Tumor necrosis factor-alpha mediates orthopedic implant osteolysis
- PMID: 9916934
- PMCID: PMC1853441
- DOI: 10.1016/s0002-9440(10)65266-2
Tumor necrosis factor-alpha mediates orthopedic implant osteolysis
Abstract
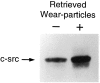
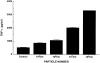
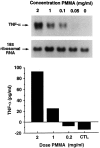
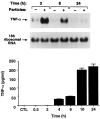
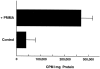
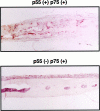
Osteolysis complicating arthroplasty reflects progressive generation of implant-derived wear particles, which prompt an inflammatory reaction attended by recruitment of osteoclasts to the prosthesis-bone interface. To identify a soluble mediator of periprosthetic osteolysis we first showed that implant particles induce c-src in murine bone marrow macrophages (BMMs), a protein specifically expressed when these cells commit to the osteoclast phenotype. The fact that tumor necrosis factor-alpha (TNF) is a potent osteoclastogenic agent while at the same time is the only soluble moiety known to be c-src inductive suggests that this cytokine may mediate implant particle-induced osteoclastogenesis. Consistent with this hypothesis, prosthesis-derived wear particles, recovered at revision arthroplasty, dose-dependently prompt TNF secretion by BMMs. Similarly, particulate polymemthylmethacrylate, the major component of orthopedic implant cement, induces BMM expression of TNF mRNA and protein in a time- and dose-dependent manner. Furthermore, failure of BMMs derived from mice deleted of both the p55 and p75 TNF receptors to express c-src in response to polymemthyl-methacrylate indicates TNF is an essential mediator of particle induction of this osteoclast specific protein. To test the hypothesis that TNF mediates implant osteolysis, we established an in vivo murine model of this condition that histologically mirrors that of man. Verifying that TNF is essential to development of particle osteolysis, mice failing to express both the p55 and p75 TNF receptors are protected from the profound bone resorption attending polymemthyl-methacrylate particle implantation on calvariae of wild-type animals. Finally, the protective effect of deletion of both TNF receptors is recapitulated in mice lacking only the p55 receptor. Thus, targeting TNF and/or its p55 receptor may arrest wear particle osteolysis.
Figures



Similar articles
-
Lipopolysaccharide-stimulated osteoclastogenesis is mediated by tumor necrosis factor via its P55 receptor.J Clin Invest. 1997 Sep 15;100(6):1557-65. doi: 10.1172/JCI119679. J Clin Invest. 1997. PMID: 9294124 Free PMC article.
-
Tumor necrosis factor-alpha mediates polymethylmethacrylate particle-induced NF-kappaB activation in osteoclast precursor cells.J Orthop Res. 2002 Mar;20(2):174-81. doi: 10.1016/S0736-0266(01)00088-2. J Orthop Res. 2002. PMID: 11918294
-
Spinal implant debris-induced osteolysis.Spine (Phila Pa 1976). 2003 Oct 15;28(20):S125-38. doi: 10.1097/00007632-200310151-00006. Spine (Phila Pa 1976). 2003. PMID: 14560184
-
The osteoclastogenic molecules RANKL and RANK are associated with periprosthetic osteolysis.J Bone Joint Surg Br. 2001 Aug;83(6):902-11. doi: 10.1302/0301-620x.83b6.10905. J Bone Joint Surg Br. 2001. PMID: 11521937 Review.
-
Wear and osteolysis in total joint replacements.Acta Orthop Scand Suppl. 1998 Feb;278:1-16. Acta Orthop Scand Suppl. 1998. PMID: 9524528 Review.
Cited by
-
microRNA Expression in Rat Apical Periodontitis Bone Lesion.Bone Res. 2013 Jun 28;1(2):170-85. doi: 10.4248/BR201302006. eCollection 2013 Jun. Bone Res. 2013. PMID: 26273501 Free PMC article.
-
Increased UHMWPE Particle-Induced Osteolysis in Fetuin-A-Deficient Mice.J Funct Biomater. 2023 Jan 4;14(1):30. doi: 10.3390/jfb14010030. J Funct Biomater. 2023. PMID: 36662077 Free PMC article.
-
Differential effects of biologic versus bisphosphonate inhibition of wear debris-induced osteolysis assessed by longitudinal micro-CT.J Orthop Res. 2008 Oct;26(10):1340-6. doi: 10.1002/jor.20620. J Orthop Res. 2008. PMID: 18404739 Free PMC article.
-
A TNF variant that associates with susceptibility to musculoskeletal disease modulates thyroid hormone receptor binding to control promoter activation.PLoS One. 2013 Sep 19;8(9):e76034. doi: 10.1371/journal.pone.0076034. eCollection 2013. PLoS One. 2013. PMID: 24069456 Free PMC article.
-
Systemic trafficking of macrophages induced by bone cement particles in nude mice.Biomaterials. 2008 Dec;29(36):4760-5. doi: 10.1016/j.biomaterials.2008.09.004. Epub 2008 Sep 27. Biomaterials. 2008. PMID: 18824259 Free PMC article.
References
-
- Harris WH: The problem is osteolysis. Clin Orthop Relat Res 1995, 311:46-53 - PubMed
-
- Anonymous: Total hip replacement. The National Institutes of Health Consensus Statement 1994, 12 Sept:12–14
-
- Jones LC, Hungerford DS: Cement disease. Clin Orthop 1987, 225:192-206 - PubMed
-
- Goldring SR, Clark CR, Wright TM: The problem in total joint arthroplasty: aseptic loosening. J Bone Jt Surg 1993, 75A:799-801 - PubMed
-
- Jiranek WA, Machado M, Jasty M, Jevsevar D, Wolfe HJ, Goldring SR, Goldberg MJ, Harris WH: Production of cytokines around loosened cemented acetabular components. J Bone Jt Surg 1993, 75A:863-879 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

