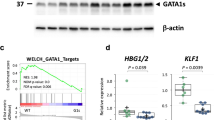
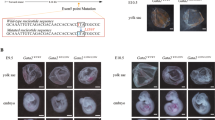
Abstract
Children with Down syndrome have a 10–20-fold elevated risk of developing leukemia, particularly acute megakaryoblastic leukemia (AMKL)1. While a subset of pediatric AMKLs is associated with the 1;22 translocation and expression of a mutant fusion protein2,3, the genetic alterations that promote Down syndrome–related AMKL (DS-AMKL) have remained elusive. Here we show that leukemic cells from every individual with DS-AMKL that we examined contain mutations in GATA1, encoding the essential hematopoietic transcription factor GATA1 (GATA binding protein 1 or globin transcription factor 1). Each mutation results in the introduction of a premature stop codon in the gene sequence that encodes the amino-terminal activation domain. These mutations prevent synthesis of full-length GATA1, but not synthesis of a shorter variant that is initiated downstream. We show that the shorter GATA1 protein, which lacks the N-terminal activation domain, binds DNA and interacts with its essential cofactor Friend of GATA1 (FOG1; encoded by ZFPM1)4 to the same extent as does full-length GATA1, but has a reduced transactivation potential. Although some reports suggest that the activation domain is dispensable in cell-culture models of hematopoiesis5,6, one study has shown that it is required for normal development in vivo7. Together, these findings indicate that loss of wildtype GATA1 constitutes one step in the pathogenesis of AMKL in Down syndrome.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Lange, B. The management of neoplastic disorders of haematopoiesis in children with Down's syndrome. Br. J. Haematol. 110, 512–524 (2000).
Dickstein, J.I., Davis, E.M. & Roulston, D. Localization of the chromosome 22 breakpoints in two cases of acute megakaryoblastic leukemia with t(1;22)(p13;q13). Cancer Genet. Cytogenet. 129, 150–154 (2001).
Ma, Z. et al. Fusion of two novel genes, RBM15 and MKL1, in the t(1;22)(p13;q13) of acute megakaryoblastic leukemia. Nature Genet. 28, 220–221 (2001).
Tsang, A.P. et al. FOG, a multitype zinc finger protein, acts as a cofactor for transcription factor GATA-1 in erythroid and megakaryocytic differentiation. Cell 90, 109–119 (1997).
Visvader, J.E., Crossley, M., Hill, J., Orkin, S.H. & Adams, J.M. The C-terminal zinc finger of GATA-1 or GATA-2 is sufficient to induce megakaryocytic differentiation of an early myeloid cell line. Mol. Cell. Biol. 15, 634–641 (1995).
Weiss, M.J., Yu, C. & Orkin, S.H. Erythroid-cell-specific properties of transcription factor GATA-1 revealed by phenotypic rescue of a gene-targeted cell line. Mol. Cell. Biol. 17, 1642–1651 (1997).
Shimizu, R., Takahashi, S., Ohneda, K., Engel, J.D. & Yamamoto, M. In vivo requirements for GATA-1 functional domains during primitive and definitive erythropoiesis. EMBO J. 20, 5250–5260 (2001).
Nichols, K.E. et al. Familial dyserythropoietic anaemia and thrombocytopenia due to an inherited mutation in GATA1. Nature Genet. 24, 266–270 (2000).
Freson, K. et al. Platelet characteristics in patients with X-linked macrothrombocytopenia because of a novel GATA1 mutation. Blood 98, 85–92 (2001).
Mehaffey, M.G., Newton, A.L., Gandhi, M.J., Crossley, M. & Drachman, J.G. X-linked thrombocytopenia caused by a novel mutation of GATA-1. Blood 98, 2681–2688 (2001).
Orkin, S.H. Diversification of haematopoietic stem cells to specific lineages. Nature Rev. Genet. 1, 57–64 (2000).
Shivdasani, R.A., Fujiwara, Y., McDevitt, M.A. & Orkin, S.H. A lineage-selective knockout establishes the critical role of transcription factor GATA-1 in megakaryocyte growth and platelet development. EMBO J. 16, 3965–3973 (1997).
Vyas, P., Ault, K., Jackson, C.W., Orkin, S.H. & Shivdasani, R.A. Consequences of GATA-1 deficiency in megakaryocytes and platelets. Blood 93, 2867–2875 (1999).
Song, W.J. et al. Haploinsufficiency of CBFA2 causes familial thrombocytopenia with propensity to develop acute myelogenous leukaemia. Nature Genet. 23, 166–175 (1999).
Michaud, J. et al. In vitro analyses of known and novel RUNX1/AML1 mutations in dominant familial platelet disorder with predisposition to acute myelogenous leukemia: implications for mechanisms of pathogenesis. Blood 99, 1364–1372 (2002).
Osato, M. et al. Biallelic and heterozygous point mutations in the runt domain of the AML1 gene associated with myeloblastic leukemias. Blood 93, 1817–1824 (1999).
Preudhomme, C. et al. High incidence of biallelic point mutations in the Runt domain of the AML1/PEBP2αB gene in Mo acute myeloid leukemia and in myeloid malignancies with acquired trisomy 21. Blood 96, 2862–2869 (2000).
Crispino, J.D., Lodish, M.B., MacKay, J.P. & Orkin, S.H. Use of altered specificity mutants to probe a specific protein–protein interaction in differentiation: the GATA-1:FOG complex. Mol. Cell 3, 219–228 (1999).
Calligaris, R., Bottardi, S., Cogoi, S., Apezteguia, I. & Santoro, C. Alternative translation initiation site usage results in two functionally distinct forms of the GATA-1 transcription factor. Proc. Natl Acad. Sci. USA 92, 11598–11602 (1995).
Komatsu, N. et al. Growth and differentiation of a human megakaryoblastic cell line, CMK. Blood 74, 42–48 (1989).
Sato, T. et al. Establishment of a human leukaemic cell line (CMK) with megakaryocytic characteristics from a Down's syndrome patient with acute megakaryoblastic leukaemia. Br. J. Haematol. 72, 184–190 (1989).
Martin, D.I. & Orkin, S.H. Transcriptional activation and DNA binding by the erythroid factor GF-1/NF-E1/Eryf 1. Genes Dev. 4, 1886–1898 (1990).
Fujiwara, Y., Browne, C.P., Cunniff, K., Goff, S.C. & Orkin, S.H. Arrested development of embryonic red cell precursors in mouse embryos lacking transcription factor GATA-1. Proc. Natl Acad. Sci. USA 93, 12355–12358 (1996).
McDevitt, M.A., Shivdasani, R.A., Fujiwara, Y., Yang, H. & Orkin, S.H. A 'knockdown' mutation created by cis-element gene targeting reveals the dependence of erythroid cell maturation on the level of transcription factor GATA-1. Proc. Natl Acad. Sci. USA 94, 6781–6785 (1997).
Takahashi, S. et al. Role of GATA-1 in proliferation and differentiation of definitive erythroid and megakaryocytic cells in vivo. Blood 92, 434–442 (1998).
Kelly, L., Clark, J. & Gilliland, D.G. Comprehensive genotypic analysis of leukemia: clinical and therapeutic implications. Curr. Opin. Oncol. 14, 10–18 (2002).
Busson-Le Coniat, M., Nguyen Khac, F., Daniel, M.T., Bernard, O.A. & Berger, R. Chromosome 21 abnormalities with AML1 amplification in acute lymphoblastic leukemia. Genes Chromosom. Cancer 32, 244–249 (2001).
Dal Cin, P. et al. Amplification of AML1 in childhood acute lymphoblastic leukemias. Genes Chromosom. Cancer 30, 407–409 (2001).
Wang, P. et al. dic(5;17): a recurring abnormality in malignant myeloid disorders associated with mutations of TP53. Genes Chromosom. Cancer 20, 282–291 (1997).
Trainor, C.D. et al. A palindromic regulatory site within vertebrate GATA-1 promoters requires both zinc-fingers of the GATA-1 DNA-binding domain for high- affinity interaction. Mol. Cell. Biol. 16, 2238–2247 (1996).
Acknowledgements
We thank K. Nichols, S. Orkin, K. Shannon and M. Weiss for insight and discussion; S. Droho and E. Smith for reviewing the manuscript; A. Fernald for assistance with the SSCP assay; and D. Tenen for advice on generating cell lysates. This work was supported, in part, by the Aplastic Anemia and MDS International Foundation (M.G.), the Cancer Research Foundation (J.D.C.) and the Picower Foundation (M.A.M.). J.D.C. is a recipient of a Burroughs Wellcome Fund Career Award in the Biomedical Sciences.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
About this article
Cite this article
Wechsler, J., Greene, M., McDevitt, M. et al. Acquired mutations in GATA1 in the megakaryoblastic leukemia of Down syndrome. Nat Genet 32, 148–152 (2002). https://doi.org/10.1038/ng955
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ng955
This article is cited by
-
Inherent genome instability underlies trisomy 21-associated myeloid malignancies
Leukemia (2024)
-
Prenatal diagnosis of Down syndrome combined with transient abnormal myelopoiesis in foetuses with a GATA1 gene variant: two case reports
Molecular Cytogenetics (2023)
-
Fasudil promotes polyploidization of megakaryoblasts in an acute megakaryocyte leukemia model
Naunyn-Schmiedeberg's Archives of Pharmacology (2023)
-
Effects of aneuploidy on cell behaviour and function
Nature Reviews Molecular Cell Biology (2022)
-
c-Mpl-del, a c-Mpl alternative splicing isoform, promotes AMKL progression and chemoresistance
Cell Death & Disease (2022)