Membrane-type 1 matrix metalloprotease (MT1-MMP) enables invasive migration of glioma cells in central nervous system white matter
- PMID: 9922462
- PMCID: PMC2132902
- DOI: 10.1083/jcb.144.2.373
Membrane-type 1 matrix metalloprotease (MT1-MMP) enables invasive migration of glioma cells in central nervous system white matter
Abstract
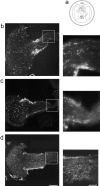
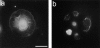
Invasive glioma cells migrate preferentially along central nervous system (CNS) white matter fiber tracts irrespective of the fact that CNS myelin contains proteins that inhibit cell migration and neurite outgrowth. Previous work has demonstrated that to migrate on a myelin substrate and to overcome its inhibitory effect, rat C6 and human glioblastoma cells require a membrane-bound metalloproteolytic activity (C6-MP) which shares several biochemical and pharmacological characteristics with MT1-MMP. We show now that MT1-MMP is expressed on the surface of rat C6 glioblastoma cells and is coenriched with C6-MP activity. Immunodepletion of C6-MP activity is achieved with an anti-MT1-MMP antibody. These data suggest that MT1-MMP and the C6-MP are closely related or identical. When mouse 3T3 fibroblasts were transfected with MT1-MMP they acquired the ability to spread and migrate on the nonpermissive myelin substrate and to infiltrate into adult rat optic nerve explants. MT1-MMP-transfected fibroblasts and C6 glioma cells were able to digest bNI-220, one of the most potent CNS myelin inhibitory proteins. Plasma membranes of both MT1-MMP-transfected fibroblasts and C6 glioma cells inactivated inhibitory myelin extracts, and this activity was sensitive to the same protease inhibitors. Interestingly, pretreatment of CNS myelin with gelatinase A/MMP-2 could not inactivate its inhibitory property. These data imply an important role of MT1-MMP in spreading and migration of glioma cells on white matter constituents in vitro and point to a function of MT1-MMP in the invasive behavior of malignant gliomas in the CNS in vivo.
Figures

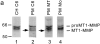
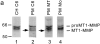



Similar articles
-
A metalloprotease activity from C6 glioma cells inactivates the myelin-associated neurite growth inhibitors and can be neutralized by antibodies.Br J Cancer. 1998 Dec;78(12):1564-72. doi: 10.1038/bjc.1998.724. Br J Cancer. 1998. PMID: 9862565 Free PMC article.
-
Glioblastoma infiltration into central nervous system tissue in vitro: involvement of a metalloprotease.J Cell Biol. 1988 Dec;107(6 Pt 1):2281-91. doi: 10.1083/jcb.107.6.2281. J Cell Biol. 1988. PMID: 3198688 Free PMC article.
-
Spreading and migration of human glioma and rat C6 cells on central nervous system myelin in vitro is correlated with tumor malignancy and involves a metalloproteolytic activity.Cancer Res. 1998 Jan 1;58(1):149-58. Cancer Res. 1998. PMID: 9426071
-
Membrane-type matrix metalloproteinases (MT-MMPs) in cell invasion.Thromb Haemost. 1997 Jul;78(1):497-500. Thromb Haemost. 1997. PMID: 9198203 Review.
-
Membrane-type matrix metalloproteinases (MT-MMPs): expression and function during glioma invasion.J Neurooncol. 2001 Jun;53(2):187-202. doi: 10.1023/a:1012213604731. J Neurooncol. 2001. PMID: 11716070 Review.
Cited by
-
Fascin-1 knock-down of human glioma cells reduces their microvilli/filopodia while improving their susceptibility to lymphocyte-mediated cytotoxicity.Am J Transl Res. 2015 Feb 15;7(2):271-84. eCollection 2015. Am J Transl Res. 2015. PMID: 25901196 Free PMC article.
-
Proteases and the biology of glioma invasion.J Neurooncol. 2002 Jan;56(2):149-58. doi: 10.1023/a:1014566604005. J Neurooncol. 2002. PMID: 11995816 Review.
-
Role of Microenvironment in Glioma Invasion: What We Learned from In Vitro Models.Int J Mol Sci. 2018 Jan 4;19(1):147. doi: 10.3390/ijms19010147. Int J Mol Sci. 2018. PMID: 29300332 Free PMC article. Review.
-
Detection of Glioblastoma Subclinical Recurrence Using Serial Diffusion Tensor Imaging.Cancers (Basel). 2020 Feb 29;12(3):568. doi: 10.3390/cancers12030568. Cancers (Basel). 2020. PMID: 32121471 Free PMC article.
-
Synergistic effects of arsenic trioxide and silibinin on apoptosis and invasion in human glioblastoma U87MG cell line.Neurochem Res. 2012 Feb;37(2):370-80. doi: 10.1007/s11064-011-0620-1. Epub 2011 Oct 4. Neurochem Res. 2012. PMID: 21969006
References
-
- Amberger VR, Paganetti PA, Seulberger H, Eldering JA, Schwab ME. Characterization of a membrane-bound metalloendoprotease of rat C6 glioblastoma cells. Cancer Res. 1994;54:4017–4025. - PubMed
-
- Amberger VR, Hensel T, Ogata N, Schwab ME. Spreading and migration of human glioma and rat C6 cells on central nervous system myelin in vitro is correlated with tumor malignancy and involves a metalloproteolytic activity. Cancer Res. 1998;58:149–158. - PubMed
-
- Atkinson SJ, Crabbe T, Cowell S, Ward RV, Butler MJ, Sato H, Seiki M, Reynolds JJ, Murphy G. Intermolecular autolytic cleavage can contribute to the activation of progelatinase A by cell membranes. J Biol Chem. 1995;270:30479–30485. - PubMed
-
- Benda ME, Lightbody J, Sato G, Levine L, Sweet W. Differential rat glia cell strain in tissue culture. Science. 1968;161:370–371. - PubMed
-
- Berens ME, Rief MD, Loo MA, Giese A. The role of extracellular matrix in human astrocytoma migration and proliferation studied in a microliter scale assay. Clin Exp Met. 1994;12:405–415. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

