The cosmetics directive provides the regulatory framework for the phasing out of animal testing for cosmetics purposes.
Specifically, it establishes
- a testing ban – prohibition to test finished cosmetic products and cosmetic ingredients on animals
- a marketing ban – prohibition to market finished cosmetic products and ingredients in the EU which were tested on animals
The same provisions are contained in the cosmetics regulation, which replaced the cosmetics directive as of 11 July 2013.
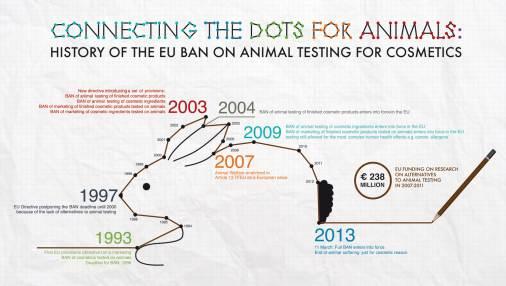
The testing ban on finished cosmetic products applies since 11 September 2004. The testing ban on ingredients or combination of ingredients applies since 11 March 2009.
The marketing ban applies since 11 March 2009 for all human health effects with the exception of repeated-dose toxicity, reproductive toxicity, and toxicokinetics. For these specific health effects, the marketing ban applies since 11 March 2013, irrespective of the availability of alternative non-animal tests.
History of the ban
Review of the 2013 implementation deadline of the marketing ban
According to article 4a (2.3) of the cosmetics directive, the European Commission was required to analyse whether one or more of the complex tests covered by the 2013 marketing ban would not be developed and validated before 11 March 2013 for technical reasons. In the case that alternative methods would not be made available, the Commission had to assess whether to make a legislative proposal in relation to the 2013 marketing ban.
First, the Commission assessed the availability of alternative methods to animal testing by 2013. A technical report was prepared, based on wide scientific expertise, subject to a public consultation, and coordinated by the Commission’s Joint Research Centre. On the basis of this technical report, the Commission reported to the European Parliament and the Council of the European Union in September 2011 that alternative methods would not yet be available by 2013.
Second, the Commission carried out an impact assessment to assess whether to make a legislative proposal given that the full replacement of animal tests by alternatives was not yet possible. A targeted stakeholder consultation was carried out between 7 December 2010 and 15 April 2011.
- Animal testing - Targeted stakeholder consultation on 2013 implementation date marketing ban (319 kB)
- Public consultation on alternative methods for cosmetics testing (243 kB)
Commission communication of 11 March 2013
On 11 March 2013, the Commission finalised the review process by adopting a Communication on the animal testing and marketing ban and the state of play of alternative methods in cosmetics. This communication confirmed the Commission’s commitment to maintaining the 2013 deadline and outlines how it intended to further support research and innovation in the area while promoting animal welfare worldwide. In addition, the EURL-CVAM technical report 2013 provides a more detailed overview of progress made in the development, validation, and regulatory acceptance of alternatives.
The adoption of the Commission Communication was announced in a press release and additional background information was provided in a questions and answers document.
The communication was accompanied by an impact assessment consisting of
- Executive summary of the impact assessment on the animal testing provisions in Cosmetics Regulation (EC) 1223/2009
- Impact assessment on the animal testing provisions in Cosmetics Regulation (EC) 1223/2009
Commission reports
- Report (2018) on the development, validation and legal acceptance of methods alternative to animal testing in the field of cosmetics
- Report (2015-2017) on the development, validation and legal acceptance of methods alternative to animal testing in the field of cosmetics
- Report (2013-2015) on the development, validation and legal acceptance of methods alternative to animal testing in the field of cosmetics
European cooperation
European Centre for the Validation of Alternative Methods
The European centre for the validation of alternative methods (ECVAM) plays a key role in the development, validation, and international recognition of alternative methods which reduce, refine, or replace the use of animals in testing. It is hosted by the Joint Research Centre's institute for health and consumer protection (IHCP) located in Ispra, Italy. Since 2011, ECVAM has become the European Union reference laboratory for alternatives to animal testing (EURL ECVAM), established under Directive 2010/63/EC on the protection of animals used for scientific purposes.
- EURL ECVAM progress report on the development, validation and regulatory acceptance of alternative methods prepared in the framework of the cosmetics regulation
- ECVAM activities: EURL ECVAM survey on in vitro methods for estimating human hepatic metabolic clearance/stability
ECVAM hosts 2 important databases in relation to alternative methods
- TSAR: tracking system for alternative test methods review, validation and approval in the context of EU regulations on chemicals
- DB-ALM: database on alternative methods
Research on alternative methods to animal testing
See an overview of research activities supported by the Commission in the AXLR8 progress reports
- Alternative testing strategies progress report 2012 (6 MB)
- Alternative testing strategies progress report 2011 (5 MB)
- Alternative testing strategies progress report 2010 (8 MB)
- Alternative testing strategies progress report 2009
The SEURAT project is a joined research project between the Commission and the cosmetics industry.
European partnership to alternative approaches to animal testing
The European partnership for alternative approaches to animal testing (EPAA) is a joint initiative between the Commission, European trade associations from 7 industry sectors, and individual companies. It was launched in November 2005 to promote the development and implementation of new 3Rs methods (replace, reduce, refine) and modern alternative approaches in safety testing.
International cooperation
Alternative methods to animal testing are one of the focuses of the 'international cooperation on cosmetics regulation' (ICCR).
An important outcome is the cooperation of international validation bodies in the framework for international cooperation on alternative test methods (ICATM) (27 kB).
Overview on the different activities to elaborate alternative testing methods by OECD
Related documents
- Reply to petitions on animal testing and cosmetics – September 2016 (141 KB)
- Timetable for phasing out of animal testing (340 kB)
- Documents related to animal testing in cosmetics (including reports on animal testing) (415 kB)
- Commission recommendation of 7 June 2006 on guidelines for claims about absence of animal tests pursuant to Directive 76/768/EEC
- Public consultation on alternative methods for cosmetics testing (243 kB)
