Abstract
Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) triggers apoptosis in tumor cells without toxicity to normal cells, but some recombinant versions of TRAIL caused hepatocyte death. We generated fully human monoclonal antibodies (mAbs) that bind specifically to TRAIL receptor 1 (TRAIL-R1) and TRAIL receptor 2 (TRAIL-R2), which mediate apoptosis signal when they ligate with TRAIL, to investigate the contribution of each receptor to induce tumor cell apoptosis and hepatocyte toxicity. All of mAbs to TRAIL-R1 and TRAIL-R2 induced cell death in several cancer cell lines susceptible to TRAIL but not in human umbilical vein endothelial cells in vitro. Both anti-TRAIL-R1 mAbs and anti-TRAIL-R2mAbs also caused cell death in hepatocytes. However, a subset of mAbs to TRAIL-R2, which was characterized by the TRAIL blocking activity, did not show strong hepatocyte toxicity. These results indicate that human normal hepatocytes are susceptible to both TRAIL-R1- and TRAIL-R2-mediated apoptosis signal.
Similar content being viewed by others
Introduction
Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)1,2 induces apoptosis in a wide variety of human cancer cell lines, but not in most normal human cells.3,4 TRAIL exhibits antitumor activity in tumor xenograft models3,4 and when administered in vivo, TRAIL shows little toxicity to normal tissues in mice and in non-human primates.4,5 However, it was reported that normal human hepatocytes, astrocytes and other brain cells were killed by recombinant soluble human TRAIL in vitro,3,6,7 which raised concern about the safety of TRAIL in cancer therapy. Recently, it was demonstrated that the method of preparing recombinant soluble ligand might be important for assessing the results of experiments with TRAIL.8,9
The mechanisms of hepatocyte toxicity by TRAIL remain unclear because of the difficulty in detection of TRAIL receptor expression in normal hepatocytes. TRAIL activates two distinct receptors, TRAIL-R1 (DR4)10 and TRAIL-R2 (DR5),11,12,13,14,15 both possess a death domain in their cytoplasmic tail that can engage the apoptotic machinery. Thus, it is presumed that TRAIL-R1 and TRAIL-R2 or both are involved in the hepatocyte toxicity. Multivalent antibodies directed to these cell surface receptors in the TNF receptor family often mimic the action of the ligand by inducing receptor aggregation and triggering signal transduction pathways.16,17,18 To discern between the signaling of these two death receptors for tumor apoptosis and hepatocyte toxicity, we generated fully human monoclonal antibodies (mAbs) specific to TRAIL-R1 and TRAIL-R2.
Our study shows that the mAbs to TRAIL-R1 and TRAIL-R2 induced apoptosis in several human cancer cells in vitro, and that hepatocyte toxicity was also induced via both TRAIL-R1 and TRAIL-R2. Furthermore, a subset of anti-TRAIL-R2 mAbs characterized by the TRAIL blocking activity was low toxic to hepatocytes, which suggests that these antibodies may provide an alternate approach to circumvent the problems of hepatocyte toxicity, and that this potent biological signaling system may be still utilized to treat human cancer.
Results
Generation of fully human mAbs to TRAIL-R1 and TRAIL-R2
KM mice contain the human chromosome fragment encoding the entire human immunoglobulin heavy-chain loci and the YAC-transgene containing the human immunoglobulin kappa light-chain gene and when immunized produce complete human antibodies.19 We generated fully human mAbs reactive with TRAIL-R1 or TRAIL-R2. All of these mAbs specifically bound to the ectodomain of TRAIL-R1 or TRAIL-R2 transfected in L929 cells as detected by flow cytometric analysis (Figure 1a and b). The selected human mAbs to TRAIL-R1 and TRAIL-R2 demonstrated relatively high affinities to their respective antigens as detected by plasmon resonance assay (Table 1).
In vitro apoptotic activity of human mAbs to TRAIL-R1 and TRAIL-R2
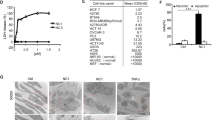
The human mAbs to TRAIL-R1 and TRAIL-R2 were potent inducers of death of Colo205 cells in vitro. In the presence of anti-human IgG, which oligomerizes mAbs, these mAbs demonstrated potent agonistic activities on Colo205 cells in a dose-dependent manner (Figure 2a), whereas mAbs alone showed little cytotoxic activity (data not shown). To determine whether the cell death induced by these mAbs upon crosslinking was apoptosis or not, we measured effector caspase activity in mAbs-treated cancer cell. In this cell line, DEVDase activation was detected within 2 h after the mAbs treatment (Figure 2b).
Apoptosis induced by mAbs via caspase activation. (a) Colo205 cells were incubated with the indicated concentrations of mAbs to TRAIL-R1, R1-A13 (▵), R1-B6 (▴), R1-B12 (◊) and TRAIL-R2, R2-E11 (□), R2-L30 (▪), R2-H48 (○), R2-F4 (•) and control antibody (♦) in the presence of goat anti-human IgG. After cells were incubated for 48 h at 37°C, the cell viability was determined. (b) Colo205 cells were incubated with mAbs to TRAIL-R1 and TRAIL-R2 at 1 μg/ml in the presence (□) or absence (▪) of goat anti-human IgG. After cells were incubated for 2 h at 37°C, the effector caspase activity was measured
These mAbs induced cell death in other human tumor cell lines that are susceptible to TRAIL, such as Jurkat, MDA-MB-231, U251, but not human umbilical vein endothelial cells (HUVECs) (Figure 3), which express TRAIL-R1 and TRAIL-R2 but resistant to TRAIL.20 Although the degree of death varied between these cell lines, the relative potency of each mAb remained the same. However, in U251 glioma cell line the mAbs did not induce death by the treatment with anti-human IgG (data not shown), but required a combination treatment with cis-diamminedichloroplatinum(II) (CDDP), a DNA-damaging agent that resulted in cell death (Figure 3). Recombinant human soluble TRAIL also required CDDP to induce cell death of U251.21 This observation demonstrates that TRAIL and mAbs to TRAIL receptors are similar in their cytotoxic activity on these carcinoma cell lines. The activation of caspase was also detected within 2 h after the mAbs treatment in these cancer cell lines (data not shown). The extent of caspase activation was different between cell lines; however, it was correlated with the cytotoxic activity induced by the mAbs. These results revealed that mAbs to TRAIL-R1 and TRAIL-R2 induced apoptosis of these carcinoma cells via caspase activation.
Effect of mAbs on viability of cancer cell lines and HUVECs. Jurkat, MDA-MB-231, U251 and HUVECs were incubated with the indicated concentrations of mAbs to TRAIL-R1, R1-A13 (▵), R1-B6 (▴), R1-B12 (◊) and TRAIL-R2, R2-E11 (□), R2-L30 (▪), R2-H48 (○), R2-F4 (•) and control antibody (♦) in the presence of goat anti-human IgG. For U251 cells, 4 μg/ml CDDP was also added to the cultures. After cells were incubated for 48 h at 37°C, the cell viability was determined
Hepatocyte toxicity of human mAbs to TRAIL-R1 and TRAIL-R2
Hepatocyte toxicity of recombinant human soluble TRAIL may limit the usefulness of this reagent for treating tumors in vivo. Some preparations of TRAIL were reported to induce apoptosis in human normal hepatocytes in vitro.6 We investigated whether the human mAbs to TRAIL-R1 and TRAIL-R2 were cytotoxic to human normal hepatocytes in the presence of anti-human IgG. All of the mAbs to TRAIL-R1 induced significant death and activated effector caspase in the hepatocyte cultures (Figure 4a and d). Although anti-TRAIL-R2mAbs also induced cell death and caspase activation in hepatocytes, they were separated into two groups, one with and the other without strong hepatocyte toxicity (Figure 4b, c, e). A subset of mAbs to TRAIL-R2, R2-E11 and R2-L30 competitively inhibited the binding of TRAIL to TRAIL-R2-Fc as detected by plasmon resonance assay (Table 1). By contrast, the other subset, R2-H48 and R2-F4, did not. These results indicated that normal human hepatocytes expressed both functional TRAIL-R1 and TRAIL-R2, and hepatocytes were susceptible to both TRAIL-R1- and TRAIL-R2-madiated apoptosis signal.
Hepatocyte toxicity of mAbs to TRAIL-R1 and TRAIL-R2 in vitro. Hepatocytes were treated with the indicated concentrations of mAbs to TRAIL-R1, R1-A13 (▵), R1-B6 (▴), R1-B12 (◊) and control antibody (♦) in the presence of goat anti-human IgG for 24 h (a), or mAbs to TRAIL-R2, R2-E11 (□), R2-L30 (▪), R2-H48 (○), R2-F4 (•) and control antibody (♦) in the presence of goat anti-human IgG for 6 h (b) or 24 h (c). Human normal hepatocytes were incubated with mAbs to TRAIL-R1 (d) and TRAIL-R2 (e) at 1 μg/ml in the presence (□) or absence (▪) of goat anti-human IgG. After cells were incubated for 2 h at 37°C, the effector caspase activity was measured
Discussion
The reported toxicity to hepatocytes is largely dependent upon the version of recombinant TRAIL.8 One possibility causing the hepatocyte toxicity of the tagged versions of TRAIL is due to poor zinc ion coordination and formation of intersubunit disulfide bonds resulting in unstable heterogeneous oligomers.8 Recently, it was suggested that these nonoptimized ligands might cause high-order multimerization of TRAIL-R1 and TRAIL-R2, leading to apoptosis in the normal cells including hepatocytes.9 However, these data also indicated that functional TRAIL-R1 and/or TRAIL-R2 were expressed in hepatocytes. Ichikawa et al.18 demonstrated that TRA-8, an anti-TRAIL-R2 mAb of mouse origin, induced cell death in tumor cells but not in normal human hepatocytes because of a lack of binding of TRA-8 by hepatocytes. They suggested that there was little to no expression of TRAIL-R2 on human hepatocytes. However, our results presented here clearly indicate that all of the mAbs to TRAIL-R1 and a subset of TRAIL-R2 mAbs strongly caused apoptosis in human hepatocytes, demonstrating that both TRAIL-R1 and TRAIL-R2 are expressed and each receptor alone is sufficient to mediate death signals in human hepatocytes.
The mechanism of action of the anti-TRAIL receptor mAb is likely to be quite complex, involving properties of the antibody as well as features inherent to the tumor, that is, the stage of differentiation of the tumor, and its resistance to death signals at the time of introducing the antibody. A hint of this interdependence is observed with the U251 tumor cells, which required both CDDP and anti-TRAIL-receptor antibodies to induce death. The anti-TRAIL-R2mAb (R2-E11) functions both as an antagonist and agonist, as an antagonist in its ability to block ligand binding (which may confer the low heptotoxicity), and as agonist through its antitumor activity. This dual behavior is a property inherent to the mechanism of activating the TNF receptors. Signaling is normally activated by receptor oligomerization caused by binding of the trivalent ligand. Antibody binding to the receptor sterically inhibits ligand access, while at the same time induces oligomerization of the receptor due to bivalent combining sites of the antibody molecule, which in turn initiates signal transduction pathways in ways similar to the ligand. In the case of TRAIL receptor and Fas, a supra-oligomerization is thought to be required for activation of death signaling.22 In agreement with the requirement of receptor oligomerization, our results indicate that anti-TRAIL receptor mAbs require a crosslinking agent to demonstrate agonistic activity in vitro. In contrast, in vivo, oligomerization of mAbs may be induced by an endogenous linker, such as the interaction of the Fc portion of mAbs with Fc receptor-bearing cells. The crosslinking of mAbs with Fc receptor on effector cells in vivo may differ from that with anti-human IgG in vitro. Therefore, the in vivo effects of endogenous linkers on mAbs to TRAIL receptors need to be further investigated.
Together, these results encourage the continued investigation of the TRAIL cytokine-receptor system for the treatment of human cancers. Fully human antibodies may avoid the potential problems arising from immunization of treated patients, and with the lack of hepatotoxicity, should prove valuable in this effort to develop effective immunotherapeutics for malignant disease.
Materials and Methods
Cell lines
Colo205 (colorectal adenocarcinoma), Jurkat (T-cell leukaemia) and MDA-MB-231 (breast adenocarcinoma) were obtained from American Type Culture Collection (ATCC, Rockville, MD, USA). U251 (glioblastoma) was obtained from RIKEN CELL BANK (RCB, Tsukuba, Japan). Cell lines were maintained in RPMI1640 or DMEM supplemented 10% fetal bovine serum, streptomycin. HUVECs were purchased from Clonetics (Walkersville, MD, USA) and maintained in the medium recommended by the supplier.
KM mice (KM MouseTM)
KM mice were generated by cross breeding double trans-chromosomic mice and transgenic mice.19 KM mice possess the human chromosome fragments containing the entire human immunoglobulin heavy-chain loci and the YAC-transgene for 50% of the human immunoglobulin kappa light-chain loci. KM mice are engineered to neither express endogenous immunoglobulin heavy chain nor kappa light chain.
Generation of fully human mAbs to TRAIL-R1 and TRAIL-R2
KM mice were immunized by standard methods with L929 cells transfected with either death domain-deleted TRAIL-R1 (amino acids 1–351) or TRAIL-R2 (amino acids 1–348) as the immunogen and fused with SP2/0 cells (ATCC, Rockville, MD, USA). Antibody-secreting hybridomas were initially screened by a plate-bound ELISA and secondarily by flow cytometry. Isotypes of mAbs were determined in an isotyping ELISA (The Binding Site, Birmingham, UK). Antibodies were purified from hybridoma culture supernatants with Protein A (Amersham Biosciences Corp., Piscataway, NJ, USA) by standard methods.
Flow cytometry
Cells (1 × 106 cells/sample) were washed with PBS containing 1% FBS and 0.1% NaN3 and incubated with 1 μg/ml of human mAbs to TRAIL-R1 and TRAIL-R2 for 1 h on ice. Cells were washed and stained with R-phycoerythrin (RPE)-labeled rabbit polyclonal anti-human kappa light chains antibodies (DAKO, Glostrup, Denmark) on ice for 1 h. After washing, cells were analyzed on FACScan flow cytometer (BD, Franklin Lakes, NJ, USA).
Cytotoxicity assay
Cancer cell lines and HUVECs were seeded in 96-well plates (1 × 104 per well) and incubated with the indicated concentrations of human mAbs to TRAIL-R1 and TRAIL-R2 with or without goat anti-human IgG (10 μg/ml) for 48 h. Cell viability was determined by CellTiter 96® AQueous Non-Radioactive Cell Proliferation Assay (Promega, Madison, WI, USA).
Human hepatocyte cultures
Human normal hepatocytes were purchased from Tissue Transformation Technologies (Edison, NJ, USA) and In Vitro Technologies (Baltimore, MD, USA). Hepatocytes were dispensed into a type-I collagen-coated plate (BD, Franklin Lakes, NJ, USA) in CM5300 medium (CEDRA, Austin, TX, USA). After overnight incubation at 37°C, the plate was washed with PBS to remove unattached cells. Hepatocytes were incubated in CM5300 containing the indicated concentrations of human mAbs to TRAIL-R1 and TRAIL-R2 with or without goat anti-human IgG (10 μg/ml) for 6 or 24 h. Hepatocyte viability was determined by CytoTox 96® Non-Radioactive Cytotoxic Assay (Promega, Madison, WI, USA).
Caspase activity
Cancer cell lines and human normal hepatocytes were seeded and incubated with human mAbs to TRAIL-R1 and TRAIL-R2 (1 μg/ml) with or without goat anti-human IgG (10 μg/ml) for 2 h. Caspase activity was measured by Apo-ONE™ Homogeneous Caspase-3/7 Assay (Promega, Madison, WI, USA) and potency of caspase activation was calculated compared to nontreated cells.
Abbreviations
- TRAIL:
-
tumor necrosis factor-related apoptosis-inducing ligand
- mAb:
-
monoclonal antibody
- TRAIL-R1:
-
TRAIL receptor 1
- TRAIL-R2:
-
TRAIL receptor 2
References
Wiley SR, Schooley K, Smolak PJ, Din WS, Huang CP, Nicholl JK, Sutherland GR, Smith TD, Rauch C and Smith CA (1995) Identification and characterization of a new member of the TNF family that induces apoptosis. Immunity 3: 673–682
Pitti RM, Marsters SA, Ruppert S, Donahue CJ, Moore A and Ashkenazi A (1996) Induction of apoptosis by Apo-2 ligand, a new member of the tumor necrosis factor cytokine family. J. Biol. Chem. 271: 12687–12690
Walczak H, Miller RE, Ariail K, Gliniak B, Griffith TS, Kubin M, Chin W, Jones J, Woodward A, Le T, Smith C, Smolak P, Goodwin RG, Rauch CT, Schuh JC and Lynch DH (1999) Tumoricidal activity of tumor necrosis factor-related apoptosis-inducing ligand in vivo. Nat. Med. 5: 157–163
Ashkenazi A, Pai RC, Fong S, Leung S, Lawrence DA, Marsters SA, Blackie C, Chang L, McMurtrey AE, Hebert A, DeForge L, Koumenis IL, Lewis D, Harris L, Bussiere J, Koeppen H, Shahrokh Z and Schwall RH (1999) Safety and antitumor activity of recombinant soluble Apo2 ligand. J. Clin. Invest. 104: 155–162
Kelley SK, Harris LA, Xie D, Deforge L, Totpal K, Bussiere J and Fox JA (2001) Preclinical studies to predict the disposition of Apo2L/tumor necrosis factor-related apoptosis-inducing ligand in humans: characterization of in vivo efficacy, pharmacokinetics, and safety. J. Pharmacol. Exp. Ther. 299: 31–38
Jo M, Kim TH, Seol DW, Esplen JE, Dorko K, Billiar TR and Strom SC (2000) Apoptosis induced in normal human hepatocytes by tumor necrosis factor-related apoptosis-inducing ligand. Nat. Med. 6: 564–567
Nitsch R, Bechmann I, Deisz RA, Haas D, Lehmann TN, Wendling U and Zipp F (2000) Human brain-cell death induced by tumour-necrosis-factor-related apoptosis-inducing ligand (TRAIL). Lancet 356: 827–828
Lawrence D, Shahrokh Z, Marsters S, Achilles K, Shih D, Mounho B, Hillan K, Totpal K, DeForge L, Schow P, Hooley J, Sherwood S, Pai R, Leung S, Khan L, Gliniak B, Bussiere J, Smith CA, Strom SS, Kelley S, Fox JA, Thomas D and Ashkenazi A (2001) Differential hepatocyte toxicity of recombinant Apo2L/TRAIL versions. Nat. Med. 7: 383–385
LeBlanc HN and Ashkenazi A (2003) Apo2L/TRAIL and its death and decoy receptors. Cell Death Differ. 10: 66–75
Pan G, O'Rourke K, Chinnaiyan AM, Gentz R, Ebner R, Ni J and Dixit VM (1997) The receptor for the cytotoxic ligand TRAIL. Science 276: 111–113
Pan G, Ni J, Wei YF, Yu G, Gentz R and Dixit VM (1997) An antagonist decoy receptor and a death domain-containing receptor for TRAIL. Science 277: 815–818
Sheridan JP, Marsters SA, Pitti RM, Gurney A, Skubatch M, Baldwin D, Ramakrishnan L, Gray CL, Baker K, Wood WI, Goddard AD, Godowski P and Ashkenazi A (1997) Control of TRAIL-induced apoptosis by a family of signaling and decoy receptors. Science 277: 818–821
Walczak H, Degli-Esposti MA, Johnson RS, Smolak PJ, Waugh JY, Boiani N, Timour MS, Gerhart MJ, Schooley KA, Smith CA, Goodwin RG and Rauch CH (1997) TRAIL-R2: a novel apoptosis-mediating receptor for TRAIL. EMBO J. 16: 5386–5397
Chaudhary PM, Eby M, Jasmin A, Bookwalter A, Murray J and Hood L (1997) Death receptor 5, a new member of the TNFR family, and DR4 induce FADD-dependent apoptosis and activate the NF-kappaB pathway. Immunity 7: 821–830
Screaton GR, Mongkolsapaya J, Xu XN, Cowper AE, McMichael AJ and Bell JI (1997) TRICK2, a new alternatively spliced receptor that transduces the cytotoxic signal from TRAIL. Curr. Biol. 7: 693–696
Griffith TS, Rauch CT, Smolak PJ, Waugh JY, Boiani N, Lynch DH, Smith CA, Goodwin RG and Kubin MZ (1999) Functional analysis of TRAIL receptors using monoclonal antibodies. J. Immunol. 162: 2597–2605
Chuntharapai A, Dodge K, Grimmer K, Schroeder K, Marsters SA, Koeppen H, Ashkenazi A and Kim KJ (2001) Isotype-dependent inhibition of tumor growth in vivo by monoclonal antibodies to death receptor 4. J. Immunol. 166: 4891–4898
Ichikawa K, Liu W, Zhao L, Wang Z, Liu D, Ohtsuka T, Zhang H, Mountz JD, Koopman WJ, Kimberly RP and Zhou T (2001) Tumoricidal activity of a novel anti-human DR5 monoclonal antibody without hepatocyte cytotoxicity. Nat. Med. 7: 954–960
Ishida I, Tomizuka K, Yoshida H, Tahara T, Takahashi N, Ohguma A, Tanaka S, Umehashi M, Maeda H, Nozaki C, Halk E and Lonberg N (2002) Production of human monoclonal and polyclonal antibodies in TransChromo (TC) animals. Cloning Stem Cells 4: 91–102
Zhang XD, Nguyen T, Thomas WD, Sanders JE and Hersey P (2000) Mechanisms of resistance of normal cells to TRAIL induced apoptosis vary between different cell types. FEBS Lett. 482: 193–199
Nagane M, Pan G, Weddle JJ, Dixit VM, Cavenee WK and Huang HJ (2000) Increased death receptor 5 expression by chemotherapeutic agents in human gliomas causes synergistic cytotoxicity with tumor necrosis factor-related apoptosis-inducing ligand in vitro and in vivo. Cancer Res. 60: 847–853
Schneider P, Holler N, Bodmer JL, Hahne M, Frei K, Fontana A and Tschopp J (1998) Conversion of membrane-bound Fas (CD95) ligand to its soluble form is associated with downregulation of its proapoptotic activity and loss of liver toxicity. J. Exp. Med. 187: 1205–1213
Acknowledgements
We thank Dr. Carl F Ware for thoughtful discussion, his critical comments and reviewing the manuscript, and Dr. Tadashi Sudo for careful reading of the manuscript. We also acknowledge Hikaru Hashida and Chiharu Tanaka for their technical assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
Edited by Dr. H Ichijo
Rights and permissions
About this article
Cite this article
Mori, E., Thomas, M., Motoki, K. et al. Human normal hepatocytes are susceptible to apoptosis signal mediated by both TRAIL-R1 and TRAIL-R2. Cell Death Differ 11, 203–207 (2004). https://doi.org/10.1038/sj.cdd.4401331
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.cdd.4401331