Abstract
All-solid-state lithium metal batteries promise high levels of safety and energy density, but their practical realization is limited by low Li reversibility, limited cell loading and demand for high-temperature and high-pressure operation, stemming from solid-state electrolyte (SSE) low-voltage reduction and high-voltage decomposition, and from lithium dendrite growth. Here we concurrently address these challenges by reporting that a family of reductive electrophiles gain electrons and cations from metal–nucleophile materials (here a Li sulfide SSE) upon contact to undergo electrochemical reduction and form interphase layers (named solid reductive-electrophile interphase) on material surfaces. The solid reductive-electrophile interphase is electron blocking and lithiophobic, prevents SSE reduction, suppresses Li dendrites and supports high-voltage cathodes. Consequently, a reductive-electrophile-treated SSE exhibits high critical capacity and Li reversibility at the anode, and enables Li(1% Mg)/SSE/LiNi0.8Co0.15Al0.05O2 all-solid-state lithium metal batteries to achieve a high coulombic efficiency (>99.9%), long cycle life (~10,000 h) and high loading (>7 mAh cm−2) at 30 °C and 2.5 MPa. This concept also extends to cathodes of other materials (for example, metal oxides), boosting the high-nickel cathode’s cycle life and expanding the operational voltage up to 4.5 V. Such solid reductive-electrophile interphase tailoring of material surfaces holds promise to accelerate all-solid-state lithium metal battery commercialization and offer solutions for a wide range of materials.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
All of the data that support the findings of this study are available in the Article. Atomic configurations of the computational models constructed in this study are available via figshare at https://doi.org/10.6084/m9.figshare.27228867 (ref. 57). Source data are provided with this paper.
Change history
27 January 2025
A Correction to this paper has been published: https://doi.org/10.1038/s41563-025-02152-7
References
Lacivita, V., Artrith, N. & Ceder, G. Structural and compositional factors that control the Li-ion conductivity in LiPON electrolytes. Chem. Mater. 30, 7077–7090 (2018).
Schwietert, T. K., Vasileiadis, A. & Wagemaker, M. First-principles prediction of the electrochemical stability and reaction mechanisms of solid-state electrolytes. JACS Au 1, 1488–1496 (2021).
Fan, X. et al. Fluorinated solid electrolyte interphase enables highly reversible solid-state Li metal battery. Sci. Adv. 4, eaau9245 (2018).
Ji, X. et al. Solid‐state electrolyte design for lithium dendrite suppression. Adv. Mater. 32, 2002741 (2020).
Park, S. H. et al. Designing 3D anode based on pore‐size‐dependent Li deposition behavior for reversible Li‐free all‐solid‐state batteries. Adv. Sci. 9, 2203130 (2022).
Cho, S. et al. Highly reversible lithium host materials for high‐energy‐density anode‐free lithium metal batteries. Adv. Funct. Mater. 32, 2208629 (2022).
Gu, D., Kim, H., Lee, J.-H. & Park, S. Surface-roughened current collectors for anode-free all-solid-state batteries. J. Energy Chem. 70, 248–257 (2022).
Walther, F. et al. Visualization of the interfacial decomposition of composite cathodes in argyrodite-based all-solid-state batteries using time-of-flight secondary-ion mass spectrometry. Chem. Mater. 31, 3745–3755 (2019).
Zhou, L. et al. High areal capacity, long cycle life 4 V ceramic all-solid-state Li-ion batteries enabled by chloride solid electrolytes. Nat. Energy 7, 83–93 (2022).
Wu, Z. et al. Growing single-crystalline seeds on lithiophobic substrates to enable fast-charging lithium-metal batteries. Nat. Energy 8, 340–350 (2023).
Zhu, Y., He, X. & Mo, Y. Origin of outstanding stability in the lithium solid electrolyte materials: insights from thermodynamic analyses based on first-principles calculations. ACS Appl. Mater. Interfaces 7, 23685–23693 (2015).
Arnold, W. et al. Synthesis of fluorine-doped lithium argyrodite solid electrolytes for solid-state lithium metal batteries. ACS Appl. Mater. Interfaces 14, 11483–11492 (2022).
Wang, M. J., Kazyak, E., Dasgupta, N. P. & Sakamoto, J. Transitioning solid-state batteries from lab to market: linking electro-chemo-mechanics with practical considerations. Joule 5, 1371–1390 (2021).
Raj, V. et al. Direct correlation between void formation and lithium dendrite growth in solid-state electrolytes with interlayers. Nat. Mater. 21, 1050–1056 (2022).
Lee, Y.-G. et al. High-energy long-cycling all-solid-state lithium metal batteries enabled by silver–carbon composite anodes. Nat. Energy 5, 299–308 (2020).
Simon, F. J., Hanauer, M., Henss, A., Richter, F. H. & Janek, J. Properties of the interphase formed between argyrodite-type Li6PS5Cl and polymer-based PEO10:LiTFSI. ACS Appl. Mater. Interfaces 11, 42186–42196 (2019).
Choi, H. J. et al. In situ formed Ag‐Li intermetallic layer for stable cycling of all‐solid‐state lithium batteries. Adv. Sci. 9, 2103826 (2022).
Su, Y. et al. A more stable lithium anode by mechanical constriction for solid state batteries. Energy Environ. Sci. 13, 908–916 (2020).
Sung, J. et al. Ultra‐thin lithium silicide interlayer for solid‐state lithium‐metal batteries. Adv. Mater. 35, 2210835 (2023).
Wang, T. et al. A self-regulated gradient interphase for dendrite-free solid-state Li batteries. Energy Environ. Sci. 15, 1325–1333 (2022).
Lee, S. et al. Design of a lithiophilic and electron-blocking interlayer for dendrite-free lithium-metal solid-state batteries. Sci. Adv. 8, eabq0153 (2022).
Wan, H. et al. Critical interphase overpotential as a lithium dendrite-suppression criterion for all-solid-state lithium battery design. Nat. Energy 8, 473–481 (2023).
Ye, L. & Li, X. A dynamic stability design strategy for lithium metal solid state batteries. Nature 593, 218–222 (2021).
Tan, D. H. S. et al. Carbon-free high-loading silicon anodes enabled by sulfide solid electrolytes. Science 373, 1494–1499 (2021).
Han, F. et al. High electronic conductivity as the origin of lithium dendrite formation within solid electrolytes. Nat. Energy 4, 187–196 (2019).
Park, R. J.-Y. et al. Semi-solid alkali metal electrodes enabling high critical current densities in solid electrolyte batteries. Nat. Energy 6, 314–322 (2021).
Banerjee, A., Wang, X., Fang, C., Wu, E. A. & Meng, Y. S. Interfaces and interphases in all-solid-state batteries with inorganic solid electrolytes. Chem. Rev. 120, 6878–6933 (2020).
Manthiram, A., Yu, X. & Wang, S. Lithium battery chemistries enabled by solid-state electrolytes. Nat. Rev. Mater. 2, 16103 (2017).
Egerton, R. F. Electron Energy-Loss Spectroscopy in the Electron Microscope (Springer Science & Business Media, 2011).
Liou, S.-C., Oleshko, V. P., Kuo, W. C.-H., Yang, T.-J. & Shu, G.-J. Investigation of the excitations of plasmons and surface exciton polaritons in monoclinic gadolinium sesquioxide by electron energy-loss spectroscopy and plasmon spectroscopic imaging. RSC Adv. 12, 10345–10354 (2022).
Li, Y. et al. Correlating structure and function of battery interphases at atomic resolution using cryoelectron microscopy. Joule 2, 2167–2177 (2018).
Chen, Y. et al. Origin of dendrite-free lithium deposition in concentrated electrolytes. Nat. Commun. 14, 2655 (2023).
Li, Y. et al. Atomic structure of sensitive battery materials and interfaces revealed by cryo–electron microscopy. Science 358, 506–510 (2017).
Cao, X. et al. Monolithic solid–electrolyte interphases formed in fluorinated orthoformate-based electrolytes minimize Li depletion and pulverization. Nat. Energy 4, 796–805 (2019).
Margolis, H. C., Kwak, S.-Y. & Yamazaki, H. Role of mineralization inhibitors in the regulation of hard tissue biomineralization: relevance to initial enamel formation and maturation. Front. Physiol. 5, 339 (2014).
Cölfen, H. & Mann, S. Higher‐order organization by mesoscale self‐assembly and transformation of hybrid nanostructures. Angew. Chem. Int. Ed. 42, 2350–2365 (2003).
Kozen, A. C., Pearse, A. J., Lin, C.-F., Noked, M. & Rubloff, G. W. Atomic layer deposition of the solid electrolyte LiPON. Chem. Mater. 27, 5324–5331 (2015).
Xia, H.-Y. et al. A new carbon-incorporated lithium phosphate solid electrolyte. J. Power Sources 514, 230603 (2021).
Tan, S. et al. Additive engineering for robust interphases to stabilize high-Ni layered structures at ultra-high voltage of 4.8 V. Nat. Energy 7, 484–494 (2022).
Shi, P. et al. Lithium difluorophosphate as a dendrite-suppressing additive for lithium metal batteries. ACS Appl. Mater. Interfaces 10, 22201–22209 (2018).
Cheng, D. et al. Unveiling the stable nature of the solid electrolyte interphase between lithium metal and LiPON via cryogenic electron microscopy. Joule 4, 2484–2500 (2020).
Kasemchainan, J. et al. Critical stripping current leads to dendrite formation on plating in lithium anode solid electrolyte cells. Nat. Mater. 18, 1105–1111 (2019).
Krauskopf, T., Mogwitz, B., Rosenbach, C., Zeier, W. G. & Janek, J. Diffusion limitation of lithium metal and Li–Mg alloy anodes on LLZO type solid electrolytes as a function of temperature and pressure. Adv. Energy Mater. 9, 1902568 (2019).
Zhao, L. et al. Taming metal–solid electrolyte interface instability via metal strain hardening. Adv. Energy Mater. 13, 2300679 (2023).
Robinson, E. A. The preparation and Raman spectrum of diphosphoryl tetrafluoride: comparison with the spectrum of diphosphoryl tetrachloride. Can. J. Chem. 40, 1725–1729 (1962).
Wang, X., Ye, L., Nan, C.-W. & Li, X. Effect of solvents on a Li10GeP2S12-based composite electrolyte via solution method for solid-state battery applications. ACS Appl. Mater. Interfaces 14, 46627–46634 (2022).
Wan, H. et al. F and N rich solid electrolyte for stable all‐solid‐state battery. Adv. Funct. Mater. 32, 2110876 (2022).
Kresse, G. & Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 54, 11169–11186 (1996).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Kohn, W. & Sham, L. J. Self-consistent equations including exchange and correlation effects. Phys. Rev. 140, A1133–A1138 (1965).
Golov, A. & Carrasco, J. Molecular-level insight into the interfacial reactivity and ionic conductivity of a Li-argyrodite Li6PS5Cl solid electrolyte at bare and coated Li-metal anodes. ACS Appl. Mater. Interfaces 13, 43734–43745 (2021).
Jorgensen, W. L., Maxwell, D. S. & Tirado-Rives, J. Development and testing of the OPLS all-atom force field on conformational energetics and properties of organic liquids. J. Am. Chem. Soc. 118, 11225–11236 (1996).
Plimpton, S. Fast parallel algorithms for short-range molecular dynamics. J. Comput. Phys. 117, 1–19 (1995).
Evans, D. J. & Holian, B. L. The Nose–Hoover thermostat. J. Chem. Phys. 83, 4069–4074 (1985).
Tang, W., Sanville, E. & Henkelman, G. A grid-based Bader analysis algorithm without lattice bias. J. Phys. Condens. Matter 21, 084204 (2009).
Wang, V., Xu, N., Liu, J.-C., Tang, G. & Geng, W.-T. VASPKIT: a user-friendly interface facilitating high-throughput computing and analysis using VASP code. Comput. Phys. Commun. 267, 108033 (2021).
Wang, Z. Atomistic configurations of solvent-LPSC slab and solvent-Li cluster. figshare https://doi.org/10.6084/m9.figshare.27228867 (2024).
Acknowledgements
The work was supported by the US Department of Energy under award number DE-AC05-76RL01830, received by C.W. We appreciate the technical support from the Maryland NanoCenter.
Author information
Authors and Affiliations
Contributions
W.Z. conceived the idea; performed experiments; and wrote the manuscript. Z.W. performed molecular simulations and density functional theory calculations. H.W. helped with the testing. A.-M.L. performed chemical synthesizing. Y.L. and Y.R. helped with cathode preparation. S.-C.L. helped with scanning transmission electron microscopy measurement. K.Z. discussed the mechanism. C.J. and B.L.L. helped with the XPS test. C.W. supervised the study and the manuscript writing. All authors discussed the results.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Materials thanks Ji-Guang Zhang and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–56, Notes 1 and 2, Tables 1–4 and Refs. 1–17.
Source data
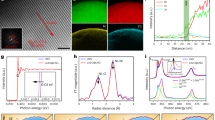
Source Data Fig. 1
Reduction potential data plotted in Fig. 1d and radar chart plotted in Fig. 1e.
Source Data Fig. 2
Thickness reference from previous reports as shown in Fig. 2f, net charge evolution plotted in Fig. 2g and XPS data plotted in Fig. 2i–k.
Source Data Fig. 3
Electrochemical data plotted in Fig. 3a–f.
Source Data Fig. 4
Electrochemical data plotted in Fig. 4a–d.
Source Data Fig. 5
XPS data plotted in Fig. 5c and electrochemical data plotted in Fig. 5d,e.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, W., Wang, Z., Wan, H. et al. Revitalizing interphase in all-solid-state Li metal batteries by electrophile reduction. Nat. Mater. (2025). https://doi.org/10.1038/s41563-024-02064-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41563-024-02064-y