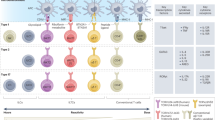
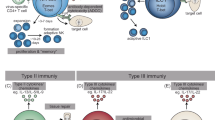
Abstract
The innate lymphoid cell (ILC) family is composed of natural killer (NK) cells, ILC1, ILC2 and ILC3, which participate in immune responses to virus, bacteria, parasites and transformed cells. ILC1, ILC2 and ILC3 subsets are mostly tissue-resident, and are profoundly imprinted by their organ of residence. They exhibit pleiotropic effects, driving seemingly paradoxical responses such as tissue repair and, alternatively, immunopathology toward allergens and promotion of tumorigenesis. Despite this, a trickle of studies now suggests that non-NK ILCs may not be overwhelmingly tumorigenic and could potentially be harnessed to drive anti-tumor responses. Here, we examine the pleiotropic behavior of ILCs in cancer and begin to unravel the gap in our knowledge that exposes a new horizon for thinking about modifying ILCs and targeting them for immunotherapy.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Ribas, A. & Wolchok, J. D. Cancer immunotherapy using checkpoint blockade. Science 359, 1350–1355 (2018).
Spits, H. et al. Innate lymphoid cells—a proposal for uniform nomenclature. Nat. Rev. Immunol. 13, 145–149 (2013).
Vosshenrich, C. A. et al. A thymic pathway of mouse natural killer cell development characterized by expression of GATA-3 and CD127. Nat. Immunol. 7, 1217–1224 (2006).
Cuff, A. O. et al. The obese liver environment mediates conversion of NK cells to a less cytotoxic ILC1-like phenotype. Front. Immunol. 10, 2180 (2019).
Gao, Y. et al. Tumor immunoevasion by the conversion of effector NK cells into type 1 innate lymphoid cells. Nat. Immunol. 18, 1004–1015 (2017). This paper demonstrates that ILC1 can express an intermediate phenotype and this hinders ILC1s, limiting their capacity to contain tumor cells in vivo.
Ricardo-Gonzalez, R. R. et al. Tissue signals imprint ILC2 identity with anticipatory function. Nat. Immunol. 10, 1093–1099 (2018).
Luci, C. et al. Influence of the transcription factor RORγt on the development of NKp46+ cell populations in gut and skin. Nat. Immunol. 10, 75–82 (2009).
Simoni, Y. et al. Human innate lymphoid cell subsets possess tissue-type based heterogeneity in phenotype and frequency. Immunity 46, 148–161 (2017).
Constantinides, M. G., McDonald, B. D., Verhoef, P. A. & Bendelac, A. A committed precursor to innate lymphoid cells. Nature 508, 397–401 (2014).
Viant, C. et al. Transforming growth factor-beta and Notch ligands act as opposing environmental cues in regulating the plasticity of type 3 innate lymphoid cells. Sci. Signal 9, ra46 (2016).
Gury-BenAri, M. et al. The spectrum and regulatory landscape of intestinal innate lymphoid cells are shaped by the microbiome. Cell 166, 1231–1246 e1213 (2016).
Rankin, L. C. et al. The transcription factor T-bet is essential for the development of NKp46+ innate lymphocytes via the Notch pathway. Nat. Immunol. 14, 389–395 (2013).
Guo, X. et al. Induction of innate lymphoid cell-derived interleukin-22 by the transcription factor STAT3 mediates protection against intestinal infection. Immunity 40, 25–39 (2014).
Sonnenberg, G. F. et al. Innate lymphoid cells promote anatomical containment of lymphoid-resident commensal bacteria. Science 336, 1321–1325 (2012).
Mao, K. et al. Innate and adaptive lymphocytes sequentially shape the gut microbiota and lipid metabolism. Nature 554, 255–259 (2018).
Pickard, J. M. et al. Rapid fucosylation of intestinal epithelium sustains host–commensal symbiosis in sickness. Nature 514, 638–641 (2014).
Huang, Y. et al. S1P-dependent interorgan trafficking of group 2 innate lymphoid cells supports host defense. Science 359, 114–119 (2018).
Stier, M. T. et al. IL-33 promotes the egress of group 2 innate lymphoid cells from the bone marrow. J. Exp. Med. 215, 263–281 (2018).
Ricardo-Gonzalez, R. R. et al. Tissue-specific pathways extrude activated ILC2s to disseminate type 2 immunity. J. Exp. Med. 217, e20191172 (2020).
Dutton, E. E. et al. Peripheral lymph nodes contain migratory and resident innate lymphoid cell populations. Sci. Immunol. 4, eaau8082 (2019).
Soriani, A., Stabile, H., Gismondi, A., Santoni, A. & Bernardini, G. Chemokine regulation of innate lymphoid cell tissue distribution and function. Cytokine Growth Factor Rev. 42, 47–55 (2018).
Karta, M. R. et al. β2 integrins rather than β1 integrins mediate Alternaria-induced group 2 innate lymphoid cell trafficking to the lung. J. Allergy Clin. Immunol. 141, 329–338 e312 (2018).
Munneke, J. M. et al. Activated innate lymphoid cells are associated with a reduced susceptibility to graft-versus-host disease. Blood 124, 812–821 (2014).
Loyon, R. et al. Peripheral innate lymphoid cells are increased in first line metastatic colorectal carcinoma patients: a negative correlation with TH1 immune responses. Front. Immunol. 10, 2121 (2019).
Bie, Q. et al. Polarization of ILC2s in peripheral blood might contribute to immunosuppressive microenvironment in patients with gastric cancer. J. Immunol. Res. 2014, 923135 (2014).
de Weerdt, I. et al. Innate lymphoid cells are expanded and functionally altered in chronic lymphocytic leukemia. Haematologica 101, e461–e464 (2016).
Irshad, S. et al. RORγt+ innate lymphoid cells promote lymph node metastasis of breast cancers. Cancer Res. 77, 1083–1096 (2017).
Jacquelot, N. et al. Blockade of the co-inhibitory molecule PD-1 unleashes ILC2-dependent antitumor immunity in melanoma. Nat. Immunol. 22, 851–864 (2021). This paper demonstrates that ILC2s tread a fine line in pro- and anti-tumoral activity. This can be manipulated experimentally but requires deeper knowledge of the molecular pathways guiding ILCs within the tumor microenvironment.
Long, A. et al. Type 2 innate lymphoid cells impede IL-33-mediated tumor suppression. J. Immunol. 201, 3456–3464 (2018).
Cortez, V. S. et al. Transforming growth factor-beta signaling guides the differentiation of innate lymphoid cells in salivary glands. Immunity 44, 1127–1139 (2016).
Park, E. et al. Toxoplasma gondii infection drives conversion of NK cells into ILC1-like cells. eLife 8, e47605 (2019).
Pikovskaya, O. et al. Cutting edge: eomesodermin is sufficient to direct type 1 innate lymphocyte development into the conventional NK lineage. J. Immunol. 196, 1449–1454 (2016).
Bernink, J. H. et al. Interleukin-12 and -23 control plasticity of CD127+ group 1 and group 3 innate lymphoid cells in the intestinal lamina propria. Immunity 43, 146–160 (2015).
Koh, J. et al. IL23-producing human lung cancer cells promote tumor growth via conversion of innate lymphoid cell 1 (ILC1) into ILC3. Clin. Cancer Res. 25, 4026–4037 (2019).
Huang, Y. et al. IL-25-responsive, lineage-negative KLRG1hi cells are multipotential ‘inflammatory’ type 2 innate lymphoid cells. Nat. Immunol. 16, 161–169 (2015).
Silver, J. S. et al. Inflammatory triggers associated with exacerbations of COPD orchestrate plasticity of group 2 innate lymphoid cells in the lungs. Nat. Immunol. 17, 626–635 (2016).
Ohne, Y. et al. IL-1 is a critical regulator of group 2 innate lymphoid cell function and plasticity. Nat. Immunol. 17, 646–655 (2016).
Bal, S. M. et al. IL-1β, IL-4 and IL-12 control the fate of group 2 innate lymphoid cells in human airway inflammation in the lungs. Nat. Immunol. 17, 636–645 (2016). The three reports of Silver et al., Ohne et al., and Bal et al. first highlighted the enormous plasticity of ILC2s regulated by cytokine receptor signalling in the inflammatory environment.
Lim, A. I. et al. IL-12 drives functional plasticity of human group 2 innate lymphoid cells. J. Exp. Med. 213, 569–583 (2016).
Zhang, K. et al. Cutting edge: Notch signaling promotes the plasticity of group-2 innate lymphoid cells. J. Immunol. 198, 1798–1803 (2017).
Cai, T. et al. IL-17-producing ST2+ group 2 innate lymphoid cells play a pathogenic role in lung inflammation. J. Allergy Clin. Immunol. 143, 229–244 e229 (2019).
Bernink, J. H. et al. c-Kit-positive ILC2s exhibit an ILC3-like signature that may contribute to IL-17-mediated pathologies. Nat. Immunol. 20, 992–1003 (2019).
Bielecki, P. et al. Skin-resident innate lymphoid cells converge on a pathogenic effector state. Nature 592, 128–132 (2021).
Parker, M. E. et al. c-Maf regulates the plasticity of group 3 innate lymphoid cells by restraining the type 1 program. J. Exp. Med. 217, e20191030 (2020).
Tizian, C. et al. c-Maf restrains T-bet-driven programming of CCR6-negative group 3 innate lymphoid cells. eLife 9, e52549 (2020).
Raykova, A. et al. Interleukins 12 and 15 induce cytotoxicity and early NK-cell differentiation in type 3 innate lymphoid cells. Blood Adv. 1, 2679–2691 (2017).
Hughes, T. et al. The transcription factor AHR prevents the differentiation of a stage 3 innate lymphoid cell subset to natural killer cells. Cell Rep. 8, 150–162 (2014).
Hanahan, D. Hallmarks of cancer: new dimensions. Cancer Discov. 12, 31–46 (2022).
Savage, P. A. et al. Recognition of a ubiquitous self antigen by prostate cancer-infiltrating CD8+ T lymphocytes. Science 319, 215–220 (2008).
Saranchova, I. et al. Type 2 innate lymphocytes actuate immunity against tumours and limit cancer metastasis. Sci. Rep. 8, 2924 (2018).
Gasteiger, G., Fan, X., Dikiy, S., Lee, S. Y. & Rudensky, A. Y. Tissue residency of innate lymphoid cells in lymphoid and nonlymphoid organs. Science 350, 981–985 (2015).
Dogra, P. et al. Tissue determinants of human NK cell development, function, and residence. Cell 180, 749–763(2020).
Bai, L. et al. Liver type 1 innate lymphoid cells develop locally via an interferon-γ-dependent loop. Science 371, eaba4177 (2021). In this paper, the generation of organ-specific ILC1 progenitors are identified, raising the notion that tissue-resident ILCs specific to tumor types may need to be considered.
Demaria, O. et al. Harnessing innate immunity in cancer therapy. Nature 574, 45–56 (2019).
Ducimetiere, L. et al. Conventional NK cells and tissue-resident ILC1s join forces to control liver metastasis. Proc. Natl Acad. Sci. USA 118, e2026271118 (2021).
Dadi, S. et al. Cancer immunosurveillance by tissue-resident innate lymphoid cells and innate-like T cells. Cell 164, 365–377 (2016).
Sun, C., Sun, H., Zhang, C. & Tian, Z. NK cell receptor imbalance and NK cell dysfunction in HBV infection and hepatocellular carcinoma. Cell. Mol. Immunol. 12, 292–302 (2015).
Han, X., Huang, T. & Han, J. Cytokines derived from innate lymphoid cells assist Helicobacter hepaticus to aggravate hepatocellular tumorigenesis in viral transgenic mice. Gut Pathog. 11, 23 (2019).
Ercolano, G. et al. Immunosuppressive mediators impair proinflammatory innate lymphoid cell function in human malignant melanoma. Cancer Immunol. Res. 8, 556–564 (2020).
Rethacker, L. et al. Specific patterns of blood ILCs in metastatic melanoma patients and their modulations in response to immunotherapy. Cancers 13, 1446 (2021).
Zhang, S., Zhao, J., Bai, X., Handley, M. & Shan, F. Biological effects of IL-15 on immune cells and its potential for the treatment of cancer. Int. Immunopharmacol. 91, 107318 (2021).
Moreno-Nieves, U. Y. et al. Landscape of innate lymphoid cells in human head and neck cancer reveals divergent NK cell states in the tumor microenvironment. Proc. Natl Acad. Sci. USA 118, e2101169118 (2021).
Delconte, R. B. et al. The helix-loop-helix protein ID2 governs NK cell fate by tuning their sensitivity to interleukin-15. Immunity 44, 103–115 (2016).
Trabanelli, S. et al. CD127+ innate lymphoid cells are dysregulated in treatment naive acute myeloid leukemia patients at diagnosis. Haematologica 100, e257–e260 (2015).
Chevalier, M. F. et al. ILC2-modulated T cell-to-MDSC balance is associated with bladder cancer recurrence. J. Clin. Invest. 127, 2916–2929 (2017).
Trabanelli, S. et al. Tumour-derived PGD2 and NKp30–B7H6 engagement drives an immunosuppressive ILC2–MDSC axis. Nat. Commun. 8, 593 (2017).
Kim, J. et al. Intratumorally establishing type 2 innate lymphoid cells blocks tumor growth. J. Immunol. 196, 2410–2423 (2016).
Moral, J. A. et al. ILC2s amplify PD-1 blockade by activating tissue-specific cancer immunity. Nature 579, 130–135 (2020). This study demonstrated the synergy between tumor-infiltrating ILC2s, CD103+ dendritic cells and T cells in the context of antibody-mediated PD-1 blockade and the capacity of this approach to relieve cell-intrinsic PD-1 inhibition to augment ILC2 expansion and promote T cell responses.
Wang, S. et al. Transdifferentiation of tumor infiltrating innate lymphoid cells during progression of colorectal cancer. Cell Res. 30, 610-622 (2020). This study describes that TGF-β signaling in colorectal cancer induces the conversion of ILC3s into IL-10-producing regulatory ILCs, which promotes tumor progression. Blockade of TGF-β signaling inhibited the transdifferentiation and tumor growth of this subset.
Kirchberger, S. et al. Innate lymphoid cells sustain colon cancer through production of interleukin-22 in a mouse model. J. Exp. Med. 210, 917–931 (2013).
Chan, I. H. et al. Interleukin-23 is sufficient to induce rapid de novo gut tumorigenesis, independent of carcinogens, through activation of innate lymphoid cells. Mucosal Immunol. 7, 842–856 (2014).
Nussbaum, K. et al. Tissue microenvironment dictates the fate and tumor-suppressive function of type 3 ILCs. J. Exp. Med. 214, 2331–2347 (2017).
Xuan, X. et al. ILC3 cells promote the proliferation and invasion of pancreatic cancer cells through IL-22/AKT signaling. Clin. Transl. Oncol. 22, 563–575 (2020).
Liu, Y. et al. NCR– group 3 innate lymphoid cells orchestrate IL-23/IL-17 axis to promote hepatocellular carcinoma development. EBioMedicine 41, 333–344 (2019).
Jacquelot, N., Tellier, J., Nutt, S. L. & Belz, G. T. Tertiary lymphoid structures and B lymphocytes in cancer prognosis and response to immunotherapies. Oncoimmunology 10, 1900508 (2021).
Carrega, P. et al. NCR+ ILC3 concentrate in human lung cancer and associate with intratumoral lymphoid structures. Nat. Commun. 6, 8280 (2015).
Cella, M. et al. A human natural killer cell subset provides an innate source of IL-22 for mucosal immunity. Nature 457, 722–725 (2009).
Satoh-Takayama, N. et al. IL-7 and IL-15 independently program the differentiation of intestinal CD3-NKp46+ cell subsets from Id2-dependent precursors. J. Exp. Med. 207, 273–280 (2010).
Moro, K. et al. Innate production of TH2 cytokines by adipose tissue-associated c-Kit+Sca-1+ lymphoid cells. Nature 463, 540–544 (2010).
Neill, D. R. et al. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature 464, 1367–1370 (2010).
Sanos, S. L. et al. RORγt and commensal microflora are required for the differentiation of mucosal interleukin 22-producing NKp46+ cells. Nat. Immunol. 10, 83–91 (2009).
Goc, J. et al. Dysregulation of ILC3s unleashes progression and immunotherapy resistance in colon cancer. Cell 184, 5015–5030 (2021). This study shows that in colorectal cancer, ILC3s show altered function and exhibit increased plasticity toward ILC1s. The authors show that MHC-class-II-expressing ILC3s protect against the development and progression of colorectal cancer while protecting against resistance to anti-PD-1 treatment.
Yudanin, N. A. et al. Spatial and temporal mapping of human innate lymphoid cells reveals elements of tissue specificity. Immunity 50, 505–519 e504 (2019).
Salimi, M. et al. Activated innate lymphoid cell populations accumulate in human tumour tissues. BMC Cancer 18, 341 (2018).
Kini Bailur, J. et al. Changes in bone marrow innate lymphoid cell subsets in monoclonal gammopathy: target for IMiD therapy. Blood Adv. 1, 2343–2347 (2017).
Trabanelli, S. et al. Human innate lymphoid cells (ILCs): Toward a uniform immune-phenotyping. Cytometry B Clin. Cytom. 94, 392–399 (2018).
Chiossone, L., Dumas, P. Y., Vienne, M. & Vivier, E. Natural killer cells and other innate lymphoid cells in cancer. Nat. Rev. Immunol. 18, 671–688 (2018).
Myers, J. A. & Miller, J. S. Exploring the NK cell platform for cancer immunotherapy. Nat. Rev. Clin. Oncol. 18, 85–100 (2021).
Daher, M. & Rezvani, K. Outlook for New CAR-based therapies with a focus on CAR NK cells: what lies beyond CAR-Engineered T cells in the race against cancer. Cancer Discov. 11, 45–58 (2021).
Vivier, E. & Malissen, B. Innate and adaptive immunity: specificities and signaling hierarchies revisited. Nat. Immunol. 6, 17–21 (2005).
Liu, E. et al. Use of CAR-transduced natural killer cells in CD19-positive lymphoid tumors. N. Engl. J. Med. 382, 545–553 (2020).
Andre, P. et al. Anti-NKG2A mAb is a checkpoint inhibitor that promotes anti-tumor immunity by unleashing both T and NK cells. Cell. 175, 1731–1743 (2018). In this study, the development of a checkpoint inhibitor that could specifically target NK cells demonstrated the feasibility of directing immunotherapy to innate as well as adaptive immune cells in enhancing therapeutic outcomes.
Gauthier, L. et al. Multifunctional natural killer cell engagers targeting NKp46 trigger protective tumor immunity. Cell 177, 1701–1713 (2019). This paper describes the development of trifunctional NK cell engagers (NKCEs) that express two activating receptors, NKp46 and CD16, together with a tumour-specific antigen. The authors show that NKCEs are more efficient than therapeutic antibodies in controlling tumor growth in murine models.
Demaria, O., Gauthier, L., Debroas, G. & Vivier, E. Natural killer cell engagers in cancer immunotherapy: next generation of immuno-oncology treatments. Eur. J. Immunol. 51, 1934–1942 (2021).
Kerbauy, L. N. et al. Combining AFM13, a bispecific CD30/CD16 antibody, with cytokine-activated blood and cord blood-derived NK cells facilitates CAR-like responses against CD30+ malignancies. Clin. Cancer Res. 27, 3744–3756 (2021).
Hernandez, D. C. et al. An in vitro platform supports generation of human innate lymphoid cells from CD34+ hematopoietic progenitors that recapitulate ex vivo identity. Immunity 54, 2417–2432(2021).
Qi, J. et al. Single-cell transcriptomic landscape reveals tumor specific innate lymphoid cells associated with colorectal cancer progression. Cell Rep. Med. 2, 100353 (2021).
Peng, L., Sun, W., Wei, F., Chen, L. & Wen, W. Interleukin-33 modulates immune responses in cutaneous melanoma in a context-specific way. Aging 13, 6740–6751 (2021).
Huang, Q. et al. Type 2 innate lymphoid cells protect against colorectal cancer progression and predict improved patient survival. Cancers 3, 559 (2021).
Wagner, M. et al. Tumor-derived lactic acid contributes to the paucity of intratumoral ILC2s. Cell Rep. 30, 2743–2757(2020).
Acknowledgements
This work was supported by grants and fellowships from the National Health and Medical Research Council (NHMRC) of Australia (APP1165443 to C. S., G. T. B. and E. V., and 1122277, 1054925, 1135898, 1123000 to G. T. B.), support from The University of Queensland Chair of Immunology (Diamantina Institute, G. T. B.), Cure Cancer Australia and Cancer Australia through the Cancer Australia Priority-driven Cancer Research Scheme (APP1163990 to N. J.) and Cancer Council NSW (RG21-05 G. T. B. and N. J.). The E.V. laboratory at CIML and Assistance-Publique des Hôpitaux de Marseille is supported by funding from the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation program (TILC, grant agreement no. 694502 and MInfla-TILC, grant agreement no. 875102, MInfla-Tilc), the Agence Nationale de la Recherche including the PIONEER Project (ANR-17-RHUS-0007), MSDAvenir, Innate Pharma and institutional grants awarded to the CIML (INSERM, CNRS, and Aix-Marseille University) and Marseille Immunopole.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
E. V. is an employee of Innate Pharma. The other authors declare no competing interests.
Peer review
Peer review information
Nature Immunology thanks Sonia Tugues and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Zoltan Fehervari was the primary editor on this article and managed its editorial process and peer review in collaboration with the rest of the editorial team
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jacquelot, N., Seillet, C., Vivier, E. et al. Innate lymphoid cells and cancer. Nat Immunol 23, 371–379 (2022). https://doi.org/10.1038/s41590-022-01127-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41590-022-01127-z