Interactions between a nuclear transporter and a subset of nuclear pore complex proteins depend on Ran GTPase
- PMID: 9891088
- PMCID: PMC116083
- DOI: 10.1128/MCB.19.2.1547
Interactions between a nuclear transporter and a subset of nuclear pore complex proteins depend on Ran GTPase
Abstract
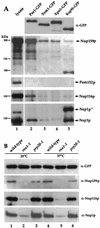
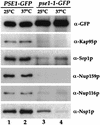
Proteins to be transported into the nucleus are recognized by members of the importin-karyopherin nuclear transport receptor family. After docking at the nuclear pore complex (NPC), the cargo-receptor complex moves through the aqueous pore channel. Once cargo is released, the importin then moves back through the channel for new rounds of transport. Thus, importin and exportin, another member of this family involved in export, are thought to continuously shuttle between the nuclear interior and the cytoplasm. In order to understand how nuclear transporters traverse the NPC, we constructed functional protein fusions between several members of the yeast importin family, including Pse1p, Sxm1p, Xpo1p, and Kap95p, and the green fluorescent protein (GFP). Complexes containing nuclear transporters were isolated by using highly specific anti-GFP antibodies. Pse1-GFP was studied in the most detail. Pse1-GFP is in a complex with importin-alpha and -beta (Srp1p and Kap95p in yeast cells) that is sensitive to the nucleotide-bound state of the Ran GTPase. In addition, Pse1p associates with the nucleoporins Nsp1p, Nup159p, and Nup116p, while Sxm1p, Xpo1p, and Kap95p show different patterns of interaction with nucleoporins. Association of Pse1p with nucleoporins also depends on the nucleotide-bound state of Ran; when Ran is in the GTP-bound state, the nucleoporin association is lost. A mutant form of Pse1p that does not bind Ran also fails to interact with nucleoporins. These data indicate that transport receptors such as Pse1p interact in a Ran-dependent manner with certain nucleoporins. These nucleoporins may represent major docking sites for Pse1p as it moves in or out of the nucleus via the NPC.
Figures
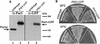

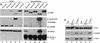



Similar articles
-
Yrb4p, a yeast ran-GTP-binding protein involved in import of ribosomal protein L25 into the nucleus.EMBO J. 1997 Oct 15;16(20):6237-49. doi: 10.1093/emboj/16.20.6237. EMBO J. 1997. PMID: 9321403 Free PMC article.
-
Specific binding of the karyopherin Kap121p to a subunit of the nuclear pore complex containing Nup53p, Nup59p, and Nup170p.J Cell Biol. 1998 Dec 28;143(7):1813-30. doi: 10.1083/jcb.143.7.1813. J Cell Biol. 1998. PMID: 9864357 Free PMC article.
-
Nuclear import of the yeast AP-1-like transcription factor Yap1p is mediated by transport receptor Pse1p, and this import step is not affected by oxidative stress.J Biol Chem. 2001 Jun 15;276(24):21863-9. doi: 10.1074/jbc.M009258200. Epub 2001 Mar 23. J Biol Chem. 2001. PMID: 11274141
-
Molecular mechanisms of nuclear protein transport.Crit Rev Eukaryot Gene Expr. 1997;7(1-2):61-72. doi: 10.1615/critreveukargeneexpr.v7.i1-2.40. Crit Rev Eukaryot Gene Expr. 1997. PMID: 9034715 Review.
-
Nuclear import and export pathways.J Cell Biochem. 1999;Suppl 32-33:76-83. doi: 10.1002/(sici)1097-4644(1999)75:32+<76::aid-jcb10>3.3.co;2-h. J Cell Biochem. 1999. PMID: 10629106 Review.
Cited by
-
The zinc-binding region of IL-2 inducible T cell kinase (Itk) is required for interaction with Gα13 and activation of serum response factor.Int J Biochem Cell Biol. 2013 Jun;45(6):1074-82. doi: 10.1016/j.biocel.2013.02.011. Epub 2013 Feb 27. Int J Biochem Cell Biol. 2013. PMID: 23454662 Free PMC article.
-
Ultrastructural imaging of endocytic sites in Saccharomyces cerevisiae by transmission electron microscopy and immunolabeling.Microsc Microanal. 2013 Apr;19(2):381-92. doi: 10.1017/S1431927612014304. Epub 2013 Mar 5. Microsc Microanal. 2013. PMID: 23458500 Free PMC article.
-
Oncogenic NRAS rapidly and efficiently induces CMML- and AML-like diseases in mice.Blood. 2006 Oct 1;108(7):2349-57. doi: 10.1182/blood-2004-08-009498. Epub 2006 Jun 8. Blood. 2006. PMID: 16763213 Free PMC article.
-
Simple kinetic relationships and nonspecific competition govern nuclear import rates in vivo.J Cell Biol. 2006 Nov 20;175(4):579-93. doi: 10.1083/jcb.200608141. J Cell Biol. 2006. PMID: 17116750 Free PMC article.
-
SAC3 may link nuclear protein export to cell cycle progression.Proc Natl Acad Sci U S A. 2000 Mar 28;97(7):3224-9. doi: 10.1073/pnas.97.7.3224. Proc Natl Acad Sci U S A. 2000. PMID: 10716708 Free PMC article.
References
-
- Aebi M, Clark M W, Vijayraghavan U, Abelson J. A yeast mutant, PRP20, altered in mRNA metabolism and maintenance of the nuclear structure, is defective in a gene homologous to the human gene RCC1 which is involved in the control of chromosome condensation. Mol Gen Genet. 1990;224:72–80. - PubMed
-
- Aitchison J D, Blobel G, Rout M P. Kap104p: A karyopherin involved in the nuclear transport of messenger RNA binding proteins. Science. 1996;274:624–627. - PubMed
-
- Arts G J, Fornerod M, Mattaj I W. Identification of a nuclear export receptor for tRNA. Curr Biol. 1998;8:305–314. - PubMed
-
- Becker J, Melchior F, Gerke V, Bischoff F R, Ponstigl H, Wittinghoffer A. RNA1 encodes a GTPase-activating protein specific for Gsp1p, the Ran/TC4 homologue of Saccharomyces cerevisiae. J Biol Chem. 1995;270:11860–11865. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
