Redundant and distinct functions for dynamin-1 and dynamin-2 isoforms
- PMID: 9864361
- PMCID: PMC2175237
- DOI: 10.1083/jcb.143.7.1871
Redundant and distinct functions for dynamin-1 and dynamin-2 isoforms
Abstract
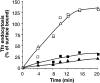
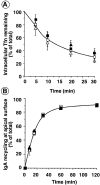
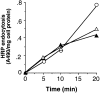
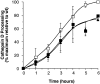
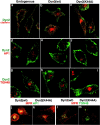
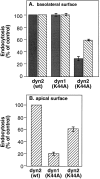
A role for dynamin in clathrin-mediated endocytosis is now well established. However, mammals express three closely related, tissue-specific dynamin isoforms, each with multiple splice variants. Thus, an important question is whether these isoforms and splice variants function in vesicle formation from distinct intracellular organelles. There are conflicting data as to a role for dynamin-2 in vesicle budding from the TGN. To resolve this issue, we compared the effects of overexpression of dominant-negative mutants of dynamin-1 (the neuronal isoform) and dynamin-2 (the ubiquitously expressed isoform) on endocytic and biosynthetic membrane trafficking in HeLa cells and polarized MDCK cells. Both dyn1(K44A) and dyn2(K44A) were potent inhibitors of receptor-mediated endocytosis; however neither mutant directly affected other membrane trafficking events, including transport mediated by four distinct classes of vesicles budding from the TGN. Dyn2(K44A) more potently inhibited receptor-mediated endocytosis than dyn1(K44A) in HeLa cells and at the basolateral surface of MDCK cells. In contrast, dyn1(K44A) more potently inhibited endocytosis at the apical surface of MDCK cells. The two dynamin isoforms have redundant functions in endocytic vesicle formation, but can be targeted to and function differentially at subdomains of the plasma membrane.
Figures
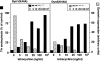

Similar articles
-
Functional comparison of the role of dynamin 2 splice variants on GLUT-4 endocytosis in 3T3L1 adipocytes.Am J Physiol Endocrinol Metab. 2000 May;278(5):E825-31. doi: 10.1152/ajpendo.2000.278.5.E825. Am J Physiol Endocrinol Metab. 2000. PMID: 10780938
-
Isoform and splice-variant specific functions of dynamin-2 revealed by analysis of conditional knock-out cells.Mol Biol Cell. 2008 Dec;19(12):5347-59. doi: 10.1091/mbc.e08-08-0890. Epub 2008 Oct 15. Mol Biol Cell. 2008. PMID: 18923138 Free PMC article.
-
An assembly-incompetent mutant establishes a requirement for dynamin self-assembly in clathrin-mediated endocytosis in vivo.Mol Biol Cell. 2004 May;15(5):2243-52. doi: 10.1091/mbc.e04-01-0015. Epub 2004 Mar 5. Mol Biol Cell. 2004. PMID: 15004222 Free PMC article.
-
Participation of dynamin in the biogenesis of cytoplasmic vesicles.FASEB J. 1999 Dec;13 Suppl 2:S243-7. doi: 10.1096/fasebj.13.9002.s243. FASEB J. 1999. PMID: 10619136 Review.
-
The dynamins: redundant or distinct functions for an expanding family of related GTPases?Proc Natl Acad Sci U S A. 1997 Jan 21;94(2):377-84. doi: 10.1073/pnas.94.2.377. Proc Natl Acad Sci U S A. 1997. PMID: 9012790 Free PMC article. Review.
Cited by
-
TIM family proteins promote the lysosomal degradation of the nuclear receptor NUR77.Sci Signal. 2012 Dec 11;5(254):ra90. doi: 10.1126/scisignal.2003200. Sci Signal. 2012. PMID: 23233528 Free PMC article.
-
An integrin-ILK-microtubule network orients cell polarity and lumen formation in glandular epithelium.Nat Cell Biol. 2013 Jan;15(1):17-27. doi: 10.1038/ncb2646. Nat Cell Biol. 2013. PMID: 23263281 Free PMC article.
-
Trafficking of the Menkes copper transporter ATP7A is regulated by clathrin-, AP-2-, AP-1-, and Rab22-dependent steps.Mol Biol Cell. 2013 Jun;24(11):1735-48, S1-8. doi: 10.1091/mbc.E12-08-0625. Epub 2013 Apr 17. Mol Biol Cell. 2013. PMID: 23596324 Free PMC article.
-
alpha-Synuclein abnormalities in mouse models of peroxisome biogenesis disorders.J Neurosci Res. 2010 Mar;88(4):866-76. doi: 10.1002/jnr.22246. J Neurosci Res. 2010. PMID: 19830841 Free PMC article.
-
Dynamin is required for recombinant adeno-associated virus type 2 infection.J Virol. 1999 Dec;73(12):10371-6. doi: 10.1128/JVI.73.12.10371-10376.1999. J Virol. 1999. PMID: 10559355 Free PMC article.
References
-
- Banting G, Maile R, Roquemore EP. The steady state distribution of humTGN46 is not significantly altered in cells defective in clathrin-mediated endocytosis. J Cell Sci. 1998;111:3451–3458. - PubMed

