Human single-chain Fv immunoconjugates targeted to a melanoma-associated chondroitin sulfate proteoglycan mediate specific lysis of human melanoma cells by natural killer cells and complement
- PMID: 9990075
- PMCID: PMC15540
- DOI: 10.1073/pnas.96.4.1627
Human single-chain Fv immunoconjugates targeted to a melanoma-associated chondroitin sulfate proteoglycan mediate specific lysis of human melanoma cells by natural killer cells and complement
Abstract
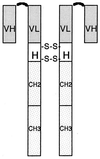
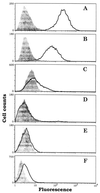
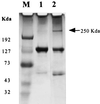
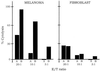
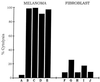
Two antimelanoma immunoconjugates containing a human single-chain Fv (scFv) targeting domain conjugated to the Fc effector domain of human IgG1 were synthesized as secreted two-chain molecules in Chinese hamster ovary and Drosophila S2 cells, and purified by affinity chromatography on protein A. The scFv targeting domains originally were isolated as melanoma-specific clones from a scFv fusion-phage library, derived from the antibody repertoire of a vaccinated melanoma patient. The purified immunoconjugates showed similar binding specificity as did the fusion-phage clones. Binding occurred to human melanoma cells but not to human melanocytes or to several other types of normal cells and tumor cells. A 250-kDa melanoma protein was immunoprecipitated by the immunoconjugates and analyzed by mass spectrometry, using two independent procedures. A screen of protein sequence databases showed an exact match of several peptide masses between the immunoprecipitated protein and the core protein of a chondroitin sulfate proteoglycan, which is expressed on the surface of most human melanoma cells. The Fc effector domain of the immunoconjugates binds natural killer (NK) cells and also the C1q protein that initiates the complement cascade; both NK cells and complement can activate powerful cytolytic responses against the targeted tumor cells. An in vitro cytolysis assay was used to test for an immunoconjugate-dependent specific cytolytic response against cultured human melanoma cells by NK cells and complement. The melanoma cells, but not the human fibroblast cells used as the control, were efficiently lysed by both NK cells and complement in the presence of the immunoconjugates. The in vitro results suggest that the immunoconjugates also could activate a specific cytolytic immune response against melanoma tumors in vivo.
Figures

Similar articles
-
Targeting tumor vasculature endothelial cells and tumor cells for immunotherapy of human melanoma in a mouse xenograft model.Proc Natl Acad Sci U S A. 1999 Jul 6;96(14):8161-6. doi: 10.1073/pnas.96.14.8161. Proc Natl Acad Sci U S A. 1999. PMID: 10393965 Free PMC article.
-
Anti-melanoma antibodies from melanoma patients immunized with genetically modified autologous tumor cells: selection of specific antibodies from single-chain Fv fusion phage libraries.Proc Natl Acad Sci U S A. 1995 Jul 3;92(14):6537-41. doi: 10.1073/pnas.92.14.6537. Proc Natl Acad Sci U S A. 1995. PMID: 7604028 Free PMC article.
-
Comparison of fusion phage libraries displaying VH or single-chain Fv antibody fragments derived from the antibody repertoire of a vaccinated melanoma patient as a source of melanoma-specific targeting molecules.Proc Natl Acad Sci U S A. 1997 Aug 19;94(17):9261-6. doi: 10.1073/pnas.94.17.9261. Proc Natl Acad Sci U S A. 1997. PMID: 9256470 Free PMC article.
-
A melanoma-specific VH antibody cloned from a fusion phage library of a vaccinated melanoma patient.Proc Natl Acad Sci U S A. 1996 Jun 25;93(13):6280-5. doi: 10.1073/pnas.93.13.6280. Proc Natl Acad Sci U S A. 1996. PMID: 8692806 Free PMC article.
-
Full-length recombinant antibodies from Escherichia coli: production, characterization, effector function (Fc) engineering, and clinical evaluation.MAbs. 2022 Jan-Dec;14(1):2111748. doi: 10.1080/19420862.2022.2111748. MAbs. 2022. PMID: 36018829 Free PMC article. Review.
Cited by
-
Immunization and immunotherapy for cancers involving infection by a human papillomavirus in a mouse model.Proc Natl Acad Sci U S A. 2002 Dec 10;99(25):16232-6. doi: 10.1073/pnas.192581299. Epub 2002 Nov 21. Proc Natl Acad Sci U S A. 2002. PMID: 12446839 Free PMC article.
-
Tissue factor (coagulation factor III): a potential double-edge molecule to be targeted and re-targeted toward cancer.Biomark Res. 2023 Jun 6;11(1):60. doi: 10.1186/s40364-023-00504-6. Biomark Res. 2023. PMID: 37280670 Free PMC article. Review.
-
The effects of small interfering RNA-targeting tissue factor on an in vitro model of neovascularization.Mol Vis. 2013 Jun 11;19:1296-303. Print 2013. Mol Vis. 2013. PMID: 23805036 Free PMC article.
-
Intratumoral injection of adenoviral vectors encoding tumor-targeted immunoconjugates for cancer immunotherapy.Proc Natl Acad Sci U S A. 2000 Aug 1;97(16):9221-5. doi: 10.1073/pnas.97.16.9221. Proc Natl Acad Sci U S A. 2000. PMID: 10922073 Free PMC article.
-
A Pharmacodynamic Analysis of Choroidal Neovascularization in a Porcine Model Using Three Targeted Drugs.Invest Ophthalmol Vis Sci. 2017 Jul 1;58(9):3732-3740. doi: 10.1167/iovs.16-21230. Invest Ophthalmol Vis Sci. 2017. PMID: 28738417 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

