Abstract
Monkeypox is a zoonotic disease that was once endemic in west and central Africa caused by monkeypox virus. However, cases recently have been confirmed in many nonendemic countries outside of Africa. WHO declared the ongoing monkeypox outbreak to be a public health emergency of international concern on July 23, 2022, in the context of the COVID-19 pandemic. The rapidly increasing number of confirmed cases could pose a threat to the international community. Here, we review the epidemiology of monkeypox, monkeypox virus reservoirs, novel transmission patterns, mutations and mechanisms of viral infection, clinical characteristics, laboratory diagnosis and treatment measures. In addition, strategies for the prevention, such as vaccination of smallpox vaccine, is also included. Current epidemiological data indicate that high frequency of human-to-human transmission could lead to further outbreaks, especially among men who have sex with men. The development of antiviral drugs and vaccines against monkeypox virus is urgently needed, despite some therapeutic effects of currently used drugs in the clinic. We provide useful information to improve the understanding of monkeypox virus and give guidance for the government and relative agency to prevent and control the further spread of monkeypox virus.
Similar content being viewed by others
Introduction
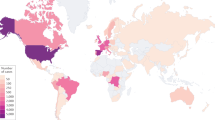
Monkeypox, an endemic disease in both West and Central Africa, before 2022 outbreak, a few cases were reported outside of Africa and these were associated with imports from endemic countries.1,2,3,4,5 However, following the initial case diagnosed in the United Kingdom, the number of monkeypox infection have dramatically increased.3,4,6,7,8 We are now facing the first multiple countries outbreak most with no clear epidemiological links to the endemic countries.9,10 World Health Organization (WHO) Director-General has announced the ongoing monkeypox outbreak a Public Health Emergency of International Concern (PHEIC).11,12 The US Centers for Disease Control and Prevention (CDC) reported that as of 13 September 2022 more than 57,995 confirmed cases had been documented in 100 countries/territories13 (Fig. 1 and Table 1).
Geographical distribution of confirmed monkeypox cases during the outbreak between January and September 2022. Confirmed cases include those laboratory-confirmed as monkeypox virus and may include cases only confirmed as orthopoxvirus. Data presented as of 12 September 2022 were obtained from CDC. Diagram generated with GraphPad Prism 9
Monkeypox virus belongs to the Poxviridae family’s Orthopoxvirus (OPV) genus, along with variola virus (VARV, also known as smallpox), vaccinia virus (VACV), camelpox virus (CMPV), and cowpox virus (CPXV),14,15,16 all of which are pathogenic for humans. There was 96.3% identity between the monkeypox virus genome’s central region, which encodes essential enzymes and structural proteins, and VARVs, which means they are highly genetically similar.17,18 In 1980, the WHO certified that smallpox had been eradicated worldwide after successful vaccination.19,20,21,22
Monkeypox virus has double-stranded DNA (dsDNA) as its genetic material that resembles brick-like particles ranging in size from 200–250 nm in diameter and thus can be seen by electron microscopy (magnified approximately ×10,000).23,24 In monkeypox virus, there are two genetic clades: West Africa (WA, Clade II) clade and Congo Basin (CB, Clade I) clade,25,26 the latter is also known as the Central African (CA) clade and more severe after infection.27,28,29,30,31,32 In 1958, monkeypox virus was first discovered in Copenhagen among laboratory monkeys, and its first monkeypox virus infection of a human occurred in 1970 in the Democratic Republic of Congo (DRC) in a 9-month-old child, with suspected smallpox.33,34,35 Monkeypox is endemic in both West Africa and Central Africa since 1970, with a mortality rate between 1 and 10%.36,37 Sequencing analysis of the current outbreak of monkeypox indicates that the virus is Western African in origin.38,39,40,41,42,43
Recently, the number of patients of monkeypox has increased dramatically, provoking great concern.44,45,46,47,48,49 For this epidemic to be controlled, monkeypox virus must be better understood and re-evaluated.50,51 Here, we review the recent investigations on monkeypox, including epidemiology of monkeypox, monkeypox virus reservoirs, novel transmission patterns, mutations and mechanisms of viral infection, clinical characteristics, laboratory diagnosis and treatment measures, as well as prevention of monkeypox infection.
Epidemiology of monkeypox
It was in 1970 that the first patient of human monkeypox was diagnosed in the DRC.33 After that, more than 400 monkeypox patients were documented in Africa between 1970 and 1990. Of these, the vast majority were diagnosed at DRC,28,52,53,54 followed by 6 cases in Central African Republic (CAR),55 2 cases in Cameroon,56,57 3 cases in Nigeria,14,58,59 2 cases in Ivory Coast,60 4 cases in Liberia,58 and 1 case each in Sierra Leone and Gabon.56 In 1990, there have been four cases of suspected monkeypox in Cameroon, one of which has been confirmed.61,62 In 1996, the DRC experienced a prolonged outbreak of human monkeypox. Initially, a patient was confirmed in February 1996, and until August of that year, a total of 71 suspected monkeypox cases were reported. As of 1999, over 500 patients of monkeypox were reported in the DRC.61,63,64,65 From February to August 2001, a total of 16 cases of monkeypox were identified in the province of Equateur in the DRC.66 Meanwhile, a study conducted by Anne et al.67 in the DRC from 2001 to 2004 examined vesicle fluids and crusted scabs from 136 people suspected of having monkeypox. They found 51 patients of monkeypox.
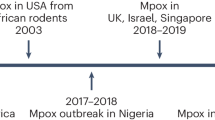
Human monkeypox attracted little attention worldwide until the first outbreak outside Africa occurred in the United States in 2003.68,69 Forty-seven cases, with confirmed (37 patients) and suspected (10 patients) monkeypox were reported, and all people infected by contact with pet prairie dogs (Cynomys spp.).70 The pets were infected after being in close proximity to imported small mammals (African rodents, rope squirrels (Funiscuirus spp.), tree squirrels (Heliosciurus spp.), Gambian giant rats (Cricetomys spp.), brushtail porcupines (Atherurus spp.), dormice (Graphiurus spp.), and striped mice (Hybomys spp.) from Ghana. According to CDC, laboratory tests using virus isolation and PCR amplification have proved that at least three dormice, two rope squirrels, and one Gambian giant rat were found to be infected by monkeypox. It was noted that no deaths occurred and no human-to-human transmission was observed,68 which was explained by the strain being a West African strain, based on genetic analysis.69 In addition, a study has conducted to evaluate the effects of the route of infection on the clinical illness of human monkeypox and demonstrated that significant symptoms of systemic diseases were more likely to occur in cases exposed to complex exposures than in cases exposed to noninvasive exposures (49.1 vs. 16.7%; P = 0.041).70 In contrast to Africa, a large number of cases confirmed in this outbreak are adults.71 In 2003, the Republic of Congo (ROC) reported the first outbreak of human monkeypox. It was recorded in this outbreak that 11 patients of monkeypox were confirmed and probable, all of whom were younger than 18 years of age, one death occurred. It is believed that the monkeypox virus has been transmitted from human to human six consecutive times, making it the longest recorded series of consecutive transmissions of monkeypox from one individual to another.72 In Unity State, Sudan, between September and December 2005, ten confirmed and nine possible patients of monkeypox were described in 5 villages (2 in Bentiu, 3 in Modin, 5 in Nuria, 5 in Rubkona, and 4 in Wang Kay).73 Between 2010 and 2018, varying numbers of monkeypox patients were documented in several African countries, including DRC,74,75 CAR,76,77,78 Cameroon,79,80 Liberia,76 Sierra Leone,81 as well as COA.82 In addition, it was reported in 2017 that Nigeria was experiencing an outbreak. 122 confirmed or possible patients of human monkeypox were reported between September 2017 and September 2018 in 17 states in Nigeria. Six deaths were recorded (case fatality rate 6%).83,84,85
Thousands of people around the world have been infected by the monkeypox outbreak in 2022, which followed several years of sporadic cases outside of Africa (United Kingdom,86,87,88 Singapore,89,90 Israel,91,92 United States93). On May 2022, multiple cases of monkeypox were identified in the United Kingdom.2 On 6 May 2022, an individual with travel ties to Nigeria was diagnosed with monkeypox.94 On 12 May, the UK Health Security Agency (UKHSA) in London confirmed two additional cases of monkeypox.95 There is no link between the two of them and they have never traveled to the endemic region. A week later, another four cases were confirmed. Unusually, there was no known contact between these patients and the previous confirmed cases.96 By polymerase chain reaction (PCR) experiment of patient swab samples, the outbreak was caused by the West African clade of monkeypox virus, which is less fatal than the other known monkeypox variant (CB, Clade I) after infection, with a case lethally rate of only 1%.39,50,97,98
Portugal, Canada, and Spain documented 14, 13, and 7 cases of monkeypox, respectively, on 18 May.99,100,101 It was also the same day that the United States identified its first monkeypox patient of 2022.102 On 19 May, Belgium and Sweden reported their first cases.103,104 Belgium announced that monkeypox cases were required to be isolated for 21 days at once. Belgium also became the first country in the world to require self-isolation of monkeypox cases. At the same time, Italy has reported first case, a traveler from the Canary Islands.105 Besides, France has reported a suspected case. Genomic analysis the virus isolated from a monkeypox virus infecting case in Portugal showed that the virus also belonged to the WA branch of evolution, closest to the virus carried by cases imported from Nigeria in 2018 and 2019.43 Two patients of monkeypox were confirmed in Australia on 20 May, both of whom had recently returned from travel to Europe.106 On the same day, the first cases were confirmed in Germany, as well as in the Netherlands and France.106,107,108,109,110 On the next day, the first cases were confirmed in both Switzerland111 and Israel,112 and the patient documented by the Israeli Ministry of Health is also the first case of monkeypox in Asia.113
After May 2022, a large number of monkeypox patients were confirmed in non-endemic countries worldwide. This unusual outbreak promoting the WHO to declare monkeypox as an “evolving threat of moderate public health concern” on June 23. Furthermore, the WHO announced that monkeypox outbreaks in many countries and regions constitute a “Public Health Emergency of International Concern” (PHEIC) on July 23, 2022.114,115 Meanwhile, monkeypox prevention and treatment guidelines have been issued in several countries around the world.116 As of 13 September 2022, 57,995 monkeypox virus infections which were laboratory confirmed have been reported in >100 countries or regions across all six WHO regions13 (Fig. 1 and Table 1). Of these, a total of 18 deaths were reported in 9 countries. In particular, a very recent news indicated that the first person of monkeypox was confirmed in Hong Kong on September 6.117 Epidemiological investigation revealed that this patient arrived in Hong Kong from the Philippines on September 5 at the age of 30 years. Consequently, as a result of the monkeypox emergence in Hong Kong, a preparedness and response plan has been activated by the Hong Kong Government. Also, this is the first reported person of monkeypox since the four cases were confirmed in Taiwan Province of China.118,119 Notably, the U.S. Centers for Disease Control and Prevention (CDC) declared monkeypox a public health emergency on August 4, 2022 in the United States.120 The monkeypox epidemic in the United States is extremely serious, as of September 6, monkeypox had since spread to every state, with a total of 20,733 cases.121,122
Monkeypox virus reservoirs
Monkeypox is a zoonotic disease whose natural reservoir remains unknown.123,124 Several researches have conducted to determine the reservoir or natural hosts of monkeypox virus, Khodakevich et al.125,126 Have reported that antibodies to the virus were found in 2 of 18 squirrels tested. The monkeypox virus was extracted from a diseased squirrel, Funisciurus anerythrus, which was the first report of monkeypox virus being isolated from a wild animal. Other researches have also suggested that squirrels of the genera Funisciurus and Heliosciurus are related to the natural cycle of monkeypox virus in DRC,127,128,129,130 as well as Rodents of the genera Cricetomys, Graphiurus, elephant shrews of the genus Petrodromus.127 In March 2012, monkeypox virus was separated from a wild-living monkey (a sooty mangabey) by Radonic et al.131 Its whole-genome sequence showed that it had significant similarities with monkeypox viruses located in Western Africa. Patrono and colleagues132 described the frequent appearance of monkeypox virus in a wild-living chimpanzees (Pan troglodytes verus, hereafter chimpanzee) population from Taï National Park, Ivory Coast. A number of animal species, mostly rodents and non-human primates, have been confirmed to be susceptible to the virus after multiple investigations,133,134 as listed in Table 2.
Novel transmission patterns
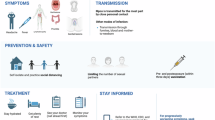
Monkeypox transmission from animals to human may include direct contact with diseased parts or body fluids of infected animals, scratching or biting by animals, eating meat from infected animals, and contact with contaminated objects.135,136,137,138,139,140,141 Person to person transmission of monkeypox is caused by close contact with a human with monkeypox virus infection, including contact respiratory secretions from those infected individuals, skin lesions or genitals, prolonged face-to-face contact, along with their bedding and clothing142,143,144 (Fig. 2). There is very limited data on monkeypox infection during pregnancy, although a study has demonstrated the existence of vertical transmission of monkeypox virus.145,146 At the General Hospital of Kole, an observational research was conducted, Mbala et al. described the fetal outcomes for one of four pregnant women.147 Of the four women, one delivered a healthy baby, one had fetal death and two delivered miscarriages in their first trimester. In addition, monkeypox virus was detected in semen sample by researchers.148 Therefore, more attention is needed cause individuals such as children and pregnant women are more susceptible with increasing cases.145,147,149,150,151,152,153,154,155,156,157 Notably, a very recent report proved that human-to-dog transmission of monkeypox virus.158 Next-generation sequencing was used to compare the DNA sequences of the monkeypox virus from the dog and the patient. The samples both contained viruses of the human monkeypox virus-1 clade, lineage B.1, that have been spreading in countries that are not endemic to the disease.
According to previous reports, monkeypox virus was not previously been highly contagious. Monkeypox of the CB clade was found to have a lower basic reproduction number (R0) than 1 between 1980 and 1984 in the Democratic Republic of the Congo.159,160 Disseminated patients of monkeypox have been recorded in Africa, usually because of close contact with wild animals, especially rodents. Such travel related monkeypox individuals have limited secondary spread, making human-to-human transmission inefficient.1,76,83 However, a recent study by Du et al.161 reported that the reproduction number was estimated to be 1.39 (95% CrI: 1.37, 1.42) by aggregating all patients in 70 countries as of July 22 2022. According to initial estimates based on analysis of the first 255 PCR-identified patients of monkeypox in Italy in 2022, the reproduction number among men who have sex with men is 2.43 (95% CI 1.82–3.26).162 Which indicates that the disease has epidemic potential. Meanwhile, the vast majority of monkeypox patients had not recently traveled to the endemic regions of Africa, such as Nigeria, the DRC as well as central and western Africa.163 Human-to-human transmission of this virus is unusually frequent in this outbreak, suggesting close contact is the most likely route of transmission.164
Another unusual feature is that the large number of cases diagnosed with monkeypox are male, and a considerable part of patients have sex with men (MSM), particularly in Canada, Spain, and the UK, suggesting that sexuality is another forms of close contact, even though monkeypox was not considered a sexually transmitted disease before.165,166,167,168,169,170,171,172,173,174,175,176,177 It is reported that higher proportion of MSM infected is due to accidental entry into the community and then sexual behavior constituting “close contact” rather than sexual transmission of the monkeypox virus itself.178 However, a study of samples from 528 confirmed cases outside endemic areas in Africa between April and June showed that 98% of patients were bisexual men or gay and 95% of those infected were suspected of transmission through sexual activity.179 A prospective cohort study in Spain indicated that 91.7% cases identifying as MSM in 181 monkeypox infection cases.180 Besides, monkeypox virus DNA was detected by PCR in seminal fluid in 29 of the 32 cases.179 On 3 August, In the Monkeypox Surveillance Bulletin published by the ECDC-WHO Regional Office for Europe, 99% (15,439/15,572) of the cases were males, with 43.4% of MSM cases.181 Meanwhile, using a branching process transmission model, researchers showed that a small minority of people have disproportionately large numbers of partners, which could explain the continued increase in monkeypox patients among MSM population.182 Another research showed a transmission number of 2.43 in the MSM community among Italian cases in May-June 2022.183 According to Endo and colleagues,182 monkeypox’s basic reproductive number (R0) may be significantly higher than 1 over the MSM sexual contact network. Monkeypox virus spread model for assessing outbreak risk in a metropolitan area indicated that if transmission efficiency increases in the higher-risk group like gays and MSMs, broader populations may be affected.184 On the other hand, Bragezzi et al.185 conducted a meta-analysis to show that sexual contact is involved in 91% cases (total 124 cases). Notably, a recent study of 21,098 monkeypox cases (data from 41 countries, as of August 23, 2022) revealed that the vast majority were MSM, with a typical rash characteristic.186 Transmission was mainly through close contact during sexual activities. Overall, all these reports suggested that MSM was up to now the most frequently suspected route of transmission.173,175,187,188,189,190,191,192,193 Consequently, targeted interventions are needed in communities with a large part of MSM individuals to prevent further transmission. And further research is demanded to determine whether sexual transmission is possible.
Monkeypox virus mutations and mechanisms of viral infection
Monkeypox virus is a species of double-stranded DNA virus which causes monkeypox in humans and other animals. It belongs to the genus Orthopoxvirus in the family Poxviridae. Among all animal viruses, poxviruses have the largest and most complex DNA genomes.194,195 There are four major elements of the virion: core, lateral bodies, outer membrane, and the outer lipoprotein envelope.196 The central core contains the viral dsDNA and core fibrils. The monkeypox virus genome consists of 197 kb with the central genomic region comprising of 101 kb.17 Both terminal variables regions include a 6379 bp terminal inverted repetition (ITR) with approximately 80 bp long hairpin loop, 70 or 54 bp short tandem repeats and unique ITR sequences NR 1 and NR 2 and the coding region.32,197,198 The virus contains about 190 nonoverlapping open reading frames (ORFs),17,197,199,200 four of which are located in the ITR sequence.197 Genes responsible for viral replication, transcription, assembly, and release are conservatively located in the genome’s central region, as they are in all orthopoxviruses.200 While most of the genes expressing virulence and host tropism are found at both ends of the genome. These terminal genes play a role in immune evasion by interfering with signaling, presentation, and recognition of antigens and apoptosis.201,202,203
Monkeypox virus has a low frequency of genomic mutations, due to the DNA double-stranded structure, and the 3’–5’ exonuclease activity of its DNA polymerase.204,205 However, the 2022 monkeypox virus diverges from the related 2018–2019 viruses by a mean of 50 single-nucleotide polymorphisms (SNPs), considering previous estimates of the substitution rate for orthopoxviruses, this is much higher than expected.39,206 Such a divergent branch could speed up the evolution of the monkeypox virus. Notably, further examination of the mutational profiles of these SNPs suggested a dramatic mutational bias, with 26 (14 non-synonymous, 10 synonymous, and 2 intergenic) and 15 (nine non-synonymous and 16 synonymous) being GA>AA and TC>TT nucleotide replacements, respectively. Several studies have indicated that mutations observed in viral genome editing may be caused by apolipoprotein B mRNA-editing catalytic polypeptide-like 3 (APOBEC3) enzymes.207,208,209,210,211,212 Another phylogenetic analysis indicated that the monkeypox virus‐2022 strains contained 46 new consensus mutations, including 24 nonsynonymous mutations, compared with the monkeypox virus-2018 strain.213 We suspected that the lower mortality and higher transmission of 2022 monkeypox virus than previous monkeypox virus may related to these viral mutations. Although these viruses are under continual adaptation, it is unknown whether these mutations contribute to help the virus evade host immunity. To further understand how these mutations affect viral function, additional research is required.
Unlike other DNA viruses, monkeypox virus replicates in the cytoplasm of the host cell.196 Poxviruses enter into the host cell may be divided into 3 steps (Fig. 3): virus attachment, hemifusion, and core entry, which occurs at the cell membrane or following endocytosis.196,214,215 A unique feature of poxviruses is that they produce two types of infectious particles: extracellular virions (EVs) and mature virions (MVs), both viral particles have different viral surface epitopes.216 Poxviruses enter cells through different mechanisms depending on their infectious forms, mature virion (MV) with single outer membrane, or enveloped virion (EV) with additional membrane and different protein composition.217,218,219,220,221,222
Schematic illustration of the transmission and clinical characteristics of monkeypox. There are many modes of transmission, animals infected with monkeypox virus (such as squirrels, rodents, monkey, and sooty mangabey), direct contact with body fluids or diseased parts of infected animals, scratching or biting by animals, consumption of meat from infected animals, sexual contact (MSM), and contact with contaminated objects, respiratory secretions from those infected individuals, skin lesions, along with their bedding and clothing. The clinical characteristics of monkeypox are depicted on the right side of the figure. General features such as lymphadenopathy, fever, headache, chills and/or sweats, sore throat, muscle ache, lack of energy, rash, and genital rashes are seen commonly. Complications of monkeypox include pneumonitis, encephalitis, keratitis, and secondary bacterial infections
Despite not being able to identify specific receptors for poxviruses, many glycosaminoglycans including heparin sulfates and chondroitin, as well as laminin, contribute to their attachment to cells.217,223,224 Up to now, many researches have been demonstrated that several vaccinia virus proteins are vital for binding to the cell surface, including D8L,225,226 A27L,227,228 A34R,229,230,231,232 A26L224 and H3L.233,234,235 It is following viral attachment that the virion binds to the membrane and fuses with the host cell, causing its core to be released into the cell’s cytoplasm. Virion core contains enzymes and factors that initiate transcription. It was proven that L1, F9, A28, A27, L5, H2 were essential viral envelope proteins for cellular entry.236,237,238,239,240,241,242,243 Virus-encoded multi-subunit DNA-dependent RNA polymerase initiates viral transcription, which is followed by host ribosome-mediated translation of early, intermediate, and late proteins. The intermediate and late stages of viral gene transcription require cooperation with host-derived transcription factors.244,245,246,247,248 Synthesis of poxvirus DNA occurs in the cytoplasm. A majority of viral particles remain in the cytoplasm as intracellular mature virions (IMVs) and are encased within the protein matrix of scabs. A second envelope (intracellular enveloped virions, IEVs) may be acquired by the remaining virions, and they are attached to the cell membrane of their host.249 As cell surface-associated enveloped virions (CEVs), they cause cell-to-cell transmission of the virus, while extracellular enveloped virions (EEVs) contribute to systemic transmission.250
Clinical characteristics
The clinical characteristics of monkeypox are quite similar to those of smallpox, but the monkeypox is clinically milder.37,251,252,253 It is crucial to realize that monkeypox and smallpox differ mainly by the occurrence of lymphadenopathy,141 since 90% of monkeypox patients suffer from lymph node enlargement.254 Monkeypox is most commonly confused with chickenpox, caused by varicella-zoster virus (VZV). In terms of their clinical characteristics, these two diseases share a great deal in common. Moreover, several studies have reported that individuals are infected both monkeypox virus and VZV.66,67,127
The incubation time of monkeypox virus is usually 5–21 days.162,183,255,256 According to Miura’s report, incubation period (18 cases) is estimated to be 8.5 days on average.255 Based on 22 possible (N = 1) and confirmed (N = 21) monkeypox patients reported in the United States through 6, 2022, Charniga and colleagues estimated the incubation time for monkeypox.256 According to their study, the incubation time from exposure to the onset of symptoms was 7.6 days on average. Besides, another research found that among 23 people with known history of exposure, the median incubation period was 7 days (between 3 and 20 days).179
In humans, monkeypox disease can be divided into two phases: the prodrome and the rash. Initial symptoms of monkeypox virus infection include headache, lack of energy, fever, chills and/or sweats, sore throat, muscle ache, and lymphadenopathy5,257,258,259,260 (Fig. 2). Usually, few days after fever and lymphadenopathy, the rash appears. Rash starts with the face and then appears the whole body, and is characterized by a few to several thousand lesions.83,91,261 As the rash progresses about 2–4 weeks, plaque is replaced by papules, blisters, pustules, scabs, followed by shedding.28,254,262 Complications of monkeypox include encephalitis, keratitis, pneumonitis, and secondary bacterial infections.28,143,254,263
In the United States, a report on the epidemiological and clinical features of monkeypox cases showed 42% of persons (n = 291 participants) has no prodromal symptoms, and 37% of patients did not have fever as of the time of assessment, despite most patients included typical features.122 And genital rashes were observed in many cases, which was also recently proven in another studies.264,265,266,267,268,269,270 Other recent reports describe similar clinical characteristics.180,271,272 Patel and colleagues272 demonstrated that all 197 monkeypox infection cases developed mucocutaneous lesions, typically in the genitals (111 of 197 patients) or the perianal areas, causing rectal pain and penile edema. Moreover, according to a case report from Spain, 78 of 181 persons had lesions in the oral and perioral region and 141 in the anogenital region. The following complications occurred in 70 of the 181 patients: 45 (24.9%) of 181 had a proctitis, 19 (10.5%) tonsillitis, and 6 (3.3%) had a bacterial skin abscess.180 The clinician should remain vigilant for monkeypox-like rashes regardless of whether the rash has disseminated or had a prodrome prior to the rash. Notably, the possibility of some monkeypox virus infections being asymptomatic has been raised by two recent case reports from Europe. It has been shown in several studies that monkeypox could go unobserved when it is propagable, so physicians and persons at high risk should be aware of this fact.171,172,273
Available data from the European Union, the United Kingdom, and the United States indicated that 28–51% have human immunodeficiency virus (HIV) infection among MSM patients with monkeypox.122,167,179,180,272,274 Currently, the risk for monkeypox infection is unknown, however, in people with HIV.
Symptoms of monkeypox last 2–4 weeks, and the disease is usually self-limiting. The lethality of monkeypox patients after infection depend on the clade of virus infected, route of infection, patient age, and patients infected with the CA clade of the virus is generally higher than the WA clade.6,70,141,275 Previous reports80,83,97,276,277,278 indicated that the case fatality rates (CFR) of monkeypox ranging from 1 to 11%. What’s more, in a systematic review that exclusively evaluated the case fatality rates (CFR) by clades, the overall rate of case fatalities was confirmed to be 8.7%, which was significantly lower for West African clade (3.6%, 95% CI 1.7–6.8%) than Central African clade (10.6%, 8.4–13.3%).1,66 Among 1958 patients, some 4% (95% CI 1–9%) of hospitalized patients had fatal outcomes.260 In the current 2022 monkeypox outbreak, it was reported that patients at least sixteen years old always have milder symptoms in the current investigations, with a pooled case fatality rate of 0.03% (1 of 2941 patients).279 Monkeypox is more often fatal in children, which is similar to smallpox.36,80 As of September 2022, a total of 15 deaths have been documented in this monkeypox global outbreak, in which 9 deaths in locations that have historically recorded monkeypox.
Laboratory diagnosis
Diagnosis of monkeypox requires a combination of clinical symptoms, epidemiological information, and laboratory tests. According to WHO recommendations, nucleic acid amplification testing (NAAT) has been conducted for monkeypox virus detection.280 Genes commonly used for conventional polymerase chain reaction (PCR) testing include hemagglutinin,281 the acidophilic-type inclusion body gene,282,283 and the crmB gene.284 Compared with other diagnostic methods, real-time PCR provides high throughput, high sensitivity, and fast results. Several genes have been developed to detect monkeypox virus, such as envelope protein gene (B6R),285 B7R gene,286 DNA polymerase gene E9L,69,285 complement binding protein C3L,278,287 DNA-dependent RNA polymerase subunit 18 (RPO18) gene,288 F3L and N3R.289 Meanwhile, recombinase polymerase amplification (RPA) method has been proven to be an alternative to real-time PCR. It was reported that the monkeypox virus-RPA-method was especially sensitive with a limit of detection (LOD) of 16 DNA molecules per microliter by targeting the tumor necrosis factor binding protein gene.290 With different target sites in the viral genome, these tests have varying sensitivities and limits of detection for monkeypox virus.291 According to Li et al.278 real-time PCR assay for generic monkeypox virus has a limit of detection for 0.7 fg (∼3.5 genomes), while monkeypox virus West Africa specific (G2R_WA) assay’s LOD is at 1.7 fg (∼8.2 genome), and monkeypox virus Congo Basin (C3L) assay’s LOD is at 9.46 fg (∼40.4 genomes). As well, Maksyutov et al.292 developed real-time PCR assay that targets viral F3L gene with a LOD of 20 copies per reaction. Several other studies reported LOD for monkeypox virus assays of 6.359 copies/mL,293 20 copies/reaction,286 11–55 fg (50–250 copies of each gene),289 2 fg (~10 viral copies),285 0.05 fg (25 copies/assay).294
Recently, a high-throughput molecular testing method for monkeypox virus was established,293 which may expand the detection capacity and reduces the detection of time. In the current outbreak, the whole genome sequencing was also used to identify the monkeypox strain.40,92,198,295 What’s more, there are several other DNA-based assays have been investigated for monkeypox virus detection, such as loop-mediated isothermal amplification (LAMP),296 restriction length fragment polymorphism (RFLP),98,297 as well as recombinase polymerase amplification (RPA).290
Laboratory methods for detecting monkeypox also include enzyme-linked immunosorbent assay (ELISA),298,299 western blot (WB)136,300 and immunohistochemistry (IHC).30,301 It is often necessary to use serologic diagnostic procedures to diagnose poxvirus when there is no virological sample. In most cases, ELISA is the most frequently used serologic test. Serological testing of the specific IgM and IgG antibodies are commonly deployed. As soon as the rash appears, IgM antibodies appear and rise approximately 2 weeks before declining and disappearing within 1 year. While IgG antibodies also generate quickly after the rash onset, rise approximately 6 weeks, and lasts for decades.302 However, the specificity is low, due to the immunologic cross-reactivity between monkeypox virus and other othopoxviruses.37,251 Electron microscopic observation can be used as an auxiliary method to detect monkeypox virus.24,303 However, due to the laborious and costly sample preparation, it is difficult to be used popularly.
Treatment
Treatment is suggested for monkeypox virus-infected individuals with severe disease at present or who may be at high risk for developing serious illness (people who are immunocompromised, pediatric population, have characteristic dermatitis or history, have skin symptoms of exfoliation, pregnant or breastfeeding woman, one or more complications), or those with monkeypox virus aberrant infections, including accidental implantation in mouth, eyes, or other anatomical areas where monkeypox virus infection that could pose a special threat (such as the genitals or anus). For most patients, treatment is symptomatic and supportive. Despite the fact that monkeypox has no specific treatment, smallpox antiviral drugs such as brincidofovir, tecovirimat, and cidofovir may have effect against monkeypox because of their similar genetics.258,304,305,306,307,308
Monkeypox life cycle and mechanisms of action of antivirals. This diagram depicts the life cycle of monkeypox virus inside a human cell. Notably, Replication cycle of monkeypox virus occurs in the cytoplasm of the host cell. Following viral attachment, virion binds and fuses with the host cell membrane, the viral core is released into the cytoplasm of the host cell. Viral particles are assembled into intracellular mature viruses (MV), then stay in the cytoplasm as intracellular mature virions of released as extracellular enveloped viruses during cell lysis. MV can also wrap an additional envelope and attached to the cell membrane, then then release through exocytosis. Cidofovir and its prodrug brincidofovir inhibit the viral DNA polymerase during DNA replication. Tecovirimat targets the VP37 protein, which is vital for envelopment of intracellular mature virus with Golgi-derived membrane to form enveloped virus (EV), prevents the virus from leaving an infected cell, hindering the spread of the virus within the body
Tecovirimat
Tecovirimat (also known as TPOXX, ST-246), a small-molecule inhibitor of virus, is effective against orthopoxviruses both in vitro and in vivo, including vaccinia virus, camelpox virus, cowpox virus, mousepox virus, variola viruses, and monkeypox virus.309 Tecovirimat targets the VP37 protein, inhibits the spread of viruses within the body by preventing them from leaving infected cells,309,310 as shown in Fig. 3. Tecovirimat inhibits neither DNA or protein synthesis nor the formation of mature virus. The mature virus remains in the host cell until cell lysis. Tecovirimat displayed s strong antiviral efficacy with an EC50 range of 0.01 to 0.07 μM.309,311,312 Several studies have demonstrated that the VP37 protein plays an essential role in encapsulating intracellular mature virus with Golgi-derived membrane to form enveloped virus.309,313,314,315,316,317
Tecovirimat has a distinguished antiviral effect against the monkeypox virus lineage responsible for the 2022 outbreak in vitro.318 Further, the effectiveness and safety of tecovirimat has been revealed in multiple animal studies.319,320,321,322,323,324 Treatment with 10 mg per kilogram of body weight of tecovirimat for 14 days in the monkeypox model could achieve >90% survival.324 In a nonhuman primate (NHP) model, by taking orally once a day, tecovirimat was found to be effective in protecting NHP from monkeypox virus illness, with reduced viral loads, fewer rashes, prolonged survival and significantly reduced mortality in the treatment group compared with the vehicle mongkeys.325 Smith et al.326 assessed the efficacy of tecovirimat against monkeypox virus challenge of 65 times the 50% lethal dose (LD50) in prairie dogs, and found that one hundred percent of animals that received tecovirimat survived challenge, while 75% of infected animals receiving vehicle alone succumbed to infection. Meanwhile, multiple phase 3 clinical trials of tecovirimat have demonstrated its safety and well tolerated (Table 3).
In 2018, the FDA approved its first use for treating smallpox after studies showed it was safe in humans and effective in animals with similar viruses.304,323,327,328 A trial in monkeypox people showed the tecovirimat (600 mg twice daily for 2 weeks orally) did not cause side effects and that viral shedding and illness lasted shorter time.329 Besides, data collected from 25 cases with diagnosed monkeypox infection had completed a course of tecovirimat therapy revealed all cases tolerated the antiviral medicine well, with minor side effects. The lesions and pain of 10 patients (40%) were completely resolved by day 7 of therapy, and 23 of 25 patients on day 21. The most frequently adverse events of therapy reported including diarrhea in 2 (8%), itching in 2 (8%), nausea in 4 (16%), headache in 5 (20%), and fatigue in 7 patients (28%).308 Moreover, several reports have also documented the efficacy and safety of tecovirimat in the treatment of monkeypox.308,330,331,332 Healthcare providers can now provide tecovirimat treatment to monkeypox patients under an expanded access Investigational New Drug (EA-IND) protocol developed by CDC and FDA. A total of 1001 cases receiving this antiviral drug have been documented, in addition, data was abstracted by the Centers for Disease Control.333
Despite this, more clinical trials are needed to determine whether this antiviral drug is effective and safe for treating monkeypox infections in people.334 A double-blind, randomized controlled study will be conducted by the National Institutes of Health’s (NIH) National Institute of Allergy and Infectious Disease (NIAID) in treating individuals confirmed with monkeypox in adults and children to evaluate the effectiveness and safety of tecovirimat.335 Meanwhile, through the AIDS Clinical Trials Group, NIH/NIAID is conducting a phase 3 double-blind, randomized controlled investigation of tecovirimat in outpatient in the United States settings to treat monkeypox.335 According to the latest report, it is currently underway or planned to conduct several clinical experiments to evaluate tecovirimat’s safety and effectiveness in treating monkeypox patients (PALM-007, PLATINUM, WHO/ARNS, and ACTG5418).336,337
Cidofovir
Since 1996, Cidofovir (CDV, also known as Vistide) has been approved for clinical use to treat cytomegalovirus (CMV) retinitis in persons with acquired immunodeficiency syndrome (AIDS).338,339,340,341 CDV is a prodrug, and it must be phosphorylated by enzymes of the cytoplasm after enter the host cells, and into CDV diphosphate (CDV-pp), which has a prolonged half-life.342,343,344 CDV-pp inhibits the viral DNA polymerase during DNA replication, as well as inhibits DNA polymerase 3′–5′ exonuclease activity.343
Many animal models and experiments in vitro have reported the effectiveness of cidofovir for treatment orthopoxviruses, including vaccinia, mousepox, as well as monkeypox.338,345,346,347,348,349,350 Between 72 to 96 h post-infection, treatment of mice inoculated with cowpox virus (CV) or vaccinia virus (VV) resulted in significant protection. And when given at least 5 days before infection or 3 days after infection with either VV or CV, a single-dose pretreatment or posttreatment with CDV at 3 to 100 mg/kg was effective.351 Another mouse model demonstrated that it was completely protective against virus-induced cutaneous lesions and against the associated mortality when started on the day of infection or the first post-infection day of topical treatment with cidofovir. In addition, systemic treatment with cidofovir caused lesions to heal and regress.346 The treatment of mice with cidofovir (for two days after 24 h of virus exposure) suppressed viral loads as well as cytokine levels in plasma and tissue, including interleukin (IL)-10, IL-6, IL-3, and IL-2.352 In non-human primate model, researches proved a significant reduction in mortality and cutaneous monkeypox lesions when CDV treatment is initiated 24 h after lethal intratracheal monkeypox virus infection.353
Various studies have shown the effectiveness of CDV against poxvirus. Patients with recalcitrant molluscum contagiosum virus (MCV) treated with intravenous cidofovir exhibited dramatic cleaning of their MCV lesions, and all three cases remain clean of recurrence.354 The use of topical CDV was found to be effective in treating molluscum contagiosum (MC) in a 12-year-old boy with Wiskott–Aldrich syndrome.355 Two children with AIDS were also successfully treated with topical 3% cidofovir in a combination vehicle (Dermovan) for recalcitrant MC.356 CDV has also been documented to be effective against cowpox and human vaccinia in additional case reports.357,358,359 Despite the lack of clinical evidence to support its use in treating monkeypox, cidofovir is generally thought to be effective. According to the CDC’s guidance, an outbreak of orthopoxviruses, including monkeypox virus, can be treated with this anti-viral medicine.
Brincidofovir
Brincidofovir (also known as CMX001 or Tembexa) is a lipid conjugate of the acyclic nucleotide phosphonate, cidofovir (CDV). After brincidofovir entry into target cells, a phospholipase enzyme cleaves the lipid ester linkage in brincidofovir, liberating CDV and activated by two sequential phosphorylations, first yield cidofovir monophosphate (CDV-P) and then yield cidofovir diphosphate (CDV-PP).360 CDV-pp suppresses the synthesis of viral DNA by inhibiting the DNA polymerase activity of several double-stranded DNA (dsDNA) viruses or by acting as an acyclic nucleotide and binding to the viral DNA strand361,362,363,364,365 (Fig. 3).
Brincidofovir has under gone numerous animal studies328,366,367,368,369,370,371,372,373,374 to demonstrate its antiviral efficacy against double-stranded DNA viruses including poxviruses such as monkeypox virus and is currently in human clinical investigations for treating other double-stranded DNA viruses (Table 3). In addition, An antiviral regimen with brincidofovir and ST-246 administered on the day of monkeypox virus infection protects mice with the STAT1-deficient C57BL/6 mouse model.375 Lethal rabbitpox models revealed that brincidofovir administration at or before the midpoint of illness could reduce the fatality rate from rabbitpox virus infection, as well as reduce viral loads, which might contribute to decrease infectivity of the virus.376,377 Studies have been conducted using the prairie dog monkeypox virus model to determine the effectiveness of anti-poxvirus therapeutics.378,379 In a monkeypox virus animal model, brincidofovir was administered orally to prairie dogs to determine its pharmacokinetics (PK). It was found that BCV treatment early in the illness tended to increase survival against a lethal monkeypox virus challenge; the earlier the treatment was initiated, the greater the chance of survival.380,381 In summary, these models suggest that treating monkeypox patients early with brincidofovir is more likely to have better outcomes.
In 2021, Brincidofovir was licensed by the FDA for treating human smallpox disease in both adults and children. To facilitate the use of brincidofovir for the treatment of monkeypox, the CDC is currently developing an Expanded Access Investigational New Drug.382 In comparison with cidofovir, brincidofovir has higher intracellular levels of the active drug, well oral bioavailability, superior antiadenoviral activity, and no nephrotoxicity.314,375,383,384,385,386,387 The complete resolution in a patient with progressive vaccinia (PV) demonstrated the effectiveness of brincidofovir in combination with other antiviral drugs.388 Recent research, however, indicates brincidofovir is associated with serious adverse effects in monkeypox patients. Over the past three years, three cases have experienced liver toxicity in the United Kingdom.329 Further studies are warranted in humans regarding the efficacy of brincidofovir against monkeypox virus.
VIGIV
Vaccinia immune globulin intravenous (VIGIV), a FDA-approved medicine, is applied to treat smallpox vaccination complications such as severe generalized vaccinia, vaccinia infections in people who have skin conditions, eczema vaccinatum, progressive vaccinia and aberrant infections induced by vaccinia virus (excluding isolated keratitis).389 Vaccinia immune globulin (VIG) is a sterile solution made up of high titers of IgG antibodies against the vaccinia virus taken from healthy persons who had been previously vaccinated against the live vaccinia virus.390 CDC allows the use of VIG for the treatment of monkeypox in an outbreak. Several studies have reported the patients received VIGIV for the treatment of orthopoxvirus infection.391,392,393 Nevertheless, data on VIG’s effectiveness in treating monkeypox virus infection are unavailable. Physicians may consider using it in severe cases. The VIG is also suitable for prophylactic use in persons with severe immunodeficiency in T cell function for whom smallpox vaccination is contraindicated after exposure to monkeypox virus.
Monkeypox prevention
To prevent getting monkeypox, CDC suggest that people need to avoid close, skin-to-skin contact with someone who has monkeypox-like rashes, avoid contact with objects and materials that a case with monkeypox has used, use hand sanitizers that contain alcohol before eating, touching the face, and wash your hands frequently after using the bathroom.
There is no vaccine specifically designed to prevent monkeypox virus infection. Owning to immunological cross protection among orthopoxviruses,194,298,394,395,396,397,398 smallpox vaccines (vaccinia virus-based) were recommended for use in the current outbreak of monkeypox.279,399,400,401,402,403,404,405 Epidemiological data on human monkeypox collected in Zaire from 1980 to 1984 revealed that there was a significant difference in attack rates between contacts without a vaccination scar and those who had been vaccinated in the past (7.2% vs. 0.9%).406 Rimoin et al. suggested that monkeypox incidence increased dramatically in the DRC 30 years after smallpox vaccination campaigns ended.407 Furthermore, their data suggested that vaccine-induced immunity lasts a long time as individuals vaccinated against smallpox >25 years ago are still at reduced risk of monkeypox today. As demonstrated by the US monkeypox outbreak of 2003, smallpox vaccination can potentially sustain cross-immune protection against West African monkeypox for decades afterward. Three individuals who had not previously been infected with monkeypox virus and had been vaccinated against smallpox for decades were unaware of their subsequent infection because none of them had any clinical characteristics associated with the monkeypox.298
Studies have reported that first-generation live vaccinia vaccines can provide approximately 85% protection against monkeypox infection due to cross-reactive antibodies that can be induced by orthopoxviruses.160 Second-generation vaccine is ACAM2000, a live attenuated vaccinia vaccine approved in the USA in August 2007 for prevention of smallpox408,409 (Fig. 4). Its effectiveness has been demonstrated in both animal models327,410,411,412 and clinical trials (Table 3).
Development of monkeypox vaccines in the future. Currently, live attenuated vaccines such as ACAM2000 and JYNNEOS are clinically recommended for the prevention of monkeypox, but these vaccines were originally designed to prevent smallpox. Therefore, there is a demand for the development of vaccines that specifically prevent monkeypox, such as inactivated vaccines, DNA vaccines, RNA vaccines, and recombinant protein vaccines. These types of vaccines have proven their efficacy and safety in the COVID-19 epidemic
Assessment of the protective effect against aerosolized monkeypox virus in cynomolgus macaques revealed that a single immunization with ACAM2000 vaccines were protected completely.410 Monkeys challenged with lethal monkeypox virus after vaccinated with the ACAM200 live attenuated smallpox vaccine, all (N = 12) vaccinated treated monkeys survived.412 In a prairie dog model, for ACAM2000, the vaccine administered at 1 day postexposure was similarly effective with the vaccine administered at 3 days postexposure, resulting in 50–62% survival, compared with only 25% survival in the unvaccinated group.411 Taken together, ACAM2000 is likely effective at preventing monkeypox; however, due to its potential for replication, it may cause severe side effects, including progressive vaccinia, eczema vaccinatum, and myopericarditis.388,409,413,414,415,416,417,418,419,420,421 A person with a weak immune system, including someone with HIV, pregnant women, and those who suffer from skin conditions like eczema, are not advised to use it.150,422
The third-generation vaccine, modified vaccinia Ankara (MVA, JYNNEOS in the United States, IMVANEX in the European Union, and IMAMUNE in Canada), was approved in individuals 18 years of age or older considered to be more susceptible for smallpox or monkeypox infection in US to prevent both smallpox and monkeypox disease in 2019.423 Unlike ACAM2000, JYNNEOS is a nonreplicating vaccine, which will not lead to the production of live virus. As a smallpox vaccine, JYNNEOS has demonstrated good immunogenicity, safety, and efficacy both in animal models and clinicals.424,425,426,427,428,429,430,431,432,433,434,435,436 It was the only currently FDA-approved vaccinia vaccine for the prevention of monkeypox disease437 (Fig. 4). Several animal model studies exhibited good protective effects against monkeypox,411,438,439,440 furthermore, in macaque challenge trials 100% protection against monkeypox infection was demonstrated, along with long lasting immunity410,441 and long lasting protective immunity for a lethal monkeypox challenge.442 Petersen et al.443 evaluated its effectiveness, immunogenicity, and safety in healthcare workers at risk of monkeypox virus infection in the DRC. Meanwhile, another human clinical investigation examining the effectiveness of monkeypox vaccination after exposure is currently underway.444 Numerous clinical trials of MVA445 have proved the efficacy and safety of JYNNEOS, as listed in Table 3.
As a result of the outbreak of monkeypox in 2022, several countries, including the United States, Spain, Germany and the United Kingdom, have announced they are buying vaccines and/or releasing vaccines them from national stockpiles to combat the epidemic. JYNNEOS is being used by the US for pre-exposure vaccination of people who are at risk of occupational exposure to orthopoxviruses according to CDC announcements made in May 2022.399,446 Meanwhile, in its interim guidance for monkeypox vaccines, WHO recommended post-exposure prophylaxis (PEP) ideally within 4 days of the first exposure, pre-exposure prophylaxis (PrEP) is recommended for health workers at risk, clinical laboratory staff performing diagnostic testing, laboratory personnel working with orthopoxviruses, and others at risk under national policy.447 IMAMUNE is approved for immunization against monkeypox and Orthopox virus by the Public Health Agency of Canada (PHAC), and IMVANEX is recommended by the European Medicine Agency (EMA) for monkeypox prevention.448,449 By July 27, 786,000 more doses are expected to be released that week after the federal government distributed 300,000 doses of vaccine to state and local health authorities and clinics throughout the country. JYNNEOS is manufactured exclusively by Bavarian Nordic, which has a limited production capacity. Recently, due to the vaccine shortage, Bavarian Nordic has expanded its capacity by working with U.S. contract manufacturer to fill smallpox/monkeypox vaccines.450 In addition, a European Union Authorization (EUA) was approved by the FDA for the JYNNEOS vaccine, allowing healthcare providers to administer it intradermally to individuals 18 years of age and older who have been considered to be more susceptible for monkeypox infection.451 Individuals vaccinated intradermally were less vaccinated (by one-fifth) than those vaccinated subcutaneously (SC). The total dose available for use could increase by five-fold as a result. A study with lower intradermal doses of MVA was found to be immunologically noninferior to the standard subcutaneous dose (NCT00914732).452
In 1975, the other smallpox vaccine, LC16m8, was licensed for active immunization against smallpox in Japan. This vaccine is derived from the Lister (Elstree) strain of vaccinia and is a live, replicating, attenuated 3rd generation vaccine.453 This high attenuation resulted from a single nucleotide deletion mutation in the B5R viral gene, resulting in a truncated membrane protein B5, which is one of the most immunogenic proteins.454,455,456 Several animal model challenge studies of safety and efficacy of LC16m8 have been conducted in mouse,454,457,458,459,460,461 rabbit,459,460 and nonhuman primates.462,463 Both the intranasal and subcutaneous inoculation models showed no symptoms of monkeypox after immunization with LC16m8.462 A single vaccination of non-human primate model revealed that LC16m8 exhibited long-lasting protection against monkeypox virus, evidence from the reduction in viremia and the IgG antibody response.464 Notably, another nonhuman primate model revealed that for immunocompromised individuals, LC16m8 is a safer and more effective alternative to ACAM2000 and Dryvax (first-generation vaccines).463 LC16m8 was shown to produce neutralizing antibodies for vaccinia, monkeypox, and variola major, as well as broad T cell responses in a phase I/II clinical trial.465 In addition, various immunogenic studies in human have confirmed a good safety profile about LC16m8.466,467,468,469,470,471,472 In summary, these results reveal that LC16m8 may be effective for preventing people against monkeypox. Japanese authorities expanded the range of indications for this vaccine to include protection against monkeypox in August 2022.473
Conclusion and perspectives
Monkeypox, COVID-19, and polio are among the three emergencies that WHO has declared worldwide at the moment. Monkeypox is now an international problem after previously being endemic to Africa. Until September 2022, more than 56000 patients have been diagnosed in the monkeypox 2022 outbreak. Monkeypox infection patients are rapidly increasing around the world, it is likely to be caused by a combination of natural and human factors. On the other hand, there has been an increase in human–wildlife contact due to climate change, deforestation, and the Ukrainian–Russian war, among other factors. In addition, it is still possible that monkeypox virus will become more prevalent due to the cessation of smallpox vaccination with vaccinia vaccine in 1980. Currently, in spite of the fact that mass vaccination is not recommended or achievable, individuals manage monkeypox may though vaccine (post-exposure prophylaxis, or pre-exposure prophylaxis), anti-viral medicines, as well as isolate or quarantine of the patients and any contacts with the patients.
Particularly, there are large number of patients have been confirmed in MSM population in this 2022 outbreak and has been related to unanticipated anal and genital lesions, suggesting the monkeypox virus may be spreading via sexual transmission.158,170,179,180,185,272 This novel transmission pattern was suspected during 2017 outbreak of human monkeypox in Nigeria, but was not proven.474 Meanwhile, MSM population has higher risk on other disease through sexual transmission, such as HIV. In Ogoina and colleagues’ study,475 it was reported that monkeypox patients in Nigeria who were HIV-positive had longer-lasting illness, and larger lesions, and a higher percentage of and genital ulcers and secondary bacterial skin infections. By contrast, Tarin-Vicente and his colleagues180 reported that there was no difference between HIV-positive patients and the rest of the population in terms of severity or progression of the disease. Therefore, more investigations are needed to understand the effectiveness of anti-viral treatment among individuals infected with both HIV and human monkeypox virus. Besides, in order to reduce monkeypox transmission within MSM groups, public health efforts must deal with challenges including homophobia, stigma, and discrimination.
Given the current global monkeypox epidemic, a race to create monkeypox -specific vaccines may occur like COVID-19 (Fig. 4). Moderna has announced that they have initiated a program to consider whether developing an mRNA monkeypox vaccine or not because of growing demand for vaccination, even though no further information on the vaccine or potential development timeline was released latter.476 Meanwhile, the U.S. Patent and Trademark Office (USPTO) has addressed a patent to the Tonix Pharmaceuticals company for its vaccine candidate namely TNX-801, which was designed to protect against smallpox and monkeypox.477 TNX-801 is a novel, horsepox-based live virus vaccine based on synthesized horsepox (sHPXV). Effectiveness and safety have been demonstrated both in mouse models previously.478 In addition, TNX-801 vaccination protects macaques from monkeypox challenge, no lesions were seen in all monkeys.479 The monkeypox virion membrane surface-binding protein E8L is vital for virus attachment to host cells, which may be also used to explore new vaccine against monkeypox.480 Importantly, knowledge of the immunological response and mechanism of monkeypox virus infection would be critical, providing new ideas for vaccine development. Identification of the genes responsible for the host-range defect of virus may engineer more effective and safe vaccines, such as C16L/B22R, C16L (MVA genome).481 For severely immunocompromised individuals, valid vaccination strategies that bypass CD4 negative cell help are needed to protect against virus.482
Global public health has been severely tested by the COVID-19 pandemic in the past two years. Confirmed cases of monkeypox are increasing rapidly worldwide especially in the US, which may have a negative impact on the global economy. With monkeypox being announced a public health emergency of international concern by WHO,11 people should raise awareness and build diagnostic capacity for the monkeypox epidemic in order to limit further spread of the virus. We should improve understanding and clinical management of monkeypox, as well as infection prevention and control skills, especially among public health personnel. At the same time, stigma and discrimination within the MSM community should be properly addressed and equitable access to treatment and vaccines should be ensured. Finally, we should initiate a global collaboration to conduct clinical investigations to test the efficacy and safety of monkeypox vaccines as well as antiviral drugs.
References
Bunge, E. M. et al. The changing epidemiology of human monkeypox-a potential threat? A systematic review. PLoS Negl. Trop. Dis. 16, e0010141 (2022).
WHO. Multi-country monkeypox outbreak in non-endemic countries. https://www.who.int/emergencies/disease-outbreak-news/item/2022-DON385 (2022).
UKHSA. Guidance: monkeypox: background information. https://www.gov.uk/guidance/monkeypox (2022).
ECDC. Epidemiological update: monkeypox multi-country outbreak. https://www.ecdc.europa.eu/en/news-events/epidemiological-update-monkeypox-multi-country-outbreak (2022).
Walter, K. & Malani, P. N. What is monkeypox? JAMA 328, 222 (2022).
ECDC. Risk assessment: monkeypox multi-country outbreak. https://www.ecdc.europa.eu/en/publications-data/risk-assessment-monkeypox-multi-country-outbreak (2022).
WHO. Monkeypox fact sheet. https://www.who.int/news-room/fact-sheets/detail/monkeypox (2022).
Kraemer, M. U. G. et al. Tracking the 2022 monkeypox outbreak with epidemiological data in real-time. Lancet Infect. Dis. 22, 941–942 (2022).
Zumla, A. et al. Monkeypox outbreaks outside endemic regions: scientific and social priorities. Lancet Infect. Dis. 22, 929–931 (2022).
Venkatesan, P. Global monkeypox outbreak. Lancet Infect. Dis. 22, 950 (2022).
WHO. WHO Director-General declares the ongoing monkeypox outbreak a public health emergency of international concern. https://www.who.int/europe/news/item/23-07-2022-who-director-general-declares-the-ongoing-monkeypox-outbreak-a-public-health-event-of-international-concern (2022).
Zarocostas, J. Monkeypox PHEIC decision hoped to spur the world to act. Lancet 400, 347 (2022).
CDC. 2022 Monkeypox outbreak global map. https://www.cdc.gov/poxvirus/monkeypox/response/2022/world-map.html (2022).
Faye, O. et al. Genomic characterisation of human monkeypox virus in Nigeria. Lancet Infect. Dis. 18, 246 (2018).
Marennikov, S. S. & Moyer, R. W. In Orthopoxviruses Pathogenic for Humans 11–18 (Springer, 2005).
Shchelkunov, S. N. An increasing danger of zoonotic orthopoxvirus infections. PLoS Pathog. 9, e1003756 (2013).
Shchelkunov, S. N. et al. Human monkeypox and smallpox viruses: genomic comparison. FEBS Lett. 509, 66–70 (2001).
Gubser, C., Hue, S., Kellam, P. & Smith, G. L. Poxvirus genomes: a phylogenetic analysis. J. Gen. Virol. 85, 105–117 (2004).
WHO. Smallpox. https://www.who.int/health-topics/smallpox#tab=tab_1 (2022).
Henderson, D. A. The eradication of smallpox-an overview of the past, present, and future. Vaccine 29(Suppl. 4), D7–D9 (2011).
Smith, G. L. & McFadden, G. Smallpox: anything to declare? Nat. Rev. Immunol. 2, 521–527 (2002).
Strassburg, M. A. The global eradication of smallpox. Am. J. Infect. Control. 10, 53–59 (1982).
Parker, S., Nuara, A., Buller, R. M. & Schultz, D. A. Human monkeypox: an emerging zoonotic disease. Future Microbiol. 2, 17–34 (2007).
Bayer-Garner, I. B. Monkeypox virus: histologic, immunohistochemical and electron-microscopic findings. J. Cutan. Pathol. 32, 28–34 (2005).
Sale, T. A., Melski, J. W. & Stratman, E. J. Monkeypox: an epidemiologic and clinical comparison of African and US disease. J. Am. Acad. Dermatol. 55, 478–481 (2006).
Saijo, M. et al. Virulence and pathophysiology of the Congo Basin and West African strains of monkeypox virus in non-human primates. J. Gen. Virol. 90, 2266–2271 (2009).
Alakunle, E. F. & Okeke, M. I. Monkeypox virus: a neglected zoonotic pathogen spreads globally. Nat. Rev. Microbiol. 20, 507–508 (2022).
Jezek, Z., Szczeniowski, M., Paluku, K. M. & Mutombo, M. Human monkeypox: clinical features of 282 patients. J. Infect. Dis. 156, 293–298 (1987).
Parker, S. & Buller, R. M. A review of experimental and natural infections of animals with monkeypox virus between 1958 and 2012. Future Virol. 8, 129–157 (2013).
Guarner, J. et al. Monkeypox transmission and pathogenesis in prairie dogs. Emerg. Infect. Dis. 10, 426–431 (2004).
Hutson, C. L. et al. Dosage comparison of Congo Basin and West African strains of monkeypox virus using a prairie dog animal model of systemic orthopoxvirus disease. Virology 402, 72–82 (2010).
Chen, N. et al. Virulence differences between monkeypox virus isolates from West Africa and the Congo basin. Virology 340, 46–63 (2005).
Ladnyj, I. D., Ziegler, P. & Kima, E. A human infection caused by monkeypox virus in Basankusu Territory, Democratic Republic of the Congo. Bull. World Health Organ. 46, 593–597 (1972).
Marennikova, S. S. et al. Isolation and properties of the causal agent of a new variola-like disease (monkeypox) in man. Bull. World Health Organ. 46, 599–611 (1972).
Von Magnus, P., Andersen, E. K., Petersen, K. B. & Birch-Andersen, A. A. A pox-like disease in cynomolgus monkeys. Acta Pathol. Microbiol. Scand. 46, 156–176 (1959).
Damon, I. K. Status of human monkeypox: clinical disease, epidemiology and research. Vaccine 29(Suppl. 4), D54–D59 (2011).
Alakunle, E., Moens, U., Nchinda, G. & Okeke, M. I. Monkeypox virus in Nigeria: infection biology, epidemiology, and evolution. Viruses. 12, 1257 (2020).
Orviz, E. et al. Monkeypox outbreak in Madrid (Spain): clinical and virological aspects. J. Infect. 85, 412–417 (2022).
Isidro, J. et al. Phylogenomic characterization and signs of microevolution in the 2022 multi-country outbreak of monkeypox virus. Nat. Med. 28, 1569–1572 (2022).
Claro, I. M. et al. Shotgun metagenomic sequencing of the first case of monkeypox virus in Brazil, 2022. Rev. Inst. Med. Trop. Sao Paulo 64, e48 (2022).
Luna, N. et al. Phylogenomic analysis of the monkeypox virus (MPXV) 2022 outbreak: emergence of a novel viral lineage? Travel Med. Infect. Dis. 49, 102402 (2022).
Minhaj, F. S. et al. Monkeypox outbreak - nine states, May 2022. MMWR Morb. Mortal. Wkly Rep. 71, 764–769 (2022).
Isidro, J., Borges, V. & Pinto, M. First draft genome sequence of Monkeypox virus associated with the suspected multi-country outbreak, May 2022 (confirmed case in Portugal). https://virological.org/t/first-draft-genome-sequence-of-monkeypox-virus-associated-with-the-suspected-multi-country-outbreak-may-2022-confirmed-case-in-portugal/799 (2022).
Thakur, V., Thakur, P., Srivastava, S. & Kumar, P. Monkeypox virus (MPX) in humans a concern: trespassing the global boundaries - correspondence. Int. J. Surg. 104, 106703 (2022).
Taylor, L. Monkeypox: concerns mount over vaccine inequity. BMJ 378, o1971 (2022).
Taylor, L. Monkeypox: WHO declares a public health emergency of international concern. BMJ 378, o1874 (2022).
Leon-Figueroa, D. A. et al. The never-ending global emergence of viral zoonoses after COVID-19? The rising concern of monkeypox in Europe, North America and beyond. Travel Med. Infect. Dis. 49, 102362 (2022).
Cheng, K., Guo, Q., Zhou, Y. & Wu, H. Concern over monkeypox outbreak: what can we learn from the top 100 highly cited articles in monkeypox research? Travel Med. Infect. Dis. 49, 102371 (2022).
Awan, U. A. et al. Monkeypox: a new threat at our doorstep! J. Infect. 85, e47–e48 (2022).
Otu, A. et al. Global human monkeypox outbreak: atypical presentation demanding urgent public health action. Lancet Microbe 3, e554–e555 (2022).
The, L. Monkeypox: a global wake-up call. Lancet 400, 337 (2022).
Jezek, Z. et al. Human monkeypox: secondary attack rates. Bull. World Health Organ. 66, 465–470 (1988).
Jezek, Z. et al. Clinico-epidemiological features of monkeypox patients with an animal or human source of infection. Bull. World Health Organ. 66, 459–464 (1988).
Jezek, Z., Grab, B., Paluku, K. M. & Szczeniowski, M. V. Human monkeypox: disease pattern, incidence and attack rates in a rural area of northern Zaire. Trop. Geogr. Med. 40, 73–83 (1988).
Khodakevich, L. et al. Monkey pox virus infection in humans in the Central African Republic. Bull. Soc. Pathol. Exot. Filiales 78, 311–320 (1985).
Meyer, A. et al. First appearance of monkey pox in human beings iControl and Prevention. Human monkeypox–Kasai Oriental, Democraticn Gabon. Med Trop. (Mars). 51, 53–57 (1991).
Jezek, Z. F. F. Human Monkeypox (Karger, 1988).
Lourie, B. et al. Human infection with monkeypox virus: laboratory investigation of six cases in West Africa. Bull. World Health Organ. 46, 633–639 (1972).
Arita, I. & Henderson, D. A. Monkeypox and whitepox viruses in West and Central Africa. Bull. World Health Organ. 53, 347–353 (1976).
Merouze, F. & Lesoin, J. J. Monkeypox: second human case observed in Ivory Coast (rural health sector of Daloa. Med Trop. (Mars). 43, 145–147 (1983).
Heymann, D. L., Szczeniowski, M. & Esteves, K. Re-emergence of monkeypox in Africa: a review of the past six years. Br. Med. Bull. 54, 693–702 (1998).
Tchokoteu, P. F. et al. Variola or a severe case of varicella? A case of human variola due to monkeypox virus in a child from the Cameroon. Ann. Soc. Belg. Med. Trop. 71, 123–128 (1991).
Centers for Disease Control and Prevention. Human monkeypox--Kasai Oriental, Democratic Republic of Congo, February 1996-October 1997. JAMA. 279, 189–190 (1998).
Mwanbal, P. T. et al. Human monkeypox in Kasai Oriental, Zaire (1996-1997). Eur. Surveill. 2, 33–35 (1997).
Mukinda, V. B. et al. Re-emergence of human monkeypox in Zaire in 1996. Monkeypox Epidemiologic Working Group. Lancet 349, 1449–1450 (1997).
Meyer, H. et al. Outbreaks of disease suspected of being due to human monkeypox virus infection in the Democratic Republic of Congo in 2001. J. Clin. Microbiol. 40, 2919–2921 (2002).
Rimoin, A. W. et al. Endemic human monkeypox, Democratic Republic of Congo, 2001-2004. Emerg. Infect. Dis. 13, 934–937 (2007).
Reynolds, M. G. et al. Spectrum of infection and risk factors for human monkeypox, United States, 2003. Emerg. Infect. Dis. 13, 1332–1339 (2007).
Reed, K. D. et al. The detection of monkeypox in humans in the Western Hemisphere. N. Engl. J. Med. 350, 342–350 (2004).
Reynolds, M. G. et al. Clinical manifestations of human monkeypox influenced by route of infection. J. Infect. Dis. 194, 773–780 (2006).
Huhn, G. D. et al. Clinical characteristics of human monkeypox, and risk factors for severe disease. Clin. Infect. Dis. 41, 1742–1751 (2005).
Learned, L. A. et al. Extended interhuman transmission of monkeypox in a hospital community in the Republic of the Congo, 2003. Am. J. Trop. Med. Hyg. 73, 428–434 (2005).
Damon, I. K., Roth, C. E. & Chowdhary, V. Discovery of monkeypox in Sudan. N. Engl. J. Med. 355, 962–963 (2006).
McCollum, A. M. et al. Human monkeypox in the Kivus, a conflict region of the Democratic Republic of the Congo. Am. J. Trop. Med. Hyg. 93, 718–721 (2015).
Nolen, L. D. et al. Extended human-to-human transmission during a monkeypox outbreak in the Democratic Republic of the Congo. Emerg. Infect. Dis. 22, 1014–1021 (2016).
Durski, K. N. et al. Emergence of monkeypox - West and Central Africa, 1970-2017. MMWR Morb. Mortal. Wkly Rep. 67, 306–310 (2018).
Kalthan, E. et al. Twelve cases of monkeypox virus outbreak in Bangassou District (Central African Republic) in December 2015. Bull. Soc. Pathol. Exot. 109, 358–363 (2016).
Berthet, N. et al. Maculopapular lesions in the Central African Republic. Lancet 378, 1354 (2011).
Sadeuh-Mba, S. A. et al. Monkeypox virus phylogenetic similarities between a human case detected in Cameroon in 2018 and the 2017-2018 outbreak in Nigeria. Infect. Genet. Evol. 69, 8–11 (2019).
Beer, E. M. & Rao, V. B. A systematic review of the epidemiology of human monkeypox outbreaks and implications for outbreak strategy. PLoS Negl. Trop. Dis. 13, e0007791 (2019).
Li, D. et al. Evaluation of the GeneXpert for human monkeypox diagnosis. Am. J. Trop. Med. Hyg. 96, 405–410 (2017).
Reynolds, M. G. et al. Detection of human monkeypox in the Republic of the Congo following intensive community education. Am. J. Trop. Med. Hyg. 88, 982–985 (2013).
Yinka-Ogunleye, A. et al. Outbreak of human monkeypox in Nigeria in 2017-18: a clinical and epidemiological report. Lancet Infect. Dis. 19, 872–879 (2019).
Yinka-Ogunleye, A. et al. Reemergence of human monkeypox in Nigeria, 2017. Emerg. Infect. Dis. 24, 1149–1151 (2018).
Kabuga, A. I. & El Zowalaty, M. E. A review of the monkeypox virus and a recent outbreak of skin rash disease in Nigeria. J. Med. Virol. 91, 533–540 (2019).
Hobson, G. et al. Family cluster of three cases of monkeypox imported from Nigeria to the United Kingdom, May 2021. Euro Surveill. 26, 2100745 (2021).
Vaughan, A. et al. Human-to-human transmission of monkeypox virus, United Kingdom, October 2018. Emerg. Infect. Dis. 26, 782–785 (2020).
Vaughan, A. et al. Two cases of monkeypox imported to the United Kingdom, September 2018. Euro Surveill. 23, 1800509 (2018).
Yong, S. E. F. et al. Imported monkeypox, Singapore. Emerg. Infect. Dis. 26, 1826–1830 (2020).
Ng, O. T. et al. A case of imported monkeypox in Singapore. Lancet Infect. Dis. 19, 1166 (2019).
Erez, N. et al. Diagnosis of imported monkeypox, Israel, 2018. Emerg. Infect. Dis. 25, 980–983 (2019).
Cohen-Gihon, I. et al. Identification and whole-genome sequencing of a monkeypox virus strain isolated in Israel. Microbiol. Resour. Announc. 9, e01524-19 (2020).
Rao, A. K. et al. Monkeypox in a traveler returning from Nigeria - Dallas, Texas, July 2021. MMWR Morb. Mortal. Wkly Rep. 71, 509–516 (2022).
WHO. Monkeypox– United Kingdom of Great Britain and Northern Ireland. https://www.who.int/emergencies/disease-outbreak-news/item/2022-DON381 (2022).
BBC. Monkeypox: two more confirmed cases of viral infection. https://www.bbc.com/news/uk-england-london-61449214 (2022).
BBC. Monkeypox: Four more cases detected in England. https://www.bbc.com/news/health-61470940 (2022).
Sklenovska, N. & Van Ranst, M. Emergence of monkeypox as the most important orthopoxvirus infection in humans. Front. Public Health 6, 241 (2018).
Likos, A. M. et al. A tale of two clades: monkeypox viruses. J. Gen. Virol. 86, 2661–2672 (2005).
Monkeypox. There are already 14 cases of monkeypox in Portugal. www.dn.pt (in European Portuguese) (2022).
Güell, O. Health confirms that the analyzes of the first seven suspected cases of monkeypox have tested positive. https://newsrnd.com/life/2022-05-18-health-confirms-that-the-analyzes-of-the-first-seven-suspected-cases-of-monkeypox-have-tested-positive.SkhHqy7v5.html (2022).
ICI.Radio-Canada.ca, Z. S. Monkey pox: at least 13 cases being examined in Montreal. https://www.archyde.com/monkey-pox-at-least-13-cases-being-examined-in-montreal/ (2022).
Sobey, R. Massachusetts confirms rare monkeypox case, the first in the US this year. In Boston Herald (2022).
First infection with monkey pox virus detected in our country. VRT NWS (2022).
A case of smallpox reported in Sweden – Public Health Agency. https://web.archive.org/web/20220519110819/https://www.folkhalsomyndigheten.se/nyheter-och-press/nyhetsarkiv/2022/maj/ett-fall-av-apkoppor-rapporterat-i-sverige/ (2022).
Italy reports first case of monkeypox infection, two more suspected. Reuters (2022).
Parkes-Hupton, H. & Johnson, S. Monkeypox confirmed in Melbourne and Sydney. ABC News. Australian Broadcasting Commission (2022).
Monkeypox cases investigated in Europe, US, Canada and Australia. BBC News (2022).
France, Germany, Belgium report first monkeypox cases amid unusual spread in Europe. https://www.msn.com/en-gb/news/world/france-germany-belgium-report-first-monkeypox-cases-amid-unusual-spread-in-europe/ar-AAXwdCU (2022).
Dutch health agency confirms first monkeypox case in the Netherlands. Reuters (2022).
Noe, S. et al. Clinical and virological features of first human monkeypox cases in Germany. Infection https://doi.org/10.1007/s15010-022-01874-z (2022).
Switzerland confirms its first case of monkeypox. https://www.reuters.com/world/europe/switzerland-confirms-its-first-case-monkeypox-2022-05-21/ (2022).
Israel confirms first case of monkeypox virus. https://www.haaretz.com/israel-news/2022-05-20/ty-article/israel-discovers-first-case-of-monkeypox-virus/00000180-e9f9-d189-af82-f9fd13df0000 (2022).
Abed Alah, M., Abdeen, S., Tayar, E. & Bougmiza, I. The story behind the first few cases of monkeypox infection in non-endemic countries, 2022. J. Infect. Public Health 15, 970–974 (2022).
Nuzzo, J. B., Borio, L. L. & Gostin, L. O. The WHO declaration of monkeypox as a global public health emergency. JAMA 328, 615–617 (2022).
Wenham, C. & Eccleston-Turner, M. Monkeypox as a PHEIC: implications for global health governance. Lancet https://doi.org/10.1016/S0140-6736(22)01437-4 (2022).
Webb, E. et al. Availability, scope and quality of monkeypox clinical management guidelines globally: a systematic review. BMJ Glob. Health. 7, e009838 (2022).
CHP investigates imported monkeypox case and alert level of the preparedness and response plan for monkeypox activated. https://www.info.gov.hk/gia/general/202209/06/P2022090600594.htm?fontSize=1 (2022).
Yang, Z. S. et al. The first monkeypox virus infection detected in Taiwan-the awareness and preparation. Int. J. Infect. Dis. 122, 991–995 (2022).
CDC. Monkeypox, Taiwan. https://www.cdc.gov.tw/Disease/SubIndex/G3A6nyt8JmqIUcUF5Pek6w (2022).
U.S. declares monkeypox outbreak a public health emergency. https://www.reuters.com/world/us/us-declare-monkeypox-public-health-emergency-washington-post-2022-08-04/ (2022).
CDC. 2022 U.S. map & case count. https://www.cdc.gov/poxvirus/monkeypox/response/2022/us-map.html (2022).
Philpott, D. et al. Epidemiologic and clinical characteristics of monkeypox cases - United States, May 17-July 22, 2022. MMWR Morb. Mortal. Wkly Rep. 71, 1018–1022 (2022).
Di Giulio, D. B. & Eckburg, P. B. Human monkeypox: an emerging zoonosis. Lancet Infect. Dis. 4, 15–25 (2004).
Mutombo, M., Arita, I. & Jezek, Z. Human monkeypox transmitted by a chimpanzee in a tropical rain-forest area of Zaire. Lancet 1, 735–737 (1983).
Khodakevich, L., Jezek, Z. & Kinzanzka, K. Isolation of monkeypox virus from wild squirrel infected in nature. Lancet 1, 98–99 (1986).
Hutson, C. L. et al. Laboratory investigations of African pouched rats (Cricetomys gambianus) as a potential reservoir host species for monkeypox virus. PLoS Negl. Trop. Dis. 9, e0004013 (2015).
Hutin, Y. J. et al. Outbreak of human monkeypox, Democratic of Congo, 1996 to 1997. Emerg. Infect. Dis. 7, 434–438 (2001).
Nolen, L. D. et al. Introduction of monkeypox into a community and household: risk factors and zoonotic reservoirs in the Democratic Republic of the Congo. Am. J. Trop. Med. Hyg. 93, 410–415 (2015).
Khodakevich, L. et al. The role of squirrels in sustaining monkeypox virus transmission. Trop. Geogr. Med. 39, 115–122 (1987).
Khodakevich, L., Jezek, Z. & Messinger, D. Monkeypox virus: ecology and public health significance. Bull. World Health Organ. 66, 747–752 (1988).
Radonic, A. et al. Fatal monkeypox in wild-living sooty mangabey, Cote d’Ivoire, 2012. Emerg. Infect. Dis. 20, 1009–1011 (2014).
Patrono, L. V. et al. Monkeypox virus emergence in wild chimpanzees reveals distinct clinical outcomes and viral diversity. Nat. Microbiol. 5, 955–965 (2020).
Doty, J. B. et al. Assessing monkeypox virus prevalence in small mammals at the human-animal interface in the Democratic Republic of the Congo. Viruses. 9, 283 (2017).
Reynolds, M. G. et al. Monkeypox re-emergence in Africa: a call to expand the concept and practice of One Health. Expert Rev. Antiinfect. Ther. 17, 129–139 (2019).
Hutson, C. L. et al. Comparison of monkeypox virus clade kinetics and pathology within the prairie dog animal model using a serial sacrifice study design. Biomed. Res. Int. 2015, 965710 (2015).
Hutson, C. L. et al. Transmissibility of the monkeypox virus clades via respiratory transmission: investigation using the prairie dog-monkeypox virus challenge system. PLoS ONE 8, e55488 (2013).
Hutson, C. L. et al. Monkeypox disease transmission in an experimental setting: prairie dog animal model. PLoS ONE 6, e28295 (2011).
Lai, C. C. et al. Monkeypox: an emerging global threat during the COVID-19 pandemic. J Microbiol. Immunol. Infect. https://doi.org/10.1016/j.jmii.2022.07.004 (2022).
Zhu, M. et al. Unusual global outbreak of monkeypox: what should we do? Front. Med. 16, 507–517 (2022).
Kaler, J. et al. Monkeypox: a comprehensive review of transmission, pathogenesis, and manifestation. Cureus 14, e26531 (2022).
Kumar, N., Acharya, A., Gendelman, H. E. & Byrareddy, S. N. The 2022 outbreak and the pathobiology of the monkeypox virus. J. Autoimmun. 131, 102855 (2022).
Pastula, D. M. & Tyler, K. L. An overview of monkeypox virus and its neuroinvasive potential. Ann. Neurol. 92, 527–531 (2022).
Brown, K. & Leggat, P. A. Human monkeypox: current state of knowledge and implications for the future. Trop. Med. Infect. Dis. 1, 8 (2016).
Vivancos, R. et al. Community transmission of monkeypox in the United Kingdom, April to May 2022. Euro Surveill. 27, 2200422 (2022).
Kisalu, N. K. & Mokili, J. L. Toward understanding the outcomes of monkeypox infection in human pregnancy. J. Infect. Dis. 216, 795–797 (2017).
Fahrni, M. L., Priyanka & Choudhary, O. P. Possibility of vertical transmission of the human monkeypox virus. Int. J. Surg. 105, 106832 (2022).
Mbala, P. K. et al. Maternal and fetal outcomes among pregnant women with human monkeypox infection in the Democratic Republic of Congo. J. Infect. Dis. 216, 824–828 (2017).
Thornhill, J. P. et al. Monkeypox virus infection in humans across 16 countries - April-June 2022. N. Engl. J. Med. 387, 679–691 (2022).
Khalil, A. et al. Monkeypox vaccines in pregnancy: lessons must be learned from COVID-19. Lancet Glob. Health 10, e1230–e1231 (2022).
Dashraath, P. et al. Guidelines for pregnant individuals with monkeypox virus exposure. Lancet 400, 21–22 (2022).
Vouga, M., Nielsen-Saines, K., Dashraath, P. & Baud, D. The monkeypox outbreak: risks to children and pregnant women. Lancet Child Adolesc. Health https://doi.org/10.1016/S2352-4642(22)00223-1 (2022).
Pomar, L., Favre, G. & Baud, D. Monkeypox infection during pregnancy: European registry to quantify maternal and fetal risks. Ultrasound Obstet. Gynecol. 60, 431 (2022).
Khalil, A. et al. Monkeypox and pregnancy: what do obstetricians need to know? Ultrasound Obstet. Gynecol. 60, 22–27 (2022).
Khalil, A. et al. Monkeypox vaccines in pregnancy: lessons must be learned from COVID-19. Lancet Glob. Health 10, e1230–e1231 (2022).
Khalil, A., Samara, A., O’Brien, P. & Ladhani, S. Call for a unified approach to Monkeypox infection in pregnancy: lessons from the COVID-19 pandemic. Nat. Commun. 13, 5038 (2022).
Dashraath, P. et al. Monkeypox and pregnancy: forecasting the risks. Am. J. Obstet. Gynecol. https://doi.org/10.1016/j.ajog.2022.08.017 (2022).
Jamieson, D. J., Jernigan, D. B., Ellis, J. E. & Treadwell, T. A. Emerging infections and pregnancy: West Nile virus, monkeypox, severe acute respiratory syndrome, and bioterrorism. Clin. Perinatol. 32, 765–776 (2005).
Seang, S. et al. Evidence of human-to-dog transmission of monkeypox virus. Lancet 400, 658–659 (2022).
Grant, R., Nguyen, L. L. & Breban, R. Modelling human-to-human transmission of monkeypox. Bull. World Health Organ. 98, 638–640 (2020).
Fine, P. E., Jezek, Z., Grab, B. & Dixon, H. The transmission potential of monkeypox virus in human populations. Int. J. Epidemiol. 17, 643–650 (1988).
Du, Z. et al. Reproduction number of monkeypox in the early stage of the 2022 multi-country outbreak. J. Travel Med. https://doi.org/10.1093/jtm/taac099 (2022).
Guzzetta, G. et al. Early estimates of monkeypox incubation period, generation time, and reproduction number, Italy, May-June 2022. Emerg. Infect. Dis. 28, 2078–2081 (2022).
Velavan, T. P. & Meyer, C. G. Monkeypox 2022 outbreak: an update. Trop. Med. Int. Health 27, 604–605 (2022).
European Centre for Disease Prevention and Control. Epidemiological update: monkeypox outbreak. https://www.ecdc.europa.eu/en/news-events/epidemiological-update-monkeypox-outbreak (2022).
Dye, C. & Kraemer, M. U. G. Investigating the monkeypox outbreak. BMJ 377, o1314 (2022).
Monkeypox is spreading among gay men worldwide. https://www.aidsmap.com/news/may-2022/monkeypox-spreading-among-gay-men-worldwide (2022).
Inigo Martinez, J. et al. Monkeypox outbreak predominantly affecting men who have sex with men, Madrid, Spain, 26 April to 16 June 2022. Euro Surveill. 27, 2200471 (2022).
Zachariou, M. Monkeypox: symptoms seen in London sexual health clinics differ from previous outbreaks, study finds. BMJ 378, o1659 (2022).
Vusirikala, A. et al. Epidemiology of early monkeypox virus transmission in sexual networks of gay and bisexual men, England, 2022. Emerg. Infect. Dis. 28, 2082–2086 (2022).
Ogoina, D. Sexual behaviours and clinical course of human monkeypox in Spain. Lancet 400, 636–637 (2022).
Ferre, V. M. et al. Detection of monkeypox virus in anorectal swabs from asymptomatic men who have sex with men in a sexually transmitted infection screening program in Paris, France. Ann. Intern. Med. https://doi.org/10.7326/M22-2183 (2022).
De Baetselier, I. et al. Retrospective detection of asymptomatic monkeypox virus infections among male sexual health clinic attendees in Belgium. Nat. Med. https://doi.org/10.1038/s41591-022-02004-w (2022).
Spicknall, I. H. et al. Modeling the impact of sexual networks in the transmission of monkeypox virus among gay, bisexual, and other men who have sex with men - United States, 2022. MMWR Morb. Mortal. Wkly Rep. 71, 1131–1135 (2022).
Sah, R. et al. Monkeypox and its possible sexual transmission: where are we now with its evidence? Pathogens 11, 924 (2022).
Heskin, J. et al. Transmission of monkeypox virus through sexual contact - a novel route of infection. J. Infect. 85, 334–363 (2022).
Delaney, K. P. et al. Strategies adopted by gay, bisexual, and other men who have sex with men to prevent monkeypox virus transmission - United States, August 2022. MMWR Morb. Mortal. Wkly Rep. 71, 1126–1130 (2022).
Antinori, A. et al. Epidemiological, clinical and virological characteristics of four cases of monkeypox support transmission through sexual contact, Italy, May 2022. Euro Surveill. 27, 2200421 (2022).
Kozlov, M. Monkeypox goes global: why scientists are on alert. Nature 606, 15–16 (2022).
Thornhill, J. P. et al. Monkeypox virus infection in humans across 16 countries - April-June 2022. N. Engl. J. Med. 387, 679–691 (2022).
Tarin-Vicente, E. J. et al. Clinical presentation and virological assessment of confirmed human monkeypox virus cases in Spain: a prospective observational cohort study. Lancet 400, 681–689 (2022).
Joint ECDC-WHO Regional Office for Europe Monkeypox Surveillance Bulletin. https://monkeypoxreport.ecdc.europa.eu/ (2022).
Endo, A. et al. Heavy-tailed sexual contact networks and monkeypox epidemiology in the global outbreak, 2022. Science 378, 90–94 (2022).
Guzzetta, G. et al. Early estimates on monkeypox incubation period, generation time and reproduction number in Italy, May-June 2022. Emerg. Infect. Dis. 28, 2078–2081 (2022).
Yuan, P. et al. Assessing transmission risks and control strategy for monkeypox as an emerging zoonosis in a metropolitan area. J. Med. Virol. https://doi.org/10.1002/jmv.28137 (2022).
Bragazzi, N. L. et al. Epidemiological trends and clinical features of the ongoing monkeypox epidemic: a preliminary pooled data analysis and literature review. J. Med. Virol. https://doi.org/10.1002/jmv.27931 (2022).
Vaughan, A. M. et al. A large multi-country outbreak of monkeypox across 41 countries in the WHO European Region, 7 March to 23 August 2022. Euro Surveill. 27, 2200620 (2022).
Graham, F. Daily briefing: how monkeypox might be spreading in sexual networks. Nature https://doi.org/10.1038/d41586-022-01726-8 (2022).
Zhu, F., Li, L. & Che, D. Monkeypox virus under COVID-19: caution for sexual transmission - correspondence. Int J. Surg. 104, 106768 (2022).
Rodriguez-Morales, A. J. & Lopardo, G. Monkeypox: another sexually transmitted infection? Pathogens. 11, 713 (2022).
Brockmeyer, N. H. As monkeypox goes sexual: a public health perspective. J. Eur. Acad. Dermatol. Venereol. 36, 1164–1166 (2022).
Alpalhao, M. et al. Monkeypox: a new (sexually transmissible) epidemic? J. Eur. Acad. Dermatol. Venereol. https://doi.org/10.1111/jdv.18424 (2022).
Laurence, J. The recent rise in sexually transmitted infections in the United States was a harbinger of the new monkeypox pandemic. AIDS Patient Care STDS 36, 333–335 (2022).
Curran, K. G. et al. HIV and sexually transmitted infections among persons with monkeypox - eight U.S. jurisdictions, May 17-July 22, 2022. MMWR Morb. Mortal. Wkly Rep. 71, 1141–1147 (2022).
Hughes, A. L., Irausquin, S. & Friedman, R. The evolutionary biology of poxviruses. Infect. Genet. Evol. 10, 50–59 (2010).
Mackett, M. & Archard, L. C. Conservation and variation in orthopoxvirus genome structure. J. Gen. Virol. 45, 683–701 (1979).
Buller, R. M. & Palumbo, G. J. Poxvirus pathogenesis. Microbiol. Rev. 55, 80–122 (1991).
Shchelkunov, S. N. et al. Analysis of the monkeypox virus genome. Virology 297, 172–194 (2002).
Vandenbogaert, M. et al. Nanopore sequencing of a monkeypox virus strain isolated from a pustular lesion in the Central African Republic. Sci. Rep. 12, 10768 (2022).
Hendrickson, R. C., Wang, C., Hatcher, E. L. & Lefkowitz, E. J. Orthopoxvirus genome evolution: the role of gene loss. Viruses 2, 1933–1967 (2010).
Kugelman, J. R. et al. Genomic variability of monkeypox virus among humans, Democratic Republic of the Congo. Emerg. Infect. Dis. 20, 232–239 (2014).
Barry, M., Wasilenko, S. T., Stewart, T. L. & Taylor, J. M. Apoptosis regulator genes encoded by poxviruses. Prog. Mol. Subcell. Biol. 36, 19–37 (2004).
Seet, B. T. et al. Poxviruses and immune evasion. Annu. Rev. Immunol. 21, 377–423 (2003).
Esteban, D. J. & Hutchinson, A. P. Genes in the terminal regions of orthopoxvirus genomes experience adaptive molecular evolution. BMC Genomics. 12, 261 (2011).
Elde, N. C. et al. Poxviruses deploy genomic accordions to adapt rapidly against host antiviral defenses. Cell 150, 831–841 (2012).
Challberg, M. D. & Englund, P. T. Purification and properties of the deoxyribonucleic acid polymerase induced by vaccinia virus. J. Biol. Chem. 254, 7812–7819 (1979).
Firth, C. et al. Using time-structured data to estimate evolutionary rates of double-stranded DNA viruses. Mol. Biol. Evol. 27, 2038–2051 (2010).
O’Toole, A. & Rambaut, Á. Initial observations about putative APOBEC3 deaminase editing driving short-term evolution of MPXV since 2017. https://virological.org/t/initial-observations-about-putative-apobec3-deaminase-editing-driving-short-term-evolution-of-mpxv-since-2017/830 (2022).
Pecori, R., Di Giorgio, S., Paulo Lorenzo, J. & Nina Papavasiliou, F. Functions and consequences of AID/APOBEC-mediated DNA and RNA deamination. Nat. Rev. Genet. 23, 505–518 (2022).
Sadeghpour, S. et al. Human APOBEC3 variations and viral infection. Viruses. 13, 1366 (2021).
Martinez, T., Shapiro, M., Bhaduri-McIntosh, S. & MacCarthy, T. Evolutionary effects of the AID/APOBEC family of mutagenic enzymes on human gamma-herpesviruses. Virus Evol. 5, vey040 (2019).
Jern, P., Russell, R. A., Pathak, V. K. & Coffin, J. M. Likely role of APOBEC3G-mediated G-to-A mutations in HIV-1 evolution and drug resistance. PLoS Pathog. 5, e1000367 (2009).
Kremer, M. et al. Vaccinia virus replication is not affected by APOBEC3 family members. Virol. J. 3, 86 (2006).
Wang, L. et al. Genomic annotation and molecular evolution of monkeypox virus outbreak in 2022. J. Med. Virol. https://doi.org/10.1002/jmv.28036 (2022).
Moss, B. Poxvirus entry and membrane fusion. Virology 344, 48–54 (2006).
Moss, B. Membrane fusion during poxvirus entry. Semin. Cell Dev. Biol. 60, 89–96 (2016).
Smith, G. L., Vanderplasschen, A. & Law, M. The formation and function of extracellular enveloped vaccinia virus. J. Gen. Virol. 83, 2915–2931 (2002).
Schmidt, F. I., Bleck, C. K. & Mercer, J. Poxvirus host cell entry. Curr. Opin. Virol. 2, 20–27 (2012).
Locker, J. K. et al. Entry of the two infectious forms of vaccinia virus at the plasma membane is signaling-dependent for the IMV but not the EEV. Mol. Biol. Cell 11, 2497–2511 (2000).
Vanderplasschen, A., Hollinshead, M. & Smith, G. L. Intracellular and extracellular vaccinia virions enter cells by different mechanisms. J. Gen. Virol. 79(Pt. 4), 877–887 (1998).
Vanderplasschen, A. & Smith, G. L. A novel virus binding assay using confocal microscopy: demonstration that the intracellular and extracellular vaccinia virions bind to different cellular receptors. J. Virol. 71, 4032–4041 (1997).
Manes, N. P. et al. Comparative proteomics of human monkeypox and vaccinia intracellular mature and extracellular enveloped virions. J. Proteome Res. 7, 960–968 (2008).
Smith, G. L. & Law, M. The exit of vaccinia virus from infected cells. Virus Res. 106, 189–197 (2004).
Moss, B. Poxvirus cell entry: how many proteins does it take? Viruses 4, 688–707 (2012).
Chiu, W. L. et al. Vaccinia virus 4c (A26L) protein on intracellular mature virus binds to the extracellular cellular matrix laminin. J. Virol. 81, 2149–2157 (2007).
Matho, M. H. et al. Structure-function characterization of three human antibodies targeting the vaccinia virus adhesion molecule D8. J. Biol. Chem. 293, 390–401 (2018).
Hsiao, J. C., Chung, C. S. & Chang, W. Vaccinia virus envelope D8L protein binds to cell surface chondroitin sulfate and mediates the adsorption of intracellular mature virions to cells. J. Virol. 73, 8750–8761 (1999).
Chung, C. S., Hsiao, J. C., Chang, Y. S. & Chang, W. A27L protein mediates vaccinia virus interaction with cell surface heparan sulfate. J. Virol. 72, 1577–1585 (1998).
Berhanu, A. et al. Vaccination of BALB/c mice with Escherichia coli-expressed vaccinia virus proteins A27L, B5R, and D8L protects mice from lethal vaccinia virus challenge. J. Virol. 82, 3517–3529 (2008).
Blasco, R., Sisler, J. R. & Moss, B. Dissociation of progeny vaccinia virus from the cell membrane is regulated by a viral envelope glycoprotein: effect of a point mutation in the lectin homology domain of the A34R gene. J. Virol. 67, 3319–3325 (1993).
Thirunavukarasu, P. et al. A rationally designed A34R mutant oncolytic poxvirus: improved efficacy in peritoneal carcinomatosis. Mol. Ther. 21, 1024–1033 (2013).
Wolffe, E. J., Katz, E., Weisberg, A. & Moss, B. The A34R glycoprotein gene is required for induction of specialized actin-containing microvilli and efficient cell-to-cell transmission of vaccinia virus. J. Virol. 71, 3904–3915 (1997).
McIntosh, A. A. & Smith, G. L. Vaccinia virus glycoprotein A34R is required for infectivity of extracellular enveloped virus. J. Virol. 70, 272–281 (1996).
Lin, C. L., Chung, C. S., Heine, H. G. & Chang, W. Vaccinia virus envelope H3L protein binds to cell surface heparan sulfate and is important for intracellular mature virion morphogenesis and virus infection in vitro and in vivo. J. Virol. 74, 3353–3365 (2000).
Davies, D. H. et al. Vaccinia virus H3L envelope protein is a major target of neutralizing antibodies in humans and elicits protection against lethal challenge in mice. J. Virol. 79, 11724–11733 (2005).
da Fonseca, F. G., Wolffe, E. J., Weisberg, A. & Moss, B. Effects of deletion or stringent repression of the H3L envelope gene on vaccinia virus replication. J. Virol. 74, 7518–7528 (2000).
Foo, C. H. et al. Vaccinia virus L1 binds to cell surfaces and blocks virus entry independently of glycosaminoglycans. Virology 385, 368–382 (2009).
Townsley, A. C., Senkevich, T. G. & Moss, B. Vaccinia virus A21 virion membrane protein is required for cell entry and fusion. J. Virol. 79, 9458–9469 (2005).
Townsley, A. C., Senkevich, T. G. & Moss, B. The product of the vaccinia virus L5R gene is a fourth membrane protein encoded by all poxviruses that is required for cell entry and cell-cell fusion. J. Virol. 79, 10988–10998 (2005).
Senkevich, T. G. & Moss, B. Vaccinia virus H2 protein is an essential component of a complex involved in virus entry and cell-cell fusion. J. Virol. 79, 4744–4754 (2005).
Diesterbeck, U. S., Gittis, A. G., Garboczi, D. N. & Moss, B. The 2.1 A structure of protein F9 and its comparison to L1, two components of the conserved poxvirus entry-fusion complex. Sci. Rep. 8, 16807 (2018).
Laliberte, J. P., Weisberg, A. S. & Moss, B. The membrane fusion step of vaccinia virus entry is cooperatively mediated by multiple viral proteins and host cell components. PLoS Pathog. 7, e1002446 (2011).
Bisht, H., Weisberg, A. S. & Moss, B. Vaccinia virus l1 protein is required for cell entry and membrane fusion. J. Virol. 82, 8687–8694 (2008).
Brown, E., Senkevich, T. G. & Moss, B. Vaccinia virus F9 virion membrane protein is required for entry but not virus assembly, in contrast to the related L1 protein. J. Virol. 80, 9455–9464 (2006).
McFadden, G. Poxvirus tropism. Nat. Rev. Microbiol. 3, 201–213 (2005).
Wright, C. F., Hubbs, A. E., Gunasinghe, S. K. & Oswald, B. W. A vaccinia virus late transcription factor copurifies with a factor that binds to a viral late promoter and is complemented by extracts from uninfected HeLa cells. J. Virol. 72, 1446–1451 (1998).
Sanz, P. & Moss, B. A new vaccinia virus intermediate transcription factor. J. Virol. 72, 6880–6883 (1998).
Rosales, R., Sutter, G. & Moss, B. A cellular factor is required for transcription of vaccinia viral intermediate-stage genes. Proc. Natl Acad. Sci. USA 91, 3794–3798 (1994).
Rosales, R., Harris, N., Ahn, B. Y. & Moss, B. Purification and identification of a vaccinia virus-encoded intermediate stage promoter-specific transcription factor that has homology to eukaryotic transcription factor SII (TFIIS) and an additional role as a viral RNA polymerase subunit. J. Biol. Chem. 269, 14260–14267 (1994).
Roberts, K. L. & Smith, G. L. Vaccinia virus morphogenesis and dissemination. Trends Microbiol. 16, 472–479 (2008).
Pauli, G. et al. Orthopox viruses: infections in humans. Transfus. Med. Hemother. 37, 351–364 (2010).
Nalca, A., Rimoin, A. W., Bavari, S. & Whitehouse, C. A. Reemergence of monkeypox: prevalence, diagnostics, and countermeasures. Clin. Infect. Dis. 41, 1765–1771 (2005).
Breman, J. G. et al. Human monkeypox, 1970-79. Bull. World Health Organ. 58, 165–182 (1980).
Mahase, E. Monkeypox: what do we know about the outbreaks in Europe and North America? BMJ 377, o1274 (2022).
Petersen, E. et al. Human monkeypox: epidemiologic and clinical characteristics, diagnosis, and prevention. Infect. Dis. Clin. North Am. 33, 1027–1043 (2019).
Miura, F. et al. Estimated incubation period for monkeypox cases confirmed in the Netherlands, May 2022. Euro Surveill. 27, 2200448 (2022).
Charniga, K. et al. Estimating the incubation period of monkeypox virus during the 2022. Preprint at medRxiv https://doi.org/10.1101/2022.06.22.22276713 (2022).
WHO. Monkeypox (World Health Organization, 2022).
Cheema, A. Y. et al. Monkeypox: a review of clinical features, diagnosis, and treatment. Cureus 14, e26756 (2022).
PR Pittman et al. Clinical characterization of human monkeypox infections in the Democratic Republic of the Congo. medRxiv, (2022).
Benites-Zapata, V. A. et al. Clinical features, hospitalisation and deaths associated with monkeypox: a systematic review and meta-analysis. Ann. Clin. Microbiol. Antimicrob. 21, 36 (2022).
McCollum, A. M. & Damon, I. K. Human monkeypox. Clin. Infect. Dis. 58, 260–267 (2014).
Singhal, T., Kabra, S. K. & Lodha, R. Monkeypox: a review. Indian J. Pediatr. 89, 955–960 (2022).
Saxena, S. K. et al. Re-emerging human monkeypox: a major public-health debacle. J. Med. Virol. https://doi.org/10.1002/jmv.27902 (2022).
Patrocinio-Jesus, R. & Peruzzu, F. Monkeypox genital lesions. N. Engl. J. Med. 387, 66 (2022).
Portela-Dias, J., Sereno, S., Falcao-Reis, I. & Rasteiro, C. Monkeypox infection with localized genital lesions in women. Am. J. Obstet. Gynecol. https://doi.org/10.1016/j.ajog.2022.08.046 (2022).
Paparizos, V. et al. Monkeypox virus infection: first reported case in Greece in a patient with a genital rash. J. Eur. Acad. Dermatol. Venereol. https://doi.org/10.1111/jdv.18521 (2022).
Hammerschlag, Y. et al. Monkeypox infection presenting as genital rash, Australia, May 2022. Euro Surveill. 27, 2200411 (2022).
Griffiths-Acha, J., Vela-Ganuza, M., Sarro-Fuente, C. & Lopez-Estebaranz, J. L. Monkeypox: a new differential diagnosis when addressing genital ulcer disease. Br. J. Dermatol. https://doi.org/10.1111/bjd.21834 (2022).
Davido, B. et al. Monkeypox 2022 outbreak: cases with exclusive genital lesions. J. Travel Med. 29, taac077 (2022).
Bociaga-Jasik, M. et al. Monkeypox present with genital ulcers-challenging clinical problem. Pol. Arch. Intern. Med. https://doi.org/10.20452/pamw.16304 (2022).
Girometti, N. et al. Demographic and clinical characteristics of confirmed human monkeypox virus cases in individuals attending a sexual health centre in London, UK: an observational analysis. Lancet Infect. Dis. 28, 1321–1328 (2022).
Patel, A. et al. Clinical features and novel presentations of human monkeypox in a central London centre during the 2022 outbreak: descriptive case series. BMJ 378, e072410 (2022).
Abbasi, J. Reports of asymptomatic monkeypox suggest that, at the very least, some infections go unnoticed. JAMA 328, 1023–1025 (2022).
Perez Duque, M. et al. Ongoing monkeypox virus outbreak, Portugal, 29 April to 23 May 2022. Euro Surveill. 27, 2200424 (2022).
Adalja, A. & Inglesby, T. A novel international monkeypox outbreak. Ann. Intern. Med. 175, 1175–1176 (2022).
Kumbhar, N. & Agarwala, P. The lurking threat of monkeypox in current times. Indian J. Med. Microbiol. https://doi.org/10.1016/j.ijmmb.2022.07.016 (2022).
Kindrachuk, J. et al. Systems kinomics demonstrates Congo Basin monkeypox virus infection selectively modulates host cell signaling responses as compared to West African monkeypox virus. Mol. Cell. Proteomics https://doi.org/10.1074/mcp.M111.015701 (2012).
Li, Y. et al. Real-time PCR assays for the specific detection of monkeypox virus West African and Congo Basin strain DNA. J. Virol. Methods 169, 223–227 (2010).
See, K. C. Vaccination for monkeypox virus infection in humans: a review of key considerations. Vaccines 10, 1342 (2022).
WHO. Laboratory testing for the monkeypox virus: Interim guidance. https://www.who.int/publications/i/item/WHO-MPX-laboratory-2022.1 (2022).
Ropp, S. L. et al. PCR strategy for identification and differentiation of small pox and other orthopoxviruses. J. Clin. Microbiol. 33, 2069–2076 (1995).
Meyer, H., Ropp, S. L. & Esposito, J. J. Gene for A-type inclusion body protein is useful for a polymerase chain reaction assay to differentiate orthopoxviruses. J. Virol. Methods 64, 217–221 (1997).
Neubauer, H. et al. Specific detection of monkeypox virus by polymerase chain reaction. J. Virol. Methods 74, 201–207 (1998).
Loparev, V. N., Massung, R. F., Esposito, J. J. & Meyer, H. Detection and differentiation of old world orthopoxviruses: restriction fragment length polymorphism of the crmB gene region. J. Clin. Microbiol. 39, 94–100 (2001).
Li, Y. et al. Detection of monkeypox virus with real-time PCR assays. J. Clin. Virol. 36, 194–203 (2006).
Shchelkunov, S. N., Shcherbakov, D. N., Maksyutov, R. A. & Gavrilova, E. V. Species-specific identification of variola, monkeypox, cowpox, and vaccinia viruses by multiplex real-time PCR assay. J. Virol. Methods 175, 163–169 (2011).
Peiro-Mestres, A. et al. Frequent detection of monkeypox virus DNA in saliva, semen, and other clinical samples from 12 patients, Barcelona, Spain, May to June 2022. Euro Surveill. 27, 2200503 (2022).
Orba, Y. et al. Orthopoxvirus infection among wildlife in Zambia. J. Gen. Virol. 96, 390–394 (2015).
Kulesh, D. A. et al. Monkeypox virus detection in rodents using real-time 3’-minor groove binder TaqMan assays on the Roche LightCycler. Lab. Invest. 84, 1200–1208 (2004).
Davi, S. D. et al. Recombinase polymerase amplification assay for rapid detection of Monkeypox virus. Diagn. Microbiol. Infect. Dis. 95, 41–45 (2019).
Jiang, Z. et al. Laboratory diagnostics for monkeypox: an overview of sensitivities from various published tests. Travel Med. Infect. Dis. 49, 102425 (2022).
Maksyutov, R. A., Gavrilova, E. V. & Shchelkunov, S. N. Species-specific differentiation of variola, monkeypox, and varicella-zoster viruses by multiplex real-time PCR assay. J. Virol. Methods 236, 215–220 (2016).
Norz, D. et al. Rapid adaptation of established high-throughput molecular testing infrastructure for monkeypox virus detection. Emerg. Infect. Dis. 28, 1765–1769 (2022).
Scaramozzino, N. et al. Real-time PCR to identify variola virus or other human pathogenic orthopox viruses. Clin. Chem. 53, 606–613 (2007).
Ulaeto, D. O., Dunning, J. & Carroll, M. W. Evolutionary implications of human transmission of monkeypox: the importance of sequencing multiple lesions. Lancet Microbe 3, e639–e640 (2022).
Iizuka, I. et al. Loop-mediated isothermal amplification-based diagnostic assay for monkeypox virus infections. J. Med. Virol. 81, 1102–1108 (2009).
Dumont, C. et al. Simple technique for in field samples collection in the cases of skin rash illness and subsequent PCR detection of orthopoxviruses and varicella zoster virus. PLoS ONE 9, e96930 (2014).
Hammarlund, E. et al. Multiple diagnostic techniques identify previously vaccinated individuals with protective immunity against monkeypox. Nat. Med. 11, 1005–1011 (2005).
Dubois, M. E., Hammarlund, E. & Slifka, M. K. Optimization of peptide-based ELISA for serological diagnostics: a retrospective study of human monkeypox infection. Vector Borne Zoonotic Dis. 12, 400–409 (2012).
Fernandez de Marco Mdel, M. et al. The highly virulent variola and monkeypox viruses express secreted inhibitors of type I interferon. FASEB J. 24, 1479–1488 (2010).
Sejvar, J. J. et al. Human monkeypox infection: a family cluster in the midwestern United States. J. Infect. Dis. 190, 1833–1840 (2004).
Karem, K. L. et al. characterization of acute-phase humoral immunity to monkeypox: use of immunoglobulin M enzyme-linked immunosorbent assay for detection of monkeypox infection during the 2003 North American outbreak. Clin. Diagn. Lab. Immunol. 12, 867–872 (2005).
Stagles, M. J. et al. The histopathology and electron microscopy of a human monkeypox lesion. Trans. R. Soc. Trop. Med. Hyg. 79, 192–202 (1985).
Sherwat, A., Brooks, J. T., Birnkrant, D. & Kim, P. Tecovirimat and the treatment of monkeypox - past, present, and future considerations. N. Engl. J. Med. 387, 579–581 (2022).
Rizk, J. G. et al. Prevention and treatment of monkeypox. Drugs 82, 957–963 (2022).
O’Shea, J. et al. Interim guidance for prevention and treatment of monkeypox in persons with HIV infection - United States, August 2022. MMWR Morb. Mortal. Wkly Rep. 71, 1023–1028 (2022).
Goyal, L., Ajmera, K., Pandit, R. & Pandit, T. Prevention and treatment of monkeypox: a step-by-step guide for healthcare professionals and general population. Cureus 14, e28230 (2022).
Desai, A. N. et al. Compassionate use of tecovirimat for the treatment of monkeypox infection. JAMA 328, 1348–1350 (2022).
Yang, G. et al. An orally bioavailable antipoxvirus compound (ST-246) inhibits extracellular virus formation and protects mice from lethal orthopoxvirus challenge. J. Virol. 79, 13139–13149 (2005).
Jordan, R. et al. ST-246 antiviral efficacy in a nonhuman primate monkeypox model: determination of the minimal effective dose and human dose justification. Antimicrob. Agents Chemother. 53, 1817–1822 (2009).
Kabanov, A. S. et al. A comparative study of the antiviral activity of chemical compounds concerning the orthopoxviruses experiments in vivo. Vopr. Virusol. 58, 39–43 (2013).
Smith, S. K. et al. In vitro efficacy of ST246 against smallpox and monkeypox. Antimicrob. Agents Chemother. 53, 1007–1012 (2009).
Bolken, T. C. & Hruby, D. E. Tecovirimat for smallpox infections. Drugs Today 46, 109–117 (2010).
De Clercq, E. Historical perspectives in the development of antiviral agents against poxviruses. Viruses 2, 1322–1339 (2010).
Blasco, R. & Moss, B. Extracellular vaccinia virus formation and cell-to-cell virus transmission are prevented by deletion of the gene encoding the 37,000-Dalton outer envelope protein. J. Virol. 65, 5910–5920 (1991).
Vliegen, I. et al. Deletion of the vaccinia virus F13L gene results in a highly attenuated virus that mounts a protective immune response against subsequent vaccinia virus challenge. Antivir. Res. 93, 160–166 (2012).
Duraffour, S. et al. ST-246 is a key antiviral to inhibit the viral F13L phospholipase, one of the essential proteins for orthopoxvirus wrapping. J. Antimicrob. Chemother. 70, 1367–1380 (2015).
Frenois-Veyrat, G. et al. Tecovirimat is highly efficient on the Monkeypox virus lineage responsible for the international 2022 outbreak. Preprint at bioRxiv https://doi.org/10.1101/2022.07.19.500484 (2022).
Sbrana, E. et al. Efficacy of the antipoxvirus compound ST-246 for treatment of severe orthopoxvirus infection. Am. J. Trop. Med. Hyg. 76, 768–773 (2007).
Quenelle, D. C. et al. Efficacy of delayed treatment with ST-246 given orally against systemic orthopoxvirus infections in mice. Antimicrob. Agents Chemother. 51, 689–695 (2007).
Russo, A. T. et al. Effects of treatment delay on efficacy of tecovirimat following lethal aerosol monkeypox virus challenge in cynomolgus macaques. J. Infect. Dis. 218, 1490–1499 (2018).
Delaune, D. & Iseni, F. Drug development against smallpox: present and future. Antimicrob. Agents Chemother. 64, e01683-19 (2020).
Laudisoit, A., Tepage, F. & Colebunders, R. Oral tecovirimat for the treatment of smallpox. N. Engl. J. Med. 379, 2084–2085 (2018).
Grosenbach, D. W. et al. Oral tecovirimat for the treatment of smallpox. N. Engl. J. Med. 379, 44–53 (2018).
Huggins, J. et al. Nonhuman primates are protected from smallpox virus or monkeypox virus challenges by the antiviral drug ST-246. Antimicrob. Agents Chemother. 53, 2620–2625 (2009).
Smith, S. K. et al. Effective antiviral treatment of systemic orthopoxvirus disease: ST-246 treatment of prairie dogs infected with monkeypox virus. J. Virol. 85, 9176–9187 (2011).
Berhanu, A. et al. Treatment with the smallpox antiviral tecovirimat (ST-246) alone or in combination with ACAM2000 vaccination is effective as a postsymptomatic therapy for monkeypox virus infection. Antimicrob. Agents Chemother. 59, 4296–4300 (2015).
Wang, X. et al. Effects of adjuvants on the immunogenicity and efficacy of a zika virus envelope domain III subunit vaccine. Vaccines 7, 161 (2019).
Adler, H. et al. Clinical features and management of human monkeypox: a retrospective observational study in the UK. Lancet Infect. Dis. 22, 1153–1162 (2022).
Peters, S. M., Hill, N. B. & Halepas, S. Oral manifestations of monkeypox: a report of 2 cases. J. Oral Maxillofac. Surg. https://doi.org/10.1016/j.joms.2022.07.147 (2022).
Matias, W. R. et al. Tecovirimat for the treatment of human monkeypox: an initial series from Massachusetts, United States. Open Forum Infect. Dis. 9, ofac377 (2022).
Lucar, J. et al. Monkeypox virus-associated severe proctitis treated with oral tecovirimat: a report of two cases. Ann. Intern. Med. https://doi.org/10.7326/L22-0300 (2022).
CDC. Demographics of patients receiving TPOXX for treatment of monkeypox. https://www.cdc.gov/poxvirus/monkeypox/response/2022/demographics-TPOXX.html (2022).
Nakoune, E. & Olliaro, P. Waking up to monkeypox. BMJ 377, o1321 (2022).
FACT SHEET: Ongoing U.S. monkeypox research activities to speed science for impact. https://www.whitehouse.gov/ostp/news-updates/2022/08/11/fact-sheet-ongoing-u-s-monkeypox-research-activities-to-speed-science-for-impact/ (2022).
Del Rio, C. & Malani, P. N. Update on the monkeypox outbreak. JAMA 328, 921–922 (2022).
Harris, E. Global monkeypox outbreaks spur drug research for the neglected disease. JAMA 328, 231–233 (2022).
De Clercq, E. Cidofovir in the treatment of poxvirus infections. Antivir. Res. 55, 1–13 (2002).
Cidofovir approved. PI Perspect 14–15 (1996).
FDA panel unanimously recommends approval of Vistide for CMV retinitis. Food and Drug Administration. J. Int. Assoc. Physicians AIDS Care. 2, 50 (1996).
FDA grants marketing clearance of Vistide for the treatment of CMV retinitis. AIDS Patient Care STDS. 10, 383–384 (1996).
Hostetler, K. Y. Alkoxyalkyl prodrugs of acyclic nucleoside phosphonates enhance oral antiviral activity and reduce toxicity: current state of the art. Antivir. Res. 82, A84–98 (2009).
Magee, W. C., Hostetler, K. Y. & Evans, D. H. Mechanism of inhibition of vaccinia virus DNA polymerase by cidofovir diphosphate. Antimicrob. Agents Chemother. 49, 3153–3162 (2005).
Aldern, K. A., Ciesla, S. L., Winegarden, K. L. & Hostetler, K. Y. Increased antiviral activity of 1-O-hexadecyloxypropyl-[2-(14)C]cidofovir in MRC-5 human lung fibroblasts is explained by unique cellular uptake and metabolism. Mol. Pharmacol. 63, 678–681 (2003).
Andrei, G. & Snoeck, R. Cidofovir activity against poxvirus infections. Viruses 2, 2803–2830 (2010).
Neyts, J., Leyssen, P., Verbeken, E. & De Clercq, E. Efficacy of cidofovir in a murine model of disseminated progressive vaccinia. Antimicrob. Agents Chemother. 48, 2267–2273 (2004).
Smee, D. F. et al. Characterization of wild-type and cidofovir-resistant strains of camelpox, cowpox, monkeypox, and vaccinia viruses. Antimicrob. Agents Chemother. 46, 1329–1335 (2002).
Yu, J. & Raj, S. M. Efficacy of three key antiviral drugs used to treat orthopoxvirus infections: a systematic review. Glob. Biosecurity https://doi.org/10.31646/gbio.12 (2019).
Baker, R. O., Bray, M. & Huggins, J. W. Potential antiviral therapeutics for smallpox, monkeypox and other orthopoxvirus infections. Antivir. Res. 57, 13–23 (2003).
Robbins, S. J. et al. The efficacy of cidofovir treatment of mice infected with ectromelia (mousepox) virus encoding interleukin-4. Antivir. Res. 66, 1–7 (2005).
Quenelle, D. C., Collins, D. J. & Kern, E. R. Efficacy of multiple- or single-dose cidofovir against vaccinia and cowpox virus infections in mice. Antimicrob. Agents Chemother. 47, 3275–3280 (2003).
Smee, D. F. et al. Differential pathogenesis of cowpox virus intranasal infections in mice induced by low and high inoculum volumes and effects of cidofovir treatment. Int. J. Antimicrob. Agents 31, 352–359 (2008).
Stittelaar, K. J. et al. Antiviral treatment is more effective than smallpox vaccination upon lethal monkeypox virus infection. Nature 439, 745–748 (2006).
Meadows, K. P., Tyring, S. K., Pavia, A. T. & Rallis, T. M. Resolution of recalcitrant molluscum contagiosum virus lesions in human immunodeficiency virus-infected patients treated with cidofovir. Arch. Dermatol. 133, 987–990 (1997).
Davies, E. G., Thrasher, A., Lacey, K. & Harper, J. Topical cidofovir for severe molluscum contagiosum. Lancet 353, 2042 (1999).
Toro, J. R., Wood, L. V., Patel, N. K. & Turner, M. L. Topical cidofovir: a novel treatment for recalcitrant molluscum contagiosum in children infected with human immunodeficiency virus 1. Arch. Dermatol. 136, 983–985 (2000).
Vora, S. et al. Severe eczema vaccinatum in a household contact of a smallpox vaccinee. Clin. Infect. Dis. 46, 1555–1561 (2008).
Graef, S. et al. Clinicopathological findings in persistent corneal cowpox infection. JAMA Ophthalmol. 131, 1089–1091 (2013).
Becker, C. et al. Cowpox virus infection in pet rat owners: not always immediately recognized. Dtsch Arztebl Int. 106, 329–334 (2009).
Cihlar, T. & Chen, M. S. Identification of enzymes catalyzing two-step phosphorylation of cidofovir and the effect of cytomegalovirus infection on their activities in host cells. Mol. Pharmacol. 50, 1502–1510 (1996).
Xiong, X., Smith, J. L. & Chen, M. S. Effect of incorporation of cidofovir into DNA by human cytomegalovirus DNA polymerase on DNA elongation. Antimicrob. Agents Chemother. 41, 594–599 (1997).
Magee, W. C., Aldern, K. A., Hostetler, K. Y. & Evans, D. H. Cidofovir and (S)-9-[3-hydroxy-(2-phosphonomethoxy)propyl]adenine are highly effective inhibitors of vaccinia virus DNA polymerase when incorporated into the template strand. Antimicrob Agents Chemother. 52, 586–597 (2008).
Kinchington, P. R. et al. Sequence changes in the human adenovirus type 5 DNA polymerase associated with resistance to the broad spectrum antiviral cidofovir. Antivir. Res. 56, 73–84 (2002).
Chou, S. et al. Viral DNA polymerase mutations associated with drug resistance in human cytomegalovirus. J. Infect. Dis. 188, 32–39 (2003).
Florescu, D. F. & Keck, M. A. Development of CMX001 (Brincidofovir) for the treatment of serious diseases or conditions caused by dsDNA viruses. Expert Rev. Antiinfect. Ther. 12, 1171–1178 (2014).
Lanier, R. et al. Development of CMX001 for the treatment of poxvirus infections. Viruses 2, 2740–2762 (2010).
Duraffour, S. et al. Emergence of cowpox: study of the virulence of clinical strains and evaluation of antivirals. PLoS ONE 8, e55808 (2013).
Parker, S. et al. Evaluation of disease and viral biomarkers as triggers for therapeutic intervention in respiratory mousepox - an animal model of smallpox. Antivir. Res. 94, 44–53 (2012).
Rice, A. D. et al. Efficacy of CMX001 as a prophylactic and presymptomatic antiviral agent in New Zealand white rabbits infected with rabbitpox virus, a model for orthopoxvirus infections of humans. Viruses 3, 63–82 (2011).
Quenelle, D. C. et al. Oral treatment of cowpox and vaccinia virus infections in mice with ether lipid esters of cidofovir. Antimicrob. Agents Chemother. 48, 404–412 (2004).
Quenelle, D. C. & Kern, E. R. Treatment of vaccinia and cowpox virus infections in mice with CMX001 and ST-246. Viruses 2, 2681–2695 (2010).
Parker, S. et al. Ectromelia virus infections of mice as a model to support the licensure of anti-orthopoxvirus therapeutics. Viruses 2, 1918–1932 (2010).
Quenelle, D. C. et al. Efficacy of CMX001 against herpes simplex virus infections in mice and correlations with drug distribution studies. J. Infect. Dis. 202, 1492–1499 (2010).
Parker, S. et al. Efficacy of therapeutic intervention with an oral ether-lipid analogue of cidofovir (CMX001) in a lethal mousepox model. Antivir. Res. 77, 39–49 (2008).
Stabenow, J. et al. A mouse model of lethal infection for evaluating prophylactics and therapeutics against Monkeypox virus. J. Virol. 84, 3909–3920 (2010).
Grossi, I. M. et al. Efficacy of delayed brincidofovir treatment against a lethal rabbitpox virus challenge in New Zealand White rabbits. Antivir. Res. 143, 278–286 (2017).
Trost, L. C. et al. The efficacy and pharmacokinetics of brincidofovir for the treatment of lethal rabbitpox virus infection: a model of smallpox disease. Antivir. Res. 117, 115–121 (2015).
Xiao, S. Y. et al. Experimental infection of prairie dogs with monkeypox virus. Emerg. Infect. Dis. 11, 539–545 (2005).
Weiner, Z. P. et al. Characterization of Monkeypox virus dissemination in the black-tailed prairie dog (Cynomys ludovicianus) through in vivo bioluminescent imaging. PLoS ONE 14, e0222612 (2019).
Hutson, C. L. et al. Pharmacokinetics and efficacy of a potential smallpox therapeutic, brincidofovir, in a lethal monkeypox virus animal model. mSphere. 6, (2021).
Crump, R., Korom, M., Buller, R. M. & Parker, S. Buccal viral DNA as a trigger for brincidofovir therapy in the mousepox model of smallpox. Antivir. Res. 139, 112–116 (2017).
Guarner, J., Del Rio, C. & Malani, P. N. Monkeypox in 2022-what clinicians need to know. JAMA 328, 139–140 (2022).
Hiwarkar, P. et al. Brincidofovir is highly efficacious in controlling adenoviremia in pediatric recipients of hematopoietic cell transplant. Blood 129, 2033–2037 (2017).
Rice, A. D. et al. Efficacy of CMX001 as a post exposure antiviral in New Zealand White rabbits infected with rabbitpox virus, a model for orthopoxvirus infections of humans. Viruses 3, 47–62 (2011).
Sudhindra, P. et al. Brincidofovir (CMX001) for the treatment of severe adenoviral pneumonia in kidney transplant recipient. Cureus 11, e5296 (2019).
Tippin, T. K., Morrison, M. E., Brundage, T. M. & Mommeja-Marin, H. Brincidofovir Is Not A Substrate For The Human Organic Anion Transporter 1: A Mechanistic Explanation For The Lack Of Nephrotoxicity Observed In Clinical Studies. Ther. Drug Monit. 38, 777–786 (2016).
Olson, V. A. et al. In vitro efficacy of brincidofovir against variola virus. Antimicrob. Agents Chemother. 58, 5570–5571 (2014).
Lederman, E. R. et al. Progressive vaccinia: case description and laboratory-guided therapy with vaccinia immune globulin, ST-246, and CMX001. J. Infect. Dis. 206, 1372–1385 (2012).
FDA. Vaccinia IMMUNE GLOBULIN INTRAVENOUS (HUMan). https://www.fda.gov/vaccines-blood-biologics/approved-blood-products/vaccinia-immune-globulin-intravenous-human (2018).
Wittek, R. Vaccinia immune globulin: current policies, preparedness, and product safety and efficacy. Int. J. Infect. Dis. 10, 193–201 (2006).
Whitehouse, E. R. et al. Novel treatment of a vaccinia virus infection from an occupational needlestick - San Diego, California, 2019. MMWR Morb. Mortal. Wkly Rep. 68, 943–946 (2019).
Lindholm, D. A. et al. Preemptive tecovirimat use in an active duty service member who presented with acute myeloid leukemia after smallpox vaccination. Clin. Infect. Dis. 69, 2205–2207 (2019).
Centers for Disease Control & Prevention. Human vaccinia infection after contact with a raccoon rabies vaccine bait -Pennsylvania, 2009. MMWR Morb. Mortal. Wkly Rep. 58, 1204–1207 (2009).
Gilchuk, I. et al. Cross-neutralizing and protective human antibody specificities to poxvirus infections. Cell 167, 684.e9–694.e9 (2016).
Edghill-Smith, Y. et al. Smallpox vaccine-induced antibodies are necessary and sufficient for protection against monkeypox virus. Nat. Med. 11, 740–747 (2005).
Stanford, M. M., McFadden, G., Karupiah, G. & Chaudhri, G. Immunopathogenesis of poxvirus infections: forecasting the impending storm. Immunol. Cell Biol. 85, 93–102 (2007).
Ichihashi, Y. & Oie, M. Epitope mosaic on the surface proteins of orthopoxviruses. Virology 163, 133–144 (1988).
McConnell, S. et al. Protection of rhesus monkeys against monkeypox by vaccinia virus immunization. Am. J. Vet. Res. 25, 192–195 (1964).
Rao, A. K. et al. Use of JYNNEOS (smallpox and monkeypox vaccine, live, nonreplicating) for preexposure vaccination of persons at risk for occupational exposure to orthopoxviruses: Recommendations of the Advisory Committee on Immunization Practices - United States, 2022. MMWR Morb. Mortal. Wkly Rep. 71, 734–742 (2022).
Petersen, E. et al. Vaccination for monkeypox prevention in persons with high-risk sexual behaviours to control on-going outbreak of monkeypox virus clade 3. Int. J. Infect. Dis. 122, 569–571 (2022).
Kupferschmidt, K. Monkeypox vaccination plans take shape amid questions. Science 376, 1142–1143 (2022).
Kozlov, M. Monkeypox vaccination begins - can the global outbreaks be contained? Nature 606, 444–445 (2022).
Chakraborty, S. et al. Monkeypox vaccines and vaccination strategies: Current knowledge and advances. An update - correspondence. Int. J. Surg, 105, 106869 (2022).
Brooks, J. T., Marks, P., Goldstein, R. H. & Walensky, R. P. Intradermal vaccination for monkeypox - benefits for individual and public health. N. Engl. J. Med. 387, 1151–1153 (2022).
Erratum: Use of JYNNEOS (smallpox and monkeypox vaccine, live, nonreplicating) for preexposure vaccination of persons at risk for occupational exposure to orthopoxviruses: Recommendations of the Advisory Committee on Immunization Practices - United States, 2022. MMWR Morb. Mortal. Wkly Rep. 71, 886 (2022).
Jezek, Z. et al. Human monkeypox: a study of 2,510 contacts of 214 patients. J. Infect. Dis. 154, 551–555 (1986).
Rimoin, A. W. et al. Major increase in human monkeypox incidence 30 years after smallpox vaccination campaigns cease in the Democratic Republic of Congo. Proc. Natl Acad. Sci. USA 107, 16262–16267 (2010).
Greenberg, R. N. & Kennedy, J. S. ACAM2000: a newly licensed cell culture-based live vaccinia smallpox vaccine. Expert Opin. Investig. Drugs 17, 555–564 (2008).
Nalca, A. & Zumbrun, E. E. ACAM2000: the new smallpox vaccine for United States strategic national stockpile. Drug Des. Dev. Ther. 4, 71–79 (2010).
Hatch, G. J. et al. Assessment of the protective effect of Imvamune and Acam2000 vaccines against aerosolized monkeypox virus in cynomolgus macaques. J. Virol. 87, 7805–7815 (2013).
Keckler, M. S. et al. IMVAMUNE((R)) and ACAM2000((R)) provide different protection against disease when administered postexposure in an intranasal monkeypox challenge prairie dog model. Vaccines 8, 396 (2020).
Russo, A. T. et al. Co-administration of tecovirimat and ACAM2000 in non-human primates: Effect of tecovirimat treatment on ACAM2000 immunogenicity and efficacy versus lethal monkeypox virus challenge. Vaccine 38, 644–654 (2020).
Reed, J. L., Scott, D. E. & Bray, M. Eczema vaccinatum. Clin. Infect. Dis. 54, 832–840 (2012).
Cassimatis, D. C. et al. Smallpox vaccination and myopericarditis: a clinical review. J. Am. Coll. Cardiol. 43, 1503–1510 (2004).
Halsell, J. S. et al. Myopericarditis following smallpox vaccination among vaccinia-naive US military personnel. JAMA 289, 3283–3289 (2003).
Bray, M. & Wright, M. E. Progressive vaccinia. Clin. Infect. Dis. 36, 766–774 (2003).
Decker, M. D. et al. Enhanced safety surveillance study of ACAM2000 smallpox vaccine among US military service members. Vaccine 39, 5541–5547 (2021).
Faix, D. J. et al. Prospective safety surveillance study of ACAM2000 smallpox vaccine in deploying military personnel. Vaccine 38, 7323–7330 (2020).
Freeman, R. & Lenz, B. Cutaneous reactions associated with ACAM2000 smallpox vaccination in a deploying U.S. Army unit. Mil. Med. 180, e152–156 (2015).
McNeil, M. M. et al. Ischemic cardiac events and other adverse events following ACAM2000((R)) smallpox vaccine in the Vaccine Adverse Event Reporting System. Vaccine 32, 4758–4765 (2014).
Beachkofsky, T. M. et al. Adverse events following smallpox vaccination with ACAM2000 in a military population. Arch. Dermatol. 146, 656–661 (2010).
CDC. Monkeypox. https://www.cdc.gov/poxvirus/monkeypox/vaccines.html (2022).
FDA. FDA approves first live, non-replicating vaccine to prevent smallpox and monkeypox. https://www.fda.gov/news-events/press-announcements/fda-approves-first-live-non-replicating-vaccine-prevent-smallpox-and-monkeypox (2019).
Frey, S. E. et al. Safety and immunogenicity of IMVAMUNE(R) smallpox vaccine using different strategies for a post event scenario. Vaccine 31, 3025–3033 (2013).
Walsh, S. R. et al. Safety and immunogenicity of modified vaccinia Ankara in hematopoietic stem cell transplant recipients: a randomized, controlled trial. J. Infect. Dis. 207, 1888–1897 (2013).
von Sonnenburg, F. et al. Safety and immunogenicity of modified vaccinia Ankara as a smallpox vaccine in people with atopic dermatitis. Vaccine 32, 5696–5702 (2014).
Overton, E. T. et al. Safety and immunogenicity of modified vaccinia Ankara-Bavarian Nordic smallpox vaccine in vaccinia-naive and experienced human immunodeficiency virus-infected individuals: an open-label, controlled clinical phase II trial. Open Forum Infect. Dis. 2, ofv040 (2015).
Greenberg, R. N. et al. Safety, immunogenicity, and surrogate markers of clinical efficacy for modified vaccinia Ankara as a smallpox vaccine in HIV-infected subjects. J. Infect. Dis. 207, 749–758 (2013).
Jones, T. IMVAMUNE, an attenuated modified vaccinia Ankara virus vaccine for smallpox infection. Curr. Opin. Mol. Ther. 10, 407–417 (2008).
Davies, D. H. et al. Antibody profiling by proteome microarray reveals the immunogenicity of the attenuated smallpox vaccine modified vaccinia virus ankara is comparable to that of Dryvax. J. Virol. 82, 652–663 (2008).
Phelps, A. L. et al. Comparative efficacy of modified vaccinia Ankara (MVA) as a potential replacement smallpox vaccine. Vaccine 25, 34–42 (2007).
Cosma, A. et al. Evaluation of modified vaccinia virus Ankara as an alternative vaccine against smallpox in chronically HIV type 1-infected individuals undergoing HAART. AIDS Res. Hum. Retroviruses 23, 782–793 (2007).
Meseda, C. A. et al. Enhanced immunogenicity and protective effect conferred by vaccination with combinations of modified vaccinia virus Ankara and licensed smallpox vaccine Dryvax in a mouse model. Virology 339, 164–175 (2005).
von Krempelhuber, A. et al. A randomized, double-blind, dose-finding Phase II study to evaluate immunogenicity and safety of the third generation smallpox vaccine candidate IMVAMUNE. Vaccine 28, 1209–1216 (2010).
Frey, S. E. et al. Clinical and immunologic responses to multiple doses of IMVAMUNE (Modified Vaccinia Ankara) followed by Dryvax challenge. Vaccine 25, 8562–8573 (2007).
Vollmar, J. et al. Safety and immunogenicity of IMVAMUNE, a promising candidate as a third generation smallpox vaccine. Vaccine 24, 2065–2070 (2006).
Bavarian-Nordic.com. Bavarian Nordic announces US FDA approval of Jynneos s (smallpox and monkeypox vaccine, live; non-replicating) for prevention of smallpox and monkeypox disease in adults. https://www.bavarian-nordic.com/investor/news/news.aspx?news=5758 (2019).
Earl, P. L. et al. Rapid protection in a monkeypox model by a single injection of a replication-deficient vaccinia virus. Proc. Natl Acad. Sci. USA 105, 10889–10894 (2008).
Keckler, M. S. et al. Establishment of the black-tailed prairie dog (Cynomys ludovicianus) as a novel animal model for comparing smallpox vaccines administered preexposure in both high- and low-dose monkeypox virus challenges. J. Virol. 85, 7683–7698 (2011).
Stittelaar, K. J. et al. Modified vaccinia virus Ankara protects macaques against respiratory challenge with monkeypox virus. J. Virol. 79, 7845–7851 (2005).
Earl, P. L. et al. Immunogenicity of a highly attenuated MVA smallpox vaccine and protection against monkeypox. Nature 428, 182–185 (2004).
Nigam, P. et al. DNA/MVA HIV-1/AIDS vaccine elicits long-lived vaccinia virus-specific immunity and confers protection against a lethal monkeypox challenge. Virology 366, 73–83 (2007).
Petersen, B. W. et al. Vaccinating against monkeypox in the Democratic Republic of the Congo. Antivir. Res. 162, 171–177 (2019).
Nguyen, L. B. L. et al. A prospective national cohort evaluating ring MVA vaccination as post-exposure prophylaxis for monkeypox. Nat. Med. https://doi.org/10.1038/d41591-022-00077-1 (2022).
Pittman, P. R. et al. Phase 3 efficacy trial of modified vaccinia Ankara as a vaccine against smallpox. N. Engl. J. Med. 381, 1897–1908 (2019).
de Nicolas-Ruanes, B. et al. Monkeypox virus case with maculopapular exanthem and proctitis during the Spanish outbreak in 2022. J. Eur. Acad. Dermatol. Venereol. 36, e658–e660 (2022).
WHO. Vaccines and immunization for monkeypox: Interim guidance, 14 June 2022. https://www.who.int/publications/i/item/who-mpx-immunization-2022.1 (2022).
EMA. EMA recommends approval of Imvanex for the prevention of monkeypox disease. https://www.ema.europa.eu/en/news/ema-recommends-approval-imvanex-prevention-monkeypox-disease
GlobeNewswire. Health Canada extends approval of IMVAMUNE to immunization against monkeypox and orthopox viruses. https://www.globenewswire.com/news-release/2020/11/12/2125193/0/en/Health-Canada-Extends-Approval-of-IMVAMUNE-to-Immunization-against-Monkeypox-and-Orthopox-Viruses.html (2020).
GlobeNewswire. Bavarian Nordic expands capacity with U.S. contract manufacturer for filling of smallpox/monkeypox vaccines. https://www.globenewswire.com/en/news-release/2022/08/18/2501182/0/en/Bavarian-Nordic-Expands-Capacity-with-U-S-Contract-Manufacturer-for-Filling-of-Smallpox-Monkeypox-Vaccines.html (2022).
FDA. Monkeypox update: FDA authorizes emergency use of JYNNEOS vaccine to increase vaccine supply. https://www.fda.gov/news-events/press-announcements/monkeypox-update-fda-authorizes-emergency-use-jynneos-vaccine-increase-vaccine-supply (2022).
Frey, S. E. et al. Comparison of lyophilized versus liquid modified vaccinia Ankara (MVA) formulations and subcutaneous versus intradermal routes of administration in healthy vaccinia-naive subjects. Vaccine 33, 5225–5234 (2015).
Takahashi-Nishimaki, F. et al. Genetic analysis of vaccinia virus Lister strain and its attenuated mutant LC16m8: production of intermediate variants by homologous recombination. J. Gen. Virol. 68, 2705–2710 (1987). Pt 10.
Morikawa, S. et al. An attenuated LC16m8 smallpox vaccine: analysis of full-genome sequence and induction of immune protection. J. Virol. 79, 11873–11891 (2005).
Yoshikawa, T. et al. Construction and characterization of bacterial artificial chromosomes harboring the full-length genome of a highly attenuated vaccinia virus LC16m8. PLoS ONE 13, e0192725 (2018).
Omura, N. et al. A novel system for constructing a recombinant highly-attenuated vaccinia virus strain (LC16m8) expressing foreign genes and its application for the generation of LC16m8-based vaccines against herpes simplex virus 2. Jpn J. Infect. Dis. 71, 229–233 (2018).
Yokote, H. et al. Safety of attenuated smallpox vaccine LC16m8 in immunodeficient mice. Clin. Vaccin. Immunol. 21, 1261–1266 (2014).
Meseda, C. A. et al. Comparative evaluation of the immune responses and protection engendered by LC16m8 and Dryvax smallpox vaccines in a mouse model. Clin. Vaccin. Immunol. 16, 1261–1271 (2009).
Empig, C. et al. Highly attenuated smallpox vaccine protects rabbits and mice against pathogenic orthopoxvirus challenge. Vaccine 24, 3686–3694 (2006).
Kidokoro, M., Tashiro, M. & Shida, H. Genetically stable and fully effective smallpox vaccine strain constructed from highly attenuated vaccinia LC16m8. Proc. Natl Acad. Sci. USA 102, 4152–4157 (2005).
Yokote, H. et al. Vaccinia virus strain LC16m8 defective in the B5R gene keeps strong protection comparable to its parental strain Lister in immunodeficient mice. Vaccine 33, 6112–6119 (2015).
Saijo, M. et al. LC16m8, a highly attenuated vaccinia virus vaccine lacking expression of the membrane protein B5R, protects monkeys from monkeypox. J. Virol. 80, 5179–5188 (2006).
Gordon, S. N. et al. Smallpox vaccine safety is dependent on T cells and not B cells. J. Infect. Dis. 203, 1043–1053 (2011).
Iizuka, I. et al. A single vaccination of nonhuman primates with highly attenuated smallpox vaccine, LC16m8, provides long-term protection against monkeypox. Jpn J. Infect. Dis. 70, 408–415 (2017).
Kennedy, J. S. et al. Safety and immunogenicity of LC16m8, an attenuated smallpox vaccine in vaccinia-naive adults. J. Infect. Dis. 204, 1395–1402 (2011).
Saito, T. et al. Clinical and immunological response to attenuated tissue-cultured smallpox vaccine LC16m8. JAMA 301, 1025–1033 (2009).
Kenner, J. et al. LC16m8: an attenuated smallpox vaccine. Vaccine 24, 7009–7022 (2006).
Nishiyama, Y. et al. Freeze-dried live attenuated smallpox vaccine prepared in cell culture “LC16-KAKETSUKEN”: post-marketing surveillance study on safety and efficacy compliant with good clinical practice. Vaccine 33, 6120–6127 (2015).
Eto, A. et al. Recent advances in the study of live attenuated cell-cultured smallpox vaccine LC16m8. Vaccine 33, 6106–6111 (2015).
Eto, A. et al. Profiling of the antibody response to attenuated LC16m8 smallpox vaccine using protein array analysis. Vaccine 37, 6588–6593 (2019).
Friedman, D. Safety and immunogenicity of LC16m8, an attenuated smallpox vaccine in vaccinia-naive adults. J. Infect. Dis. 206, 1149–1150 (2012).
Johnson, B. F. et al. Serological responses in humans to the smallpox vaccine LC16m8. J. Gen. Virol. 92, 2405–2410 (2011).
Healthworld.com. Japan approves smallpox vaccine to prevent monkeypox. https://health.economictimes.indiatimes.com/news/industry/japan-approves-smallpox-vaccine-to-prevent-monkeypox/93321404 (2022).
Ogoina, D. et al. The 2017 human monkeypox outbreak in Nigeria-Report of outbreak experience and response in the Niger Delta University Teaching Hospital, Bayelsa State, Nigeria. PLoS ONE 14, e0214229 (2019).
Ogoina, D. et al. Clinical course and outcome of human monkeypox in Nigeria. Clin. Infect. Dis. 71, e210–e214 (2020).
ABC NEWS. Moderna considering creating an mRNA monkeypox vaccine amid growing demand for shots. https://abcnews.go.com/Health/moderna-creating-mrna-monkeypox-vaccine-amid-growing-demand/story?id=87875414 (2022).
Tonix Pharmaceuticals, TNX-801. https://www.tonixpharma.com/tnx-801/ (2022).
Noyce, R. S., Lederman, S. & Evans, D. H. Construction of an infectious horsepox virus vaccine from chemically synthesized DNA fragments. PLoS ONE 13, e0188453 (2018).
Noyce, R et al. Synthetic chimeric horsepox virus (scHPXV) vaccination protects macaques from monkeypox. https://www.tonixpharma.com/wp-content/uploads/2021/11/Synthetic-Chimeric-Horsepox-Virus-scHPXV-Vaccination-Protects-Macaques-from-Monkeypox.pdf (2020).
Gao, A. & Gao. S. In silico identification of non-cross-reactive epitopes for monkeypox cell surface-binding protein. Preprint at Research Square https://doi.org/10.21203/rs.3.rs-1693979/v1 (2022).
Peng, C. & Moss, B. Repair of a previously uncharacterized second host-range gene contributes to full replication of modified vaccinia virus Ankara (MVA) in human cells. Proc. Natl Acad. Sci. USA 117, 3759–3767 (2020).
Edghill-Smith, Y. et al. Smallpox vaccine does not protect macaques with AIDS from a lethal monkeypox virus challenge. J. Infect. Dis. 191, 372–381 (2005).
Hutson, C. L. et al. Monkeypox zoonotic associations: insights from laboratory evaluation of animals associated with the multi-state US outbreak. Am. J. Trop. Med. Hyg. 76, 757–768 (2007).
Marennikova, S. S. & Seluhina, E. M. Susceptibility of some rodent species to monkeypox virus, and course of the infection. Bull. World Health Organ. 53, 13–20 (1976).
Arita, I. & Henderson, D. A. Smallpox and monkeypox in non-human primates. Bull. World Health Organ. 39, 277–283 (1968).
Shelukhina, E. M., Shenkman, L. S., Rozina, E. E. & Marennikova, S. S. Possible mechanism of orthopoxvirus preservation in nature. Vopr. Virusol. 368–372 (1979).
Earl, P. L., Americo, J. L., Cotter, C. A. & Moss, B. Comparative live bioluminescence imaging of monkeypox virus dissemination in a wild-derived inbred mouse (Mus musculus castaneus) and outbred African dormouse (Graphiurus kelleni). Virology 475, 150–158 (2015).
Americo, J. L., Moss, B. & Earl, P. L. Identification of wild-derived inbred mouse strains highly susceptible to monkeypox virus infection for use as small animal models. J. Virol. 84, 8172–8180 (2010).
Earl, P. L., Americo, J. L. & Moss, B. Genetic studies of the susceptibility of classical and wild-derived inbred mouse strains to monkeypox virus. Virology 481, 161–165 (2015).
Jezek, Z. & Fenner, F. Human monkeypox. Monogr. Virol. 17, 1–140 (1988).
Reynolds, M. G., Carroll, D. S. & Karem, K. L. Factors affecting the likelihood of monkeypox’s emergence and spread in the post-smallpox era. Curr. Opin. Virol. 2, 335–343 (2012).
Falendysz, E. A. et al. Further assessment of monkeypox virus infection in gambian pouched rats (Cricetomys gambianus) using in vivo bioluminescent imaging. PLoS Negl. Trop. Dis. 9, e0004130 (2015).
Falendysz, E. A. et al. Evaluation of monkeypox virus infection of black-tailed prairie dogs (Cynomys ludovicianus) using in vivo bioluminescent imaging. J. Wildl. Dis. 50, 524–536 (2014).
Marennikova, S. S., Shelukhina, E. M. & Zhukova, O. A. Experimental infection of squirrels Sciurus vulgaris by monkey pox virus. Acta Virol. 33, 399 (1989).
Sergeev, A. A. et al. Using the ground squirrel (Marmota bobak) as an animal model to assess monkeypox drug efficacy. Transbound. Emerg. Dis. 64, 226–236 (2017).
Sbrana, E., Xiao, S. Y., Newman, P. C. & Tesh, R. B. Comparative pathology of North American and central African strains of monkeypox virus in a ground squirrel model of the disease. Am. J. Trop. Med Hyg. 76, 155–164 (2007).
Reynolds, M. G. et al. A silent enzootic of an orthopoxvirus in Ghana, West Africa: evidence for multi-species involvement in the absence of widespread human disease. Am. J. Trop. Med. Hyg. 82, 746–754 (2010).
Khodakevich, L. et al. Monkeypox virus in relation to the ecological features surrounding human settlements in Bumba zone, Zaire. Trop. Geogr. Med. 39, 56–63 (1987).
Gispen, R., Brand-Saathof, B. B. & Hekker, A. C. Monkeypox-specific antibodies in human and simian sera from the Ivory Coast and Nigeria. Bull. World Health Organ. 53, 355–360 (1976).
Mucker, E. M. et al. Susceptibility of marmosets (Callithrix jacchus) to monkeypox virus: a low dose prospective model for monkeypox and smallpox disease. PLoS ONE 10, e0131742 (2015).
Breman, J. G. et al. Human poxvirus disease after smallpox eradication. Am. J. Trop. Med. Hyg. 26, 273–281 (1977).
Arita, I. et al. Outbreaks of monkeypox and serological surveys in nonhuman primates. Bull. World Health Organ. 46, 625–631 (1972).
Sauer, R. M. et al. Studies on a pox disease of monkeys. I. Pathology. Am. J. Vet. Res. 21, 377–380 (1960).
Breman, J. G., Bernadou, J. & Nakano, J. H. Poxvirus in West African nonhuman primates: serological survey results. Bull. World Health Organ. 55, 605–612 (1977).
Peters, J. C. An epizootic of monkey pox at Rotterdam zoo. Int. Zoo. Yearb. 6, 274–275 (1966).
Elizaga, M. L. et al. Prospective surveillance for cardiac adverse events in healthy adults receiving modified vaccinia Ankara vaccines: a systematic review. PLoS ONE 8, e54407 (2013).
Overton, E. T. et al. A randomized phase II trial to compare safety and immunogenicity of the MVA-BN smallpox vaccine at various doses in adults with a history of AIDS. Vaccine 38, 2600–2607 (2020).
Greenberg, R. N. et al. A randomized, double-blind, placebo-controlled phase II trial investigating the safety and immunogenicity of modified vaccinia ankara smallpox vaccine (MVA-BN(R)) in 56-80-year-old subjects. PLoS ONE 11, e0157335 (2016).
Overton, E. T. et al. Immunogenicity and safety of three consecutive production lots of the non replicating smallpox vaccine MVA: a randomised, double blind, placebo controlled phase III trial. PLoS ONE 13, e0195897 (2018).
Zitzmann-Roth, E. M. et al. Cardiac safety of modified vaccinia Ankara for vaccination against smallpox in a young, healthy study population. PLoS ONE 10, e0122653 (2015).
Seaman, M. S. et al. Effect of vaccination with modified vaccinia Ankara (ACAM3000) on subsequent challenge with Dryvax. J. Infect. Dis. 201, 1353–1360 (2010).
Wilck, M. B. et al. Safety and immunogenicity of modified vaccinia Ankara (ACAM3000): effect of dose and route of administration. J. Infect. Dis. 201, 1361–1370 (2010).
Jang, H. C. et al. A randomized, double-blind, controlled clinical trial to evaluate the efficacy and safety of CJ-50300, a newly developed cell culture-derived smallpox vaccine, in healthy volunteers. Vaccine 28, 5845–5849 (2010).
Frey, S. E. et al. Human antibody responses following vaccinia immunization using protein microarrays and correlation with cell-mediated immunity and antibody-dependent cellular cytotoxicity responses. J. Infect. Dis. 224, 1372–1382 (2021).
Acknowledgements
This work was supported by National Natural Science Foundation of China (81972878, 82172733), the National Key Research and Development Program of China (2016YFC1303403, 2020YFC0860200), and Key Research and Development Program of Sichuan Province (2022ZDZX0024, 2017SZDZX0012).
Author information
Authors and Affiliations
Contributions
Y.H. and L.M. collected references and drafted the manuscript. Y.H. revised the paper. L.M. drew the figures and tables. W.W. reviewed the article and provided professional guidance. All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Huang, Y., Mu, L. & Wang, W. Monkeypox: epidemiology, pathogenesis, treatment and prevention. Sig Transduct Target Ther 7, 373 (2022). https://doi.org/10.1038/s41392-022-01215-4
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41392-022-01215-4
This article is cited by
-
Assessment of MPOX infection-related knowledge levels, concerns, and associated factors: a community-based cross-sectional study
BMC Public Health (2025)
-
Capsule network approach for monkeypox (CAPSMON) detection and subclassification in medical imaging system
Scientific Reports (2025)
-
Mpox treatment evolution: past milestones, present advances, and future directions
Naunyn-Schmiedeberg's Archives of Pharmacology (2025)
-
Longitudinal viral shedding and antibody response characteristics of men with acute infection of monkeypox virus: a prospective cohort study
Nature Communications (2024)
-
Evolutionary trajectory and characteristics of Mpox virus in 2023 based on a large-scale genomic surveillance in Shenzhen, China
Nature Communications (2024)