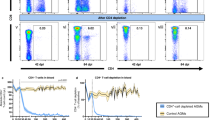
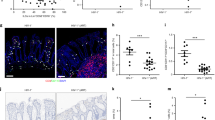
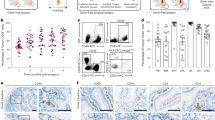
Abstract
Chronic activation of the immune system is a hallmark of progressive HIV infection and better predicts disease outcome than plasma viral load, yet its etiology remains obscure. Here we show that circulating microbial products, probably derived from the gastrointestinal tract, are a cause of HIV-related systemic immune activation. Circulating lipopolysaccharide, which we used as an indicator of microbial translocation, was significantly increased in chronically HIV-infected individuals and in simian immunodeficiency virus (SIV)-infected rhesus macaques (P ≤ 0.002). We show that increased lipopolysaccharide is bioactive in vivo and correlates with measures of innate and adaptive immune activation. Effective antiretroviral therapy seemed to reduce microbial translocation partially. Furthermore, in nonpathogenic SIV infection of sooty mangabeys, microbial translocation did not seem to occur. These data establish a mechanism for chronic immune activation in the context of a compromised gastrointestinal mucosal surface and provide new directions for therapeutic interventions that modify the consequences of acute HIV infection.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Lane, H.C. et al. Abnormalities of B-cell activation and immunoregulation in patients with the acquired immunodeficiency syndrome. N. Engl. J. Med. 309, 453–458 (1983).
Hellerstein, M. et al. Directly measured kinetics of circulating T lymphocytes in normal and HIV-1-infected humans. Nat. Med. 5, 83–89 (1999).
Hazenberg, M.D. et al. T-cell division in human immunodeficiency virus (HIV)-1 infection is mainly due to immune activation: a longitudinal analysis in patients before and during highly active antiretroviral therapy (HAART). Blood 95, 249–255 (2000).
Valdez, H. & Lederman, M.M. Cytokines and cytokine therapies in HIV infection. AIDS Clin. Rev. 187–228 (1997).
Giorgi, J.V. et al. Shorter survival in advanced human immunodeficiency virus type 1 infection is more closely associated with T lymphocyte activation than with plasma virus burden or virus chemokine coreceptor usage. J. Infect. Dis. 179, 859–870 (1999).
Brenchley, J.M., Price, D.A. & Douek, D.C. HIV disease: fallout from a mucosal catastrophe? Nat. Immunol. 7, 235–239 (2006).
Grossman, Z., Meier-Schellersheim, M., Paul, W.E. & Picker, L.J. Pathogenesis of HIV infection: what the virus spares is as important as what it destroys. Nat. Med. 12, 289–295 (2006).
Mattapallil, J.J. et al. Massive infection and loss of memory CD4+ T cells in multiple tissues during acute SIV infection. Nature 434, 1093–1097 (2005).
Veazey, R.S. et al. Gastrointestinal tract as a major site of CD4+ T cell depletion and viral replication in SIV infection. Science 280, 427–431 (1998).
Guadalupe, M. et al. Severe CD4+ T-cell depletion in gut lymphoid tissue during primary human immunodeficiency virus type 1 infection and substantial delay in restoration following highly active antiretroviral therapy. J. Virol. 77, 11708–11717 (2003).
Brenchley, J.M. et al. CD4+ T cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract. J. Exp. Med. 200, 749–759 (2004).
Mehandru, S. et al. Primary HIV-1 infection is associated with preferential depletion of CD4+ T lymphocytes from effector sites in the gastrointestinal tract. J. Exp. Med. 200, 761–770 (2004).
Picker, L.J. et al. Insufficient production and tissue delivery of CD4+ memory T cells in rapidly progressive simian immunodeficiency virus infection. J. Exp. Med. 200, 1299–1314 (2004).
George, M.D., Reay, E., Sankaran, S. & Dandekar, S. Early antiretroviral therapy for simian immunodeficiency virus infection leads to mucosal CD4+ T-cell restoration and enhanced gene expression regulating mucosal repair and regeneration. J. Virol. 79, 2709–2719 (2005).
Kotler, D.P. HIV infection and the gastrointestinal tract. AIDS 19, 107–117 (2005).
Sharpstone, D. et al. Small intestinal transit, absorption, and permeability in patients with AIDS with and without diarrhoea. Gut 45, 70–76 (1999).
Macpherson, A.J. & Harris, N.L. Interactions between commensal intestinal bacteria and the immune system. Nat. Rev. Immunol. 4, 478–485 (2004).
Cooke, K.R., Olkiewicz, K., Erickson, N. & Ferrara, J.L. The role of endotoxin and the innate immune response in the pathophysiology of acute graft versus host disease. J. Endotoxin Res. 8, 441–448 (2002).
Caradonna, L. et al. Enteric bacteria, lipopolysaccharides and related cytokines in inflammatory bowel disease: biological and clinical significance. J. Endotoxin Res. 6, 205–214 (2000).
Takeda, K., Kaisho, T. & Akira, S. Toll-like receptors. Annu. Rev. Immunol. 21, 335–376 (2003).
Hill, G.R. et al. Differential roles of IL-1 and TNF-α on graft-versus-host disease and graft versus leukemia. J. Clin. Invest. 104, 459–467 (1999).
Schietroma, M. et al. Intestinal and systemic endotoxaemia after laparotomic or laparoscopic cholecystectomy. Chir. Ital. 58, 171–177 (2006).
Wellmann, W., Fink, P.C., Benner, F. & Schmidt, F.W. Endotoxaemia in active Crohn's disease. Treatment with whole gut irrigation and 5-aminosalicylic acid. Gut 27, 814–820 (1986).
Wyatt, J., Vogelsang, H., Hubl, W., Waldhoer, T. & Lochs, H. Intestinal permeability and the prediction of relapse in Crohn's disease. Lancet 341, 1437–1439 (1993).
Schietroma, M., Carlei, F., Cappelli, S. & Amicucci, G. Intestinal permeability and systemic endotoxemia after laparotomic or laparoscopic cholecystectomy. Ann. Surg. 243, 359–363 (2006).
Kitchens, R.L. & Thompson, P.A. Modulatory effects of sCD14 and LBP on LPS-host cell interactions. J. Endotoxin Res. 11, 225–229 (2005).
Lien, E. et al. Elevated levels of serum-soluble CD14 in human immunodeficiency virus type 1 (HIV-1) infection: correlation to disease progression and clinical events. Blood 92, 2084–2092 (1998).
Nomura, F. et al. Cutting edge: endotoxin tolerance in mouse peritoneal macrophages correlates with down-regulation of surface toll-like receptor 4 expression. J. Immunol. 164, 3476–3479 (2000).
Strutz, F., Heller, G., Krasemann, K., Krone, B. & Muller, G.A. Relationship of antibodies to endotoxin core to mortality in medical patients with sepsis syndrome. Intensive Care Med. 25, 435–444 (1999).
Cohen, I.R. & Norins, L.C. Natural human antibodies to gram-negative bacteria: immunoglobulins G, A, and M. Science 152, 1257–1259 (1966).
Barclay, G.R. Endogenous endotoxin-core antibody (EndoCAb) as a marker of endotoxin exposure and a prognostic indicator: a review. Prog. Clin. Biol. Res. 392, 263–272 (1995).
Titanji, K. et al. Loss of memory B cells impairs maintenance of long-term serological memory during HIV-1 infection. Blood 108, 1580–1587 (2006).
Ito, T., Kanzler, H., Duramad, O., Cao, W. & Liu, Y.J. Specialization, kinetics, and repertoire of type 1 interferon responses by human plasmacytoid predendritic cells. Blood 107, 2423–2431 (2006).
Aziz, N. et al. Stability of plasma levels of cytokines and soluble activation markers in patients with human immunodeficiency virus infection. J. Infect. Dis. 179, 843–848 (1999).
Haas, D.W. et al. Proinflammatory cytokine and human immunodeficiency virus RNA levels during early Mycobacterium avium complex bacteremia in advanced AIDS. J. Infect. Dis. 177, 1746–1749 (1998).
MacArthur, R.D. et al. Effects of mycobacterium avium complex-infection treatment on cytokine expression in human immunodeficiency virus-infected persons: results of AIDS clinical trials group protocol 853. J. Infect. Dis. 181, 1486–1490 (2000).
Beignon, A.S. et al. Endocytosis of HIV-1 activates plasmacytoid dendritic cells via Toll-like receptor-viral RNA interactions. J. Clin. Invest. 115, 3265–3275 (2005).
Caron, G. et al. Direct stimulation of human T cells via TLR5 and TLR7/8: flagellin and R-848 up-regulate proliferation and IFN-gamma production by memory CD4+ T cells. J. Immunol. 175, 1551–1557 (2005).
Kovacs, J.A. et al. Identification of dynamically distinct subpopulations of T lymphocytes that are differentially affected by HIV. J. Exp. Med. 194, 1731–1741 (2001).
Lederman, M.M. et al. Immunologic responses associated with 12 weeks of combination antiretroviral therapy consisting of zidovudine, lamivudine, and ritonavir: results of AIDS clinical trials group protocol 315. J. Infect. Dis. 178, 70–79 (1998).
Bafica, A., Scanga, C.A., Schito, M., Chaussabel, D. & Sher, A. Influence of coinfecting pathogens on HIV expression: evidence for a role of Toll-like receptors. J. Immunol. 172, 7229–7234 (2004).
Morris, L. et al. HIV-1 antigen-specific and -nonspecific B cell responses are sensitive to combination antiretroviral therapy. J. Exp. Med. 188, 233–245 (1998).
Emu, B. et al. Phenotypic, functional, and kinetic parameters associated with apparent T-cell control of human immunodeficiency virus replication in individuals with and without antiretroviral treatment. J. Virol. 79, 14169–14178 (2005).
Holmes, C.L., Russell, J.A. & Walley, K.R. Genetic polymorphisms in sepsis and septic shock: role in prognosis and potential for therapy. Chest 124, 1103–1115 (2003).
Sankaran, S. et al. Gut mucosal T cell responses and gene expression correlate with protection against disease in long-term HIV-1-infected nonprogressors. Proc. Natl. Acad. Sci. USA 102, 9860–9865 (2005).
Silvestri, G. et al. Nonpathogenic SIV infection of sooty mangabeys is characterized by limited bystander immunopathology despite chronic high-level viremia. Immunity 18, 441–452 (2003).
Zaunders, J.J. et al. Increased turnover of CCR5+ and redistribution of CCR5- CD4 T lymphocytes during primary human immunodeficiency virus type 1 infection. J. Infect. Dis. 183, 736–743 (2001).
Skoner, D.P., Gentile, D.A., Patel, A. & Doyle, W.J. Evidence for cytokine mediation of disease expression in adults experimentally infected with influenza A virus. J. Infect. Dis. 180, 10–14 (1999).
Veazey, R.S. et al. Protection of macaques from vaginal SHIV challenge by vaginally delivered inhibitors of virus-cell fusion. Nature 438, 99–102 (2005).
Fort, M.M. et al. A synthetic TLR4 antagonist has anti-inflammatory effects in two murine models of inflammatory bowel disease. J. Immunol. 174, 6416–6423 (2005).
Acknowledgements
We would like to thank G. Barclay for information on the origins of EndoCab. The authors would like to acknowledge the Bad Boys of Cleveland (BBC), J. Spritzler, R. Seder and R. Koup for advice and discussion. This study was supported by grants from the US National Institutes of Health (AI 25879, AI 38858 and AI 36219 to M.M.L.; ROI AI052755 and AI066998 to G.S.; and AI052745, AI41531, P30 AI27763, P30 MH62246 and MO1-RR0083-37 to the UCSF cohort study) and the Yerkes Primate Center (RR-00165 to G.S.). O.L. is supported by grants from the French National Agency for Research on Aids and Viral Hepatitis (ANRS) and is a member of the ANRS EP36 study group. D.A.P. is a Senior Clinical Fellow of the Medical Research Council (UK). This research was supported in part by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases (NIAID), US National Institutes of Health.
Author information
Authors and Affiliations
Contributions
J.M.B., D.A.P., T.E.A., Z.K., E.B., D.A. and D.C.D. conducted the in vitro experiments; J.M.B. and D.C.D conducted the data analyses; T.W.S., O.L., B.R., L.T.-J., A.L., J.N.M., F.M.H., M.M.L. and S.G.D. procured samples and recruited subjects for the human clinical studies; G.S., S.R. and L.J.P. led the studies in SIV-infected monkeys. All authors contributed to the project's planning and writing of the manuscript; D.C.D. supervised the project.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
Plasma LBP and sCD14 levels. (PDF 89 kb)
Supplementary Fig. 2
Stimulation of PBMC from uninfected individuals with plasma from HIV-infected and uninfected individuals. (PDF 176 kb)
Supplementary Fig. 3
EndoCAb and plasma LPS in controllers. (PDF 544 kb)
Rights and permissions
About this article
Cite this article
Brenchley, J., Price, D., Schacker, T. et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med 12, 1365–1371 (2006). https://doi.org/10.1038/nm1511
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm1511