Estrogen-induced activation of mitogen-activated protein kinase in cerebral cortical explants: convergence of estrogen and neurotrophin signaling pathways
- PMID: 9952396
- PMCID: PMC6786041
- DOI: 10.1523/JNEUROSCI.19-04-01179.1999
Estrogen-induced activation of mitogen-activated protein kinase in cerebral cortical explants: convergence of estrogen and neurotrophin signaling pathways
Abstract
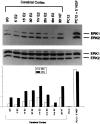
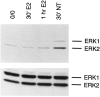
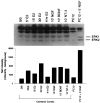
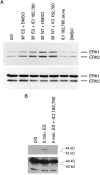
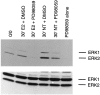
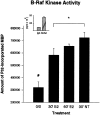
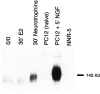
We have shown that estrogen elicits a selective enhancement of the growth and differentiation of axons and dendrites (neurites) in the developing CNS. We subsequently demonstrated widespread colocalization of estrogen and neurotrophin receptors (trk) within developing forebrain neurons and reciprocal transcriptional regulation of these receptors by their ligands. Using organotypic explants of the cerebral cortex, we tested the hypothesis that estrogen/neurotrophin receptor coexpression also may result in convergence or cross-coupling of their signaling pathways. Estradiol elicited rapid (within 5-15 min) tyrosine phosphorylation/activation of the mitogen-activated protein (MAP) kinases, ERK1 and ERK2, that persisted for at least 2 hr. This extracellular signal-regulated protein kinase (ERK) activation was inhibited successfully by the MEK1 inhibitor PD98059, but not by the estrogen receptor (ER) antagonist ICI 182,780, and did not appear to result from estradiol-induced activation of trk. Furthermore, we also found that estradiol elicited an increase in B-Raf kinase activity. The latter and subsequent downstream events leading to ERK activation may be a consequence of our documentation of a multimeric complex consisting of, at least, the ER, hsp90, and B-Raf. These novel findings provide an alternative mechanism for some of the estrogen actions in the developing CNS and could explain not only some of the very rapid effects of estrogen but also the ability of estrogen and neurotrophins to regulate the same broad array of cytoskeletal and growth-associated genes involved in neurite growth and differentiation.
Figures
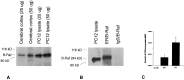


Similar articles
-
Novel mechanisms of estrogen action in the brain: new players in an old story.Front Neuroendocrinol. 1999 Apr;20(2):97-121. doi: 10.1006/frne.1999.0177. Front Neuroendocrinol. 1999. PMID: 10328986 Review.
-
Estrogen-induced activation of the mitogen-activated protein kinase cascade in the cerebral cortex of estrogen receptor-alpha knock-out mice.J Neurosci. 2000 Mar 1;20(5):1694-700. doi: 10.1523/JNEUROSCI.20-05-01694.2000. J Neurosci. 2000. PMID: 10684871 Free PMC article.
-
Estrogen receptor protein interaction with phosphatidylinositol 3-kinase leads to activation of phosphorylated Akt and extracellular signal-regulated kinase 1/2 in the same population of cortical neurons: a unified mechanism of estrogen action.J Neurosci. 2006 Sep 13;26(37):9439-47. doi: 10.1523/JNEUROSCI.1443-06.2006. J Neurosci. 2006. PMID: 16971528 Free PMC article.
-
Differential regulation of mitogen-activated protein kinases ERK1/2 and ERK5 by neurotrophins, neuronal activity, and cAMP in neurons.J Neurosci. 2001 Jan 15;21(2):434-43. doi: 10.1523/JNEUROSCI.21-02-00434.2001. J Neurosci. 2001. PMID: 11160424 Free PMC article.
-
Novel sites and mechanisms of oestrogen action in the brain.Novartis Found Symp. 2000;230:56-69; discussion 69-73. doi: 10.1002/0470870818.ch6. Novartis Found Symp. 2000. PMID: 10965502 Review.
Cited by
-
Estradiol signaling in the regulation of reproduction and energy balance.Front Neuroendocrinol. 2012 Oct;33(4):342-63. doi: 10.1016/j.yfrne.2012.08.004. Epub 2012 Sep 7. Front Neuroendocrinol. 2012. PMID: 22981653 Free PMC article. Review.
-
Role of protein phosphatases in estrogen-mediated neuroprotection.J Neurosci. 2005 Aug 3;25(31):7191-8. doi: 10.1523/JNEUROSCI.1328-05.2005. J Neurosci. 2005. PMID: 16079401 Free PMC article.
-
Integration of the extranuclear and nuclear actions of estrogen.Mol Endocrinol. 2005 Aug;19(8):1951-9. doi: 10.1210/me.2004-0390. Epub 2005 Feb 10. Mol Endocrinol. 2005. PMID: 15705661 Free PMC article. Review.
-
Estrogen actions on mitochondria--physiological and pathological implications.Mol Cell Endocrinol. 2008 Aug 13;290(1-2):51-9. doi: 10.1016/j.mce.2008.04.013. Epub 2008 May 2. Mol Cell Endocrinol. 2008. PMID: 18571833 Free PMC article. Review.
-
Membrane-localised oestrogen receptor alpha and beta influence neuronal activity through activation of metabotropic glutamate receptors.J Neuroendocrinol. 2009 Mar;21(4):257-62. doi: 10.1111/j.1365-2826.2009.01838.x. J Neuroendocrinol. 2009. PMID: 19207809 Free PMC article. Review.
References
-
- Barnier JV, Papin C, Eychene A, Lecoq O, Calothy G. The mouse B-raf gene encodes multiple protein isoforms with tissue-specific expression. J Biol Chem. 1995;270:23381–23389. - PubMed
-
- Chiaia N, Foy M, Teyler TJ. The hamster hippocampal slice. II. Neuroendocrine modulation. Behav Neurosci. 1983;97:839–843. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
