and green round-shape dots scenario 4. All interventions compared against scenario 1.
Incremental net benefit (INB)
In the INB computation for the pairwise comparison of the KRTs, 5 of the 24 comparisons had negative INB, see Table 2. Thus, the intervention was not cost-effective relative to the comparator. It was also observed that the pair with HD as the comparator and KT as the intervention yielded the highest INB in each study. This was consistent despite differences in country income level, WTP-threshold, time horizon and perspective across all studies10,11,27,44,47,48.
For the scenario analysis approach, it was noted that one study18 yielded negative INB, indicating that all scenarios were not cost-effective compared to Scenario 1. This study was from a lower-MIC. All the other studies conducted in upper-MICs and HIC had positive INB. Scenario 4 had the highest INB overall, and included one study from upper-MIC and HIC10,46, signifying the greatest cost-effectiveness relative to threshold, compared to Scenario 1. This was followed by Scenario 320,45,50.
Discussion
This systematic review included thirteen studies to assess the cost-effectiveness of KRTs for ESKD by exploring two different approaches: pairwise comparison of KRT modalities and specific scenario analyses that reflected differential proportions for each KRT modality.
For the pairwise comparison approach, our findings suggested that KT tended to be the most cost-effective treatment option compared to HD and PD. PD was less costly than HD across all country income levels and offered comparable effectiveness. In the specific scenario approaches, increasing patient proportions for the provision of KT to between 12% and 34%, and PD to between 20% and 40%, while decreasing HD to between 26% and 68% provided the most cost-effective coverage for ESKD patients.
These findings prioritize KT and PD as the key strategies to enhance KRT sustainability in national health coverage systems, as is the current provision in many countries. Italy, the Netherlands and the United States of America (USA) have developed policies to increase kidney organ donation from both living and deceased donors53,54. Hong Kong and Thailand implement ‘PD-first’ policies for patients newly diagnosed with ESKD55. Canada, Spain, China, Taiwan and Mexico support PD-favored policies promoted through incentivizing PD uptake through increased reimbursement rates, training of personnel, and providing PD machines and supplies56. In Hong Kong, for example, the policy is to promote CAPD as the initial choice, while reserving APD for individuals with elevated membrane transfer levels or those with specific psychosocial requirements. APD machine subsidies are offered to promote this approach. During the COVID-19 pandemic, two health care reform initiatives were passed in the USA to increase support for home-based dialysis thereby reducing risk of exposure to the virus57. These policy-related changes have potential to tip the cost-effectiveness in favour of PD.
Although KT is considered the gold standard and most cost-effective modality, the promotion of KT remains the most challenging due to limited donor availability. Three studies explored incorporation of alternative forms of KT, i.e., cadaveric organ donation and pre-emptive KT. Though beneficial, these were less cost-effective compared to living-donor KT. Worldwide, the current KT requirement is 255 per million population. HICs especially from Eastern and Central Europe have greater coverage of KT at 363 pmp compared to upper-middle (80 pmp) and lower-middle (27 pmp) income countries from Africa and Latin America9.
As such, PD may represent a more feasible KRT for lower-MICs and LICs that face limitations due to infrastructural and professional capacity for performing KT. However, expanding PD, particularly in developing countries, may be limited due to the high cost of importing PD fluids and disposables58. Local manufacturing and volume-based negotiated pricing for these consumables are highly encouraged56. Additionally, there would be an institutional learning curve as the practice of PD in most of these countries is not widespread.
Nevertheless, despite the cost-effective benefits of KT and PD, the availability of HD within healthcare systems is especially important for patients with cardiovascular complications and those in need of intensive monitoring by healthcare professionals for better clinical outcomes59. Our findings suggest maintaining HD populations between 26 and 68% would be the most cost-effective option.
Quality assessment using the ECOBIAS checklist indicated that despite more than half of the studies included, having more than 60% of items graded as low risk of bias, the listing of studies in a trial register was not applicable to all studies and variable checking for double counting was unclear. Biases related to the model, information on internal consistency for mathematical logic was unclear for all studies, while consideration of all four principles of uncertainty, as well as synthesis of treatment effects using meta-analytic techniques, indicated that no studies were considered to have a low risk of bias.
Our study has several strengths. To the best of our knowledge, this is the first study to summarize cost-effectiveness of all strategies of KRT, and also consider specific scenarios for varying ESKD patient population proportions under each KRT modality. This was possible through the computation of INB instead of ICER as the economic effect measure, given the limitations associated with ICER calculation and interpretation. Despite the challenges of synthesizing economic evaluation studies, with varying levels of income, WTP thresholds, measurements of costs and utilities; stratification of these studies based on the approach used and converting monetary units to a common standard currency (US $2022), enabled indirect comparisons across both studies and countries. This study will be particularly important to LICs and lower-MICs, where cost is paramount to patients’ choice of dialysis modality.
Our study also had limitations. KRT cost-effectiveness findings may be less generalizable especially with lower-MICs and LICs, as the majority of studies included originated from HICs and upper-MICs. In addition, as data on measures of dispersion was not provided across all studies, pooling INB data by applying meta-analysis could not be undertaken, which represents a future opportunity. Our recommendations for economic evaluation studies would be to comprehensively report how costs were valued, the currency and year of conversion, as well as to report the mean values and the dispersions for the main categories of costs and outcomes of interest, to allow for comparability across studies.
Conclusion
While KT was the most cost-effective KRT identified from the pairwise comparisons and an increase in the provision of both KT and PD coverage was supported from the specified scenario analyses, variability in cost-effectiveness of KRTs across country income levels was noted. These modalities were cost-effective in HIC and upper-MIC, but this was not applicable for lower-MICs and LICs. In addition, PD is a more cost-effective modality compared to HD, especially in regions where KT is not widely available. However, PD is often underutilized in lower-middle and low-income countries, which exacerbates the strain on healthcare budgets.
Data availability
The datasets used and/or analysed during the current study available upon request from the corresponding author.
Change history
31 October 2024
A Correction to this paper has been published: https://doi.org/10.1038/s41598-024-77061-x
References
Harris, D. C. H. et al. Increasing access to integrated ESKD care as part of universal health coverage. Kidney Int. 95, S1–S33. https://doi.org/10.1016/j.kint.2018.12.005 (2019).
Jones-Hughes, T. et al. Immunosuppressive therapy for kidney transplantation in adults: a systematic review and economic model. Health Technol. Assess. 20, 1–594. https://doi.org/10.3310/hta20620 (2016).
Teerawattananon, Y., Dabak, S. V., Khoe, L. C., Bayani, D. B. S. & Isaranuwatchai, W. To include or not include: renal dialysis policy in the era of universal health coverage. BMJ 368, m82. https://doi.org/10.1136/bmj.m82 (2020).
Thurlow, J. S. et al. Global epidemiology of end-stage kidney disease and disparities in kidney replacement therapy. Am. J. Nephrol. 52, 98–107. https://doi.org/10.1159/000514550 (2021).
Bello, A. K. et al. ISN Global Kidney Health Atlas (Ihrnternational Society of Nepology, 2023).
Howell, M., Walker, R. C. & Howard, K. Cost effectiveness of dialysis modalities: a systematic review of economic evaluations. Appl. Health Econ. Health Policy 17, 315–330. https://doi.org/10.1007/s40258-018-00455-2 (2019).
Liyanage, T. et al. Worldwide access to treatment for end-stage kidney disease: a systematic review. Lancet 385, 1975–1982. https://doi.org/10.1016/S0140-6736(14)61601-9 (2015).
Ruiz-Ortega, M., Rayego-Mateos, S., Lamas, S., Ortiz, A. & Rodrigues-Diez, R. R. Targeting the progression of chronic kidney disease. Nat. Rev. Nephrol. 16, 269–288. https://doi.org/10.1038/s41581-019-0248-y (2020).
Bello, A. K. et al. Global Kidney Health Atlas (International Society of Nephrology, 2019).
Yang, F., Liao, M., Wang, P. & Liu, Y. Cost-effectiveness analysis of renal replacement therapy strategies in Guangzhou city, southern China. BMJ Open 11, e039653. https://doi.org/10.1136/bmjopen-2020-039653 (2021).
Moradpour, A. Economic evaluation of end stage renal disease treatments in Iran. Clin. Epidemiol. Global Health 8, 199–204 (2020).
O’Connell, P. J. et al. The role of kidney transplantation as a component of integrated care for chronic kidney disease. Kidney Int. Suppl. 10, e78–e85. https://doi.org/10.1016/j.kisu.2019.11.006 (2020).
Muralidharan, A. & White, S. The need for kidney transplantation in low- and middle-income countries in 2012: an epidemiological perspective. Transplantation 99, 476–481. https://doi.org/10.1097/TP.0000000000000657 (2015).
Tonelli, M. et al. Systematic review: kidney transplantation compared with Dialysis in clinically relevant outcomes. Am. J. Transplant. 11, 2093–2109. https://doi.org/10.1111/j.1600-6143.2011.03686.x (2011).
Saran, R. et al. US Renal Data System 2018 Annual Data Report: Epidemiology of kidney disease in the United States. Am. J. Kidney Dis. 73, A7–A8. https://doi.org/10.1053/j.ajkd.2019.01.001 (2019).
Naicker, S. Burden of end-stage renal disease in sub-saharan Africa. Clin. Nephrol. 74 (Suppl 1), 13–16. https://doi.org/10.5414/cnp74s013 (2010).
Ikechi Okpechi, A. N. et al. A roadmap for kidney care in Africa: an analysis of International Society of Nephrology–Global Kidney Health Atlas Africa data describing current gaps and opportunities. Afr. J. Nephrol. 25 (2022).
Bayani, D. B. S. et al. Filtering for the best policy: an economic evaluation of policy options for kidney replacement coverage in the Philippines. Nephrology (Carlton) 26, 170–177. https://doi.org/10.1111/nep.13830 (2021).
Villa, G. et al. Cost-effectiveness analysis of the Spanish renal replacement therapy program. Perit. Dial Int. 32, 192–199. https://doi.org/10.3747/pdi.2011.00037 (2012).
Howard, K. et al. The cost-effectiveness of increasing kidney transplantation and home-based dialysis. Nephrology (Carlton) 14, 123–132. https://doi.org/10.1111/j.1440-1797.2008.01073.x (2009).
Elshahat, S. et al. The impact of chronic kidney disease on developed countries from a health economics perspective: a systematic scoping review. PLoS One 15, e0230512. https://doi.org/10.1371/journal.pone.0230512 (2020).
Mushi, L., Marschall, P. & Flessa, S. The cost of dialysis in low and middle-income countries: a systematic review. BMC Health Serv. Res. 15, 506. https://doi.org/10.1186/s12913-015-1166-8 (2015).
Purnell, T. S. et al. Comparison of life participation activities among adults treated by hemodialysis, peritoneal dialysis, and kidney transplantation: a systematic review. Am. J. Kidney Dis. 62, 953–973. https://doi.org/10.1053/j.ajkd.2013.03.022 (2013).
Chung, R. et al. Economic evaluations in kidney transplantation: frequency, characteristics, and quality-a systematic review. Transplantation 97, 1027–1033. https://doi.org/10.1097/TP.0000000000000079 (2014).
Senanayake, S., Graves, N., Healy, H., Baboolal, K. & Kularatna, S. Cost-utility analysis in chronic kidney disease patients undergoing kidney transplant; what pays? A systematic review. Cost Eff. Resour. Alloc. 18, 18. https://doi.org/10.1186/s12962-020-00213-z (2020).
Fu, R., Sekercioglu, N., Berta, W. & Coyte, P. C. Cost-effectiveness of deceased-donor renal transplant versus dialysis to treat end-stage renal disease: a systematic review. Transpl. Direct. 6, e522. https://doi.org/10.1097/TXD.0000000000000974 (2020).
Yang, F., Liao, M., Wang, P., Yang, Z. & Liu, Y. The cost-effectiveness of kidney replacement therapy modalities: a systematic review of full economic evaluations. Appl. Health Econ. Health Policy 19, 163–180. https://doi.org/10.1007/s40258-020-00614-4 (2021).
O’Mahony, J. F. Does cost-effectiveness analysis really need to abandon the incremental cost-effectiveness ratio to embrace net benefit? Pharmacoeconomics 38, 777–779. https://doi.org/10.1007/s40273-020-00931-5 (2020).
Hoch, J. S. & Dewa, C. S. Advantages of the net benefit regression framework for economic evaluations of interventions in the workplace: a case study of the cost-effectiveness of a collaborative mental health care program for people receiving short-term disability benefits for psychiatric disorders. J. Occup. Environ. Med. 56, 441–445. https://doi.org/10.1097/JOM.0000000000000130 (2014).
Santoli, G., Nurchis, M. C., Calabro, G. E. & Damiani, G. Incremental net benefit and incremental cost-effectiveness ratio of COVID-19 Vaccination campaigns: systematic review of cost-effectiveness evidence. Vaccines (Basel). https://doi.org/10.3390/vaccines11020347 (2023).
Paulden, M. Calculating and interpreting ICERs and net benefit. Pharmacoeconomics 38, 785–807. https://doi.org/10.1007/s40273-020-00914-6 (2020).
Page, M. J. et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 372, n71. https://doi.org/10.1136/bmj.n71 (2021).
Adarkwah, C. C., van Gils, P. F., Hiligsmann, M. & Evers, S. M. Risk of bias in model-based economic evaluations: the ECOBIAS checklist. Expert Rev. Pharmacoecon. Outcomes Res. 16, 513–523. https://doi.org/10.1586/14737167.2015.1103185 (2016).
Husereau, D. et al. Consolidated health economic evaluation reporting standards 2022 (CHEERS 2022) statement: updated reporting guidance for health economic evaluations. BJOG 129, 336–344. https://doi.org/10.1111/1471-0528.17012 (2022).
Willan, A. R. Incremental net benefit in the analysis of economic data from clinical trials, with application to the CADET-Hp trial. Eur. J. Gastroenterol. Hepatol. 16, 543–549. https://doi.org/10.1097/00042737-200406000-00006 (2004).
Willan, A. R., Chen, E. B., Cook, R. J. & Lin, D. Y. Incremental net benefit in randomized clinical trials with quality-adjusted survival. Stat. Med. 22, 353–362. https://doi.org/10.1002/sim.1347 (2003).
Lopez-Villegas, A., Catalan-Matamoros, D., Peiro, S., Lappegard, K. T. & Lopez-Liria, R. Cost-utility analysis of telemonitoring versus conventional hospital-based follow-up of patients with pacemakers. The NORDLAND randomized clinical trial. PLoS One 15, e0226188. https://doi.org/10.1371/journal.pone.0226188 (2020).
Robinson, L. A., Hammitt, J. K., Chang, A. Y. & Resch, S. Understanding and improving the one and three times GDP per capita cost-effectiveness thresholds. Health Policy Plan. 32, 141–145. https://doi.org/10.1093/heapol/czw096 (2017).
IMF. International Monetary Fund Home Page. https://www.imf.org/en/Publications/WEO/weo-database/2022/October (2022).
Crespo, C., Monleon, A., Diaz, W. & Rios, M. Comparative efficiency research (COMER): meta-analysis of cost-effectiveness studies. BMC Med. Res. Methodol. 14, 139. https://doi.org/10.1186/1471-2288-14-139 (2014).
Cohen, D. J. & Reynolds, M. R. Interpreting the results of cost-effectiveness studies. J. Am. Coll. Cardiol. 52, 2119–2126. https://doi.org/10.1016/j.jacc.2008.09.018 (2008).
Bank, T. W. The World by Income and Region. https://datatopics.worldbank.org/world-development-indicators/the-world-by-income-and-region.html (2021).
Jensen, C. E., Sorensen, P. & Petersen, K. D. In Denmark kidney transplantation is more cost-effective than dialysis. Dan. Med. J. 61, A4796 (2014).
Kontodimopoulos, N. & Niakas, D. An estimate of lifelong costs and QALYs in renal replacement therapy based on patients’ life expectancy. Health Policy 86, 85–96. https://doi.org/10.1016/j.healthpol.2007.10.002 (2008).
de Wit, G. A., Ramsteijn, P. G. & de Charro, F. T. Economic evaluation of end stage renal disease treatment. Health Policy 44, 215–232. https://doi.org/10.1016/s0168-8510(98)00017-7 (1998).
Haller, M., Gutjahr, G., Kramar, R., Harnoncourt, F. & Oberbauer, R. Cost-effectiveness analysis of renal replacement therapy in Austria. Nephrol. Dial Transpl. 26, 2988–2995. https://doi.org/10.1093/ndt/gfq780 (2011).
Rosselli, D., Rueda, J. D. & Diaz, C. E. Cost-effectiveness of kidney transplantation compared with chronic dialysis in end-stage renal disease. Saudi J. Kidney Dis. Transpl. 26, 733–738. https://doi.org/10.4103/1319-2442.160175 (2015).
Arredondo, A., Rangel, R. & de Icaza, E. Cost-effectiveness of interventions for end-stage renal disease. Rev. Saude Publica 32, 556–565. https://doi.org/10.1590/s0034-89101998000600009 (1998).
Sesso, R. et al. Cost-effectiveness analysis of the treatment of end-stage renal disease in Brazil. Int. J. Technol. Assess. Health Care 6, 107–114. https://doi.org/10.1017/s0266462300008965 (1990).
Shimizu, U. et al. Cost-effectiveness achieved through changing the composition of renal replacement therapy in Japan. J. Med. Econ. 15, 444–453. https://doi.org/10.3111/13696998.2011.653512 (2012).
Teerawattananon, Y., Mugford, M. & Tangcharoensathien, V. Economic evaluation of palliative management versus peritoneal dialysis and hemodialysis for end-stage renal disease: evidence for coverage decisions in Thailand. Value Health 10, 61–72. https://doi.org/10.1111/j.1524-4733.2006.00145.x (2007).
PPTMaps. Editable map of the world with country borders. PPTMaps. https://www.pptmaps.com/Editable-map-of-the-world-with-country-borders.html (2024).
Barnieh, L. et al. A scoping review for strategies to increase living kidney donation. Clin. J. Am. Soc. Nephrol. 12, 1518–1527. https://doi.org/10.2215/CJN.01470217 (2017).
Testa, G. & Siegler, M. Increasing the supply of kidneys for transplantation by making living donors the preferred source of donor kidneys. Medicine (Baltim) 93, e318. https://doi.org/10.1097/MD.0000000000000318 (2014).
Tang, S. C. W. & Lai, K. N. Peritoneal dialysis: the ideal bridge from conservative therapy to kidney transplant. J. Nephrol. 33, 1189–1194. https://doi.org/10.1007/s40620-020-00787-0 (2020).
Liu, F. X. et al. A global overview of the impact of peritoneal dialysis first or favored policies: an opinion. Perit. Dial Int. 35, 406–420. https://doi.org/10.3747/pdi.2013.00204 (2015).
Brown, E. A. & Perl, J. Increasing peritoneal dialysis use in response to the COVID-19 pandemic: will it go viral? J. Am. Soc. Nephrol. 31, 1928–1930. https://doi.org/10.1681/ASN.2020050729 (2020).
Li, P. K. et al. Peritoneal dialysis first policy in Hong Kong for 35 years: global impact. Nephrology (Carlton) 27, 787–794. https://doi.org/10.1111/nep.14042 (2022).
Bello, A. K. et al. Epidemiology of haemodialysis outcomes. Nat. Rev. Nephrol. 18, 378–395. https://doi.org/10.1038/s41581-022-00542-7 (2022).
Acknowledgements
The authors would like to thank the Thailand International Cooperation Agency (TICA) and Mahidol University Health Technology Assessment (MUHTA) Graduate Program for providing scholarship and training in Health Technology Assessment (HTA)’s PhD degree as well as research facility.
Funding
This work was supported by funding from Mahidol University and the International Decision Support Initiative (iDSI) through the doctoral study in Mahidol University Health Technology Assessment (MUHTA) Graduate Program. This work was produced as part of the iDSI (www.idsihealth.org), which supports countries to get the best value for money from health spending. iDSI receives funding support from the Bill & Melinda Gates Foundation and the UK Department for International Development (Grant No. OPP1087363). PN and TO received a scholarship from the Thailand International Cooperation Agency (TICA) for studying Health Technology Assessment at Mahidol University. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
PN, SY, UC and AT conceptualized, designed and developed the search strategy for the study. PN, SY and BSB screened the studies and formulated a reporting method. PN and TO extracted data and carried out quality assessment. PN drafted the manuscript, with critical revision from SY, BSB, UC and AT. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this Article was revised: In the original version of this Article, Usa Chaikledkaew was omitted as a co-corresponding author. Correspondence and requests for materials should also be addressed to usa.chi@mahidol.ac.th.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nyokabi, P., Youngkong, S., Bagepally, B.S. et al. A systematic review and quality assessment of economic evaluations of kidney replacement therapies in end-stage kidney disease. Sci Rep 14, 23018 (2024). https://doi.org/10.1038/s41598-024-73735-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-73735-8