The replicative capacities of large E1B-null group A and group C adenoviruses are independent of host cell p53 status
- PMID: 9971789
- PMCID: PMC104451
- DOI: 10.1128/JVI.73.3.2074-2083.1999
The replicative capacities of large E1B-null group A and group C adenoviruses are independent of host cell p53 status
Abstract
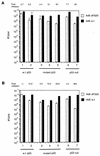
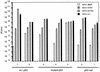
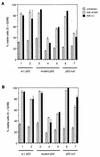
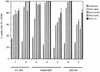
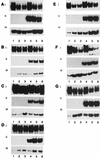
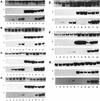
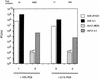
Recent reports suggest that an early region 1B (E1B) 55, 000-molecular-weight polypeptide (55K)-null adenovirus type 5 (Ad5) mutant (dl1520) can replicate to the same extent as wild-type (wt) Ad5 in cells either deficient or mutated in p53, implicating p53 in limiting viral replication in vivo. In contrast, we show here that the replicative capacity of Ad5 dl1520 is wholly independent of host cell p53 status, as is the replicative capacity of comparable Ad12 E1B 54K-null adenoviruses (Ad12 dl620 and Ad12 hr703). Furthermore, we show that there is no requirement for complex formation between p53 and Ad5 E1B 55K or Ad12 E1B 54K for a productive infection, such that wt Ad5 and wt Ad12 will both replicate in cells which are null for p53. In addition, we find that these Ad5 and Ad12 mutant viruses induce S phase irrespective of the p53 status of the cell and that, therefore, S-phase induction does not correlate with the replicative capacity of the virus. Interestingly, the replicative capacities of the large E1B-null adenoviruses correlated positively with the ability to express E1B 19K and were related to the ability to repress premature adenovirus-induced apoptosis. Infection of primary human cells indicated that Ad5 dl1520, wt Ad5, and wt Ad12 replicated better in cycling normal human skin fibroblasts (HSFs) than in quiescent HSFs. Thus, the cell cycle status of the host cell, upon infection, also influences viral yield.
Figures
Similar articles
-
Control of p53 expression by adenovirus 12 early region 1A and early region 1B 54K proteins.Virology. 1996 Apr 1;218(1):23-34. doi: 10.1006/viro.1996.0162. Virology. 1996. PMID: 8615026
-
Enhanced expression of p53 in human cells infected with mutant adenoviruses.Virology. 1994 Sep;203(2):229-40. doi: 10.1006/viro.1994.1480. Virology. 1994. PMID: 8053147
-
Consequences of disruption of the interaction between p53 and the larger adenovirus early region 1B protein in adenovirus E1 transformed human cells.Oncogene. 2000 Jan 20;19(3):452-62. doi: 10.1038/sj.onc.1203316. Oncogene. 2000. PMID: 10656694
-
Genetically modified adenoviruses against gliomas: from bench to bedside.Neurology. 2004 Aug 10;63(3):418-26. doi: 10.1212/01.wnl.0000133302.15022.7f. Neurology. 2004. PMID: 15304571 Review.
-
Interactions between adenovirus proteins and the p53 pathway: the development of ONYX-015.Semin Cancer Biol. 2000 Dec;10(6):453-9. doi: 10.1006/scbi.2000.0336. Semin Cancer Biol. 2000. PMID: 11170867 Review.
Cited by
-
The human adenovirus type 5 E1B 55 kDa protein obstructs inhibition of viral replication by type I interferon in normal human cells.PLoS Pathog. 2012;8(8):e1002853. doi: 10.1371/journal.ppat.1002853. Epub 2012 Aug 9. PLoS Pathog. 2012. PMID: 22912576 Free PMC article.
-
Tumor-specific, replication-competent adenovirus vectors overexpressing the adenovirus death protein.J Virol. 2000 Jul;74(13):6147-55. doi: 10.1128/jvi.74.13.6147-6155.2000. J Virol. 2000. PMID: 10846098 Free PMC article.
-
A role for E1B-AP5 in ATR signaling pathways during adenovirus infection.J Virol. 2008 Aug;82(15):7640-52. doi: 10.1128/JVI.00170-08. Epub 2008 May 14. J Virol. 2008. PMID: 18480432 Free PMC article.
-
Reduced infectivity of adenovirus type 5 particles and degradation of entering viral genomes associated with incomplete processing of the preterminal protein.J Virol. 2012 Dec;86(24):13554-65. doi: 10.1128/JVI.02337-12. Epub 2012 Oct 3. J Virol. 2012. PMID: 23035217 Free PMC article.
-
The nuclear export signal within the E4orf6 protein of adenovirus type 5 supports virus replication and cytoplasmic accumulation of viral mRNA.J Virol. 2000 Jan;74(2):764-72. doi: 10.1128/jvi.74.2.764-772.2000. J Virol. 2000. PMID: 10623738 Free PMC article.
References
-
- Barker D D, Berk A J. Adenovirus proteins from both E1B reading frames are required for transformation of rodent cells by viral infection and DNA transfection. Virology. 1987;156:107–121. - PubMed
-
- Bernards R, De Leeuw M G W, Houweling A, van der Eb A J. Role of the adenovirus early region 1B tumor antigens in transformation and lytic infection. Virology. 1986;150:126–139. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

