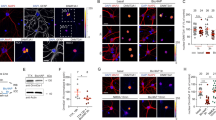
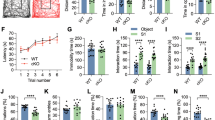
Abstract
DNA methylation–dependent epigenetic mechanisms underlie the development and function of the mammalian brain. MeCP2 is highly expressed in neurons and functions as a molecular linker between DNA methylation, chromatin remodeling and transcription regulation. Previous in vitro studies have shown that neuronal activity–induced phosphorylation (NAIP) of methyl CpG–binding protein 2 (MeCP2) precedes its release from the Bdnf promoter and the ensuing Bdnf transcription. However, the in vivo function of this phosphorylation event remains elusive. We generated knock-in mice that lack NAIP of MeCP2 and found that they performed better in hippocampus-dependent memory tests, presented enhanced long-term potentiation at two synapses in the hippocampus and showed increased excitatory synaptogenesis. At the molecular level, the phospho-mutant MeCP2 protein bound more tightly to several MeCP2 target gene promoters and altered the expression of these genes. Our results suggest that NAIP of MeCP2 is required for modulating dynamic functions of the adult mouse brain.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Miller, C.A. & Sweatt, J.D. Covalent modification of DNA regulates memory formation. Neuron 53, 857–869 (2007).
Miller, C.A. et al. Cortical DNA methylation maintains remote memory. Nat. Neurosci. 13, 664–666 (2010).
Feng, J. et al. Dnmt1 and Dnmt3a maintain DNA methylation and regulate synaptic function in adult forebrain neurons. Nat. Neurosci. 13, 423–430 (2010).
Lewis, J.D. et al. Purification, sequence, and cellular localization of a novel chromosomal protein that binds to methylated DNA. Cell 69, 905–914 (1992).
Nan, X., Campoy, F.J. & Bird, A. MeCP2 is a transcriptional repressor with abundant binding sites in genomic chromatin. Cell 88, 471–481 (1997).
Jones, P.L. et al. Methylated DNA and MeCP2 recruit histone deacetylase to repress transcription. Nat. Genet. 19, 187–191 (1998).
Bird, A.P. & Wolffe, A.P. Methylation-induced repression: belts, braces, and chromatin. Cell 99, 451–454 (1999).
Chahrour, M. et al. MeCP2, a key contributor to neurological disease, activates and represses transcription. Science 320, 1224–1229 (2008).
Yasui, D.H. et al. Integrated epigenomic analyses of neuronal MeCP2 reveal a role for long-range interaction with active genes. Proc. Natl. Acad. Sci. USA 104, 19416–19421 (2007).
Amir, R.E. et al. Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2. Nat. Genet. 23, 185–188 (1999).
Van den Veyver, I.B. & Zoghbi, H.Y. Methyl-CpG-binding protein 2 mutations in Rett syndrome. Curr. Opin. Genet. Dev. 10, 275–279 (2000).
Wan, M. et al. Rett syndrome and beyond: recurrent spontaneous and familial MECP2 mutations at CpG hotspots. Am. J. Hum. Genet. 65, 1520–1529 (1999).
Xiang, F. et al. Mutation screening in Rett syndrome patients. J. Med. Genet. 37, 250–255 (2000).
Hagberg, B. Rett's syndrome: prevalence and impact on progressive severe mental retardation in girls. Acta Paediatr. Scand. 74, 405–408 (1985).
Hagberg, B. & Hagberg, G. Rett syndrome: epidemiology and geographical variability. Eur. Child Adolesc. Psychiatry 6 (suppl. 1), 5–7 (1997).
Chen, W.G. et al. Derepression of BDNF transcription involves calcium-dependent phosphorylation of MeCP2. Science 302, 885–889 (2003).
Zhou, Z. et al. Brain-specific phosphorylation of MeCP2 regulates activity-dependent Bdnf transcription, dendritic growth, and spine maturation. Neuron 52, 255–269 (2006).
Tao, J. et al. Phosphorylation of MeCP2 at Serine 80 regulates its chromatin association and neurological function. Proc. Natl. Acad. Sci. USA 106, 4882–4887 (2009).
Deng, J.V. et al. MeCP2 in the nucleus accumbens contributes to neural and behavioral responses to psychostimulants. Nat. Neurosci. 13, 1128–1136 (2010).
Martinowich, K. et al. DNA methylation-related chromatin remodeling in activity-dependent BDNF gene regulation. Science 302, 890–893 (2003).
Collins, A.L. et al. Mild overexpression of MeCP2 causes a progressive neurological disorder in mice. Hum. Mol. Genet. 13, 2679–2689 (2004).
Chao, H.T., Zoghbi, H.Y. & Rosenmund, C. MeCP2 controls excitatory synaptic strength by regulating glutamatergic synapse number. Neuron 56, 58–65 (2007).
Skene, P.J. et al. Neuronal MeCP2 is expressed at near histone-octamer levels and globally alters the chromatin state. Mol. Cell 37, 457–468 (2010).
Acknowledgements
We thank A. Messing, J. Svaren and J. Yin for critically reading the manuscript, S. Marwaha for assistance with mouse work, and M. Andrzejewski for assistance with statistical analysis. H.L. was supported by a pre-doctoral fellowship from the Stem Cell and Regenerative Medicine Center at the University of Wisconsin, Madison. E.C.W. was supported by a predoctoral training grant (5T32GM07133) and a graduate student fellowship from the Friends of the Waisman Center. Q.C. was supported by a Young Investigator Award from the National Alliance for Research on Schizophrenia and Depression. This work was partially supported by grants from the National Institute of Child Health and Human Development (R01 HD064743 and R21 HD066560 to Q.C., and P30 HD03352 to the Waisman Center).
Author information
Authors and Affiliations
Contributions
Q.C. directed the studies. H.L., X.Z. and Q.C. conceived and designed the experiments. H.L. performed the western blot, mouse behavioral tests, ChIP experiments, gene expression studies, imaging and image analysis. K.F.C. performed mouse behavioral tests. X.Z. performed all of the electrophysiology experiments, neuronal culture and hippocampal slice experiments, and immunostaining. E.C.W. performed immunostaining. The paper was written by Q.C., H.L. and X.Z. and commented on by all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–8 (PDF 4800 kb)
Rights and permissions
About this article
Cite this article
Li, H., Zhong, X., Chau, K. et al. Loss of activity-induced phosphorylation of MeCP2 enhances synaptogenesis, LTP and spatial memory. Nat Neurosci 14, 1001–1008 (2011). https://doi.org/10.1038/nn.2866
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.2866