A bioluminescence resonance energy transfer (BRET) system: application to interacting circadian clock proteins
- PMID: 9874787
- PMCID: PMC15108
- DOI: 10.1073/pnas.96.1.151
A bioluminescence resonance energy transfer (BRET) system: application to interacting circadian clock proteins
Abstract
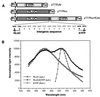
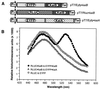
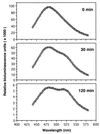
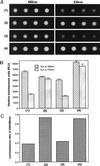
We describe a method for assaying protein interactions that offers some attractive advantages over previous assays. This method, called bioluminescence resonance energy transfer (BRET), uses a bioluminescent luciferase that is genetically fused to one candidate protein, and a green fluorescent protein mutant fused to another protein of interest. Interactions between the two fusion proteins can bring the luciferase and green fluorescent protein close enough for resonance energy transfer to occur, thus changing the color of the bioluminescent emission. By using proteins encoded by circadian (daily) clock genes from cyanobacteria, we use the BRET technique to demonstrate that the clock protein KaiB interacts to form homodimers. BRET should be particularly useful for testing protein interactions within native cells, especially with integral membrane proteins or proteins targeted to specific organelles.
Figures
Similar articles
-
Circadian rhythms of cyanobacteria: monitoring the biological clocks of individual colonies by bioluminescence.J Bacteriol. 1994 Apr;176(7):1881-5. doi: 10.1128/jb.176.7.1881-1885.1994. J Bacteriol. 1994. PMID: 8144454 Free PMC article.
-
Bioluminescence resonance energy transfer-based imaging of protein-protein interactions in living cells.Nat Protoc. 2019 Apr;14(4):1084-1107. doi: 10.1038/s41596-019-0129-7. Epub 2019 Mar 25. Nat Protoc. 2019. PMID: 30911173
-
Combining protein complementation assays with resonance energy transfer to detect multipartner protein complexes in living cells.Methods. 2008 Jul;45(3):214-8. doi: 10.1016/j.ymeth.2008.06.006. Epub 2008 Jun 27. Methods. 2008. PMID: 18586102
-
Monitoring Ligand-Activated Protein-Protein Interactions Using Bioluminescent Resonance Energy Transfer (BRET) Assay.Methods Mol Biol. 2016;1473:3-15. doi: 10.1007/978-1-4939-6346-1_1. Methods Mol Biol. 2016. PMID: 27518618 Review.
-
Circadian programs in cyanobacteria: adaptiveness and mechanism.Annu Rev Microbiol. 1999;53:389-409. doi: 10.1146/annurev.micro.53.1.389. Annu Rev Microbiol. 1999. PMID: 10547696 Review.
Cited by
-
A Perspective on Studying G-Protein-Coupled Receptor Signaling with Resonance Energy Transfer Biosensors in Living Organisms.Mol Pharmacol. 2015 Sep;88(3):589-95. doi: 10.1124/mol.115.098897. Epub 2015 May 13. Mol Pharmacol. 2015. PMID: 25972446 Free PMC article. Review.
-
Monitoring Intracellular pH Change with a Genetically Encoded and Ratiometric Luminescence Sensor in Yeast and Mammalian Cells.Methods Mol Biol. 2016;1461:117-30. doi: 10.1007/978-1-4939-3813-1_9. Methods Mol Biol. 2016. PMID: 27424899 Free PMC article.
-
Exonuclease III protection assay with FRET probe for detecting DNA-binding proteins.Nucleic Acids Res. 2005 Feb 1;33(2):e23. doi: 10.1093/nar/gni021. Nucleic Acids Res. 2005. PMID: 15687381 Free PMC article.
-
Luciferase-Based Biosensors in the Era of the COVID-19 Pandemic.ACS Nanosci Au. 2021 Aug 9;1(1):15-37. doi: 10.1021/acsnanoscienceau.1c00009. eCollection 2021 Dec 15. ACS Nanosci Au. 2021. PMID: 37579261 Free PMC article. Review.
-
Screening of Protein-Protein Interaction Modulators Using BRET-Based Technology.Methods Mol Biol. 2022;2525:173-183. doi: 10.1007/978-1-0716-2473-9_12. Methods Mol Biol. 2022. PMID: 35836067
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

