Enhancement of human immunodeficiency virus type 1 infection by the CC-chemokine RANTES is independent of the mechanism of virus-cell fusion
- PMID: 9847374
- PMCID: PMC103875
- DOI: 10.1128/JVI.73.1.684-694.1999
Enhancement of human immunodeficiency virus type 1 infection by the CC-chemokine RANTES is independent of the mechanism of virus-cell fusion
Abstract
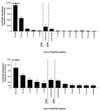
We have studied the effects of CC-chemokines on human immunodeficiency virus type 1 (HIV-1) infection, focusing on the infectivity enhancement caused by RANTES. High RANTES concentrations increase the infectivity of HIV-1 isolates that use CXC-chemokine receptor 4 for entry. However, RANTES can have a similar enhancing effect on macrophagetropic viruses that enter via CC-chemokine receptor 5 (CCR5), despite binding to the same receptor as the virus. Furthermore, RANTES enhances the infectivity of HIV-1 pseudotyped with the envelope glycoprotein of murine leukemia virus or vesicular stomatitis virus, showing that the mechanism of enhancement is independent of the route of virus-cell fusion. The enhancing effects of RANTES are not mediated via CCR5 or other known chemokine receptors and are not mimicked by MIP-1alpha or MIP-1beta. The N-terminally modified derivative aminooxypentane RANTES (AOP-RANTES) efficiently inhibits HIV-1 infection via CCR5 but otherwise mimics RANTES by enhancing viral infectivity. There are two mechanisms of enhancement: one apparent when target cells are pretreated with RANTES (or AOP-RANTES) for several hours, and the other apparent when RANTES (or AOP-RANTES) is added during virus-cell absorption. We believe that the first mechanism is related to cellular activation by RANTES, whereas the second is an increase in virion attachment to target cells.
Figures

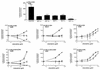
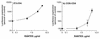
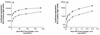
Similar articles
-
Genetic subtype-independent inhibition of human immunodeficiency virus type 1 replication by CC and CXC chemokines.J Virol. 1998 Jan;72(1):396-404. doi: 10.1128/JVI.72.1.396-404.1998. J Virol. 1998. PMID: 9420238 Free PMC article.
-
Aminooxypentane addition to the chemokine macrophage inflammatory protein-1alpha P increases receptor affinities and HIV inhibition.J Biol Chem. 2000 Dec 15;275(50):39254-61. doi: 10.1074/jbc.M006768200. J Biol Chem. 2000. PMID: 11005816
-
CC CKR5: a RANTES, MIP-1alpha, MIP-1beta receptor as a fusion cofactor for macrophage-tropic HIV-1.Science. 1996 Jun 28;272(5270):1955-8. doi: 10.1126/science.272.5270.1955. Science. 1996. PMID: 8658171
-
Rational design of novel HIV-1 entry inhibitors by RANTES engineering.Vaccine. 2008 Jun 6;26(24):3008-15. doi: 10.1016/j.vaccine.2007.12.023. Epub 2008 Jan 10. Vaccine. 2008. PMID: 18243436 Free PMC article. Review.
-
Molecular Role of HIV-1 Human Receptors (CCL5-CCR5 Axis) in neuroAIDS: A Systematic Review.Microorganisms. 2024 Apr 12;12(4):782. doi: 10.3390/microorganisms12040782. Microorganisms. 2024. PMID: 38674726 Free PMC article. Review.
Cited by
-
Efficacy of silk fibroin biomaterial vehicle for in vivo mucosal delivery of Griffithsin and protection against HIV and SHIV infection ex vivo.J Int AIDS Soc. 2020 Oct;23(10):e25628. doi: 10.1002/jia2.25628. J Int AIDS Soc. 2020. PMID: 33073530 Free PMC article.
-
Ex Vivo Evaluation of Mucosal Responses to Vaccination with ALVAC and AIDSVAX of Non-Human Primates.Vaccines (Basel). 2022 Jan 25;10(2):187. doi: 10.3390/vaccines10020187. Vaccines (Basel). 2022. PMID: 35214645 Free PMC article.
-
Higher macrophage inflammatory protein (MIP)-1alpha and MIP-1beta levels from CD8+ T cells are associated with asymptomatic HIV-1 infection.Proc Natl Acad Sci U S A. 2000 Dec 5;97(25):13812-7. doi: 10.1073/pnas.240469997. Proc Natl Acad Sci U S A. 2000. PMID: 11095721 Free PMC article.
-
Hemofiltrate CC chemokine 1[9-74] causes effective internalization of CCR5 and is a potent inhibitor of R5-tropic human immunodeficiency virus type 1 strains in primary T cells and macrophages.Antimicrob Agents Chemother. 2002 Apr;46(4):982-90. doi: 10.1128/AAC.46.4.982-990.2002. Antimicrob Agents Chemother. 2002. PMID: 11897579 Free PMC article.
-
Characterization of structure, dynamics, and detergent interactions of the anti-HIV chemokine variant 5P12-RANTES.Biophys J. 2013 Dec 3;105(11):2586-97. doi: 10.1016/j.bpj.2013.10.025. Biophys J. 2013. PMID: 24314089 Free PMC article.
References
-
- Alkhatib G, Combadiere C, Broder C C, Feng Y, Kennedy P E, Murphy P M, Berger E A. CC CKR5: a RANTES, MIP-1α, MIP-1β receptor as a fusion cofactor for macrophage-tropic HIV-1. Science. 1996;272:1955–1958. - PubMed
-
- Amara A, Le Gall S, Schwartz O, Salamero J, Montes M, Loetscher P, Baggiolini M, Virelizier J-L, Arenzana-Seisdedos F. HIV coreceptor downregulation as antiviral principle: SDF-1α-dependent internalization of the chemokine receptor CXCR4 contributes to inhibition of HIV replication. J Exp Med. 1997;186:139–146. - PMC - PubMed
-
- Arenzana-Seisedos F, Virelizier J-L, Rousset D, Clark-Lewis I, Loetscher P, Moser B, Baggiolini M. HIV blocked by chemokine antagonist. Nature. 1996;383:400. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

